Role of Post-Transcriptional Regulation in Learning and Memory in Mammals
Abstract
1. Introduction
2. Post-Transcriptional Regulation and Synaptic Plasticity during the Nervous System’s Development
2.1. Prelocalization of mRNAs and the Role of RNA-Binding Proteins and miRNAs in Normal Development
2.1.1. RNA Processing and Localization
2.1.2. The Developmental Impact of RNA-Binding Proteins
2.1.3. The Role of miRNAs in Brain Development
2.1.4. The Developmental Role of mRNA Modifications
2.1.5. Prelocalization of mRNAs and the Role of RNA-Binding Proteins in Developmental Pathologies
3. Post-Transcriptional Regulation and Synaptic Plasticity in the Adult Brain: Learning and Memory
3.1. Prelocalized mRNAs and RNA-Binding Proteins in the Normal Adult Brain
3.2. Translational Control at the Synapses: Signals and Mechanisms
3.3. Inverse Traffic from the Synapse to the Nucleus
3.4. Alterations of mRNA and RNA-Binding Protein Prelocalization in Different Pathologies of the Adult Brain
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orgel, L.E. Evolution of the genetic apparatus. J. Mol. Biol. 1968, 38, 381–393. [Google Scholar] [CrossRef]
- Gilbert, W. The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef]
- Spirin, A.S. Omnipotent RNA. FEBS Lett. 2002, 530, 4–8. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Kazakov, S.A.; Johnston, B.H.; Landweber, L.F. The RNA world on ice: A new scenario for the emergence of RNA information. J. Mol. Evol. 2005, 61, 264–273. [Google Scholar] [CrossRef]
- Taylor, W.R. Transcription and translation in an RNA world. Philos. Trans. Soc. B 2006, 361, 1751–1760. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Schirò, G.; Di Liegro, I. RNA-Binding Proteins as Epigenetic Regulators of Brain Functions and Their Involvement in Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 14622. [Google Scholar] [CrossRef]
- Rufo, C.M.; Moroz, Y.S.; Moroz, O.V.; Stöhr, J.; Smith, T.A.; Hu, X.; DeGrado, W.F.; Korendovych, I.V. Short peptides self-assemble to produce catalytic amyloids. Nat. Chem. 2014, 6, 303–309. [Google Scholar] [CrossRef]
- Greenwald, J.; Kwiatkowski, W.; Riek, R. Peptide Amyloids in the origin of life. J. Mol. Biol. 2018, 430, 3735–3750. [Google Scholar] [CrossRef]
- Carny, O.; Gazit, E. A model for the role of short self-assembled peptides in the very early stages of the origin of life. FASEB J. 2005, 19, 1051–1055. [Google Scholar] [CrossRef]
- Singh, P.P.; Banerji, A. Case for an RNA-prion world: A hypothesis based on conformational diversity. J. Biol. Phys. 2011, 37, 185–188. [Google Scholar] [CrossRef][Green Version]
- Maury, C.P.J. Amyloid and the origin of life: Self-replicating catalytic amyloids as prebiotic informational and protometabolic entities. Cell. Mol. Life Sci. 2018, 75, 1499–1507. [Google Scholar] [CrossRef]
- Zozulia, O.; Dolan, M.A.; Korendovych, I.V. Catalytic peptide assemblies. Chem. Soc. Rev. 2018, 47, 3621–3639. [Google Scholar] [CrossRef]
- Ohashi, R.; Shiina, N. Cataloguing and Selection of mRNAs Localized to Dendrites in Neurons and Regulated by RNA-Binding Proteins in RNA Granules. Biomolecules 2020, 10, 167. [Google Scholar] [CrossRef]
- Kedersha, N.; Stoecklin, G.; Ayodele, M.; Yacono, P.; Lykke-Andersen, J.; Fritzler, M.J.; Scheuner, D.; Kaufman, R.J.; Golan, D.E.; Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005, 169, 871–884. [Google Scholar] [CrossRef]
- Kiebler, M.A.; Bassell, G.J. Neuronal RNA granules: Movers and makers. Neuron 2006, 51, 685–690. [Google Scholar] [CrossRef]
- Derrigo, M.; Cestelli, A.; Savettieri, G.; Di Liegro, I. RNA-protein interactions in the control of stability and localization of messenger RNA (Review). Int. J. Mol. Med. 2000, 5, 111–123. [Google Scholar] [CrossRef]
- Hutten, S.; Sharangdhar, T.; Kiebler, M. Unmasking the messenger. RNA Biol. 2014, 11, 992–997. [Google Scholar] [CrossRef]
- Nam, J.-W.; Choi, S.-W.; You, B.-H. Incredible RNA: Dual Functions of Coding and Noncoding. Mol. Cells 2016, 39, 367–374. [Google Scholar] [CrossRef]
- Sudhakaran, I.; Ramaswami, M. Long-term memory consolidation: The role of RNA-binding proteins with prion-like domains. RNA Biol. 2017, 14, 568–586. [Google Scholar] [CrossRef]
- Si, K. Prions: What are they good for? Annu. Rev. Cell. Dev. Biol. 2015, 31, 149–169. [Google Scholar] [CrossRef]
- Si, K.; Kandel, E.R. The Role of Functional Prion-Like Proteins in the Persistence of Memory. Cold Spring Harb. Perspect. Biol. 2016, 8, a021774. [Google Scholar] [CrossRef]
- Danilov, L.G.; Sukhanova, X.V.; Rogoza, T.M.; Antonova, E.Y.; Trubitsina, N.P.; Zhouravleva, G.A.; Bondarev, S.A. Identification of New FG-Repeat Nucleoporins with Amyloid Properties. Int. J. Mol. Sci. 2023, 24, 8571. [Google Scholar] [CrossRef]
- Bolognani, F.; Perrone-Bizzozero, N.I. RNA-protein interactions and control of mRNA stability in neurons. J. Neurosci. Res. 2008, 86, 481–489. [Google Scholar] [CrossRef]
- Ravanidis, S.; Kattan, F.G.; Doxakis, E. Unraveling the Pathways to Neuronal Homeostasis and Disease: Mechanistic Insights into the Role of RNA-Binding Proteins and Associated Factors. Int. J. Mol. Sci. 2018, 19, 2280. [Google Scholar] [CrossRef]
- Landinez-Macias, M.; Urwyler, O. The Fine Art of Writing a Message: RNA Metabolism in the Shaping and Remodeling of the Nervous System. Front. Mol. Neurosci. 2021, 14, 755686. [Google Scholar] [CrossRef]
- Guan, W.; Bellemin, S.; Bouchet, M.; Venkatasubramanian, L.; Guillermin, C.; Laurençon, A.; Kabir, C.; Darnas, A.; Godin, C.; Urdy, S.; et al. Post-transcriptional regulation of transcription factor codes in immature neurons drives neuronal diversity. Cell Rep. 2022, 39, 110992. [Google Scholar] [CrossRef]
- Parra, A.S.; Johnston, C.A. Emerging Roles of RNA-Binding Proteins in Neurodevelopment. J. Dev. Biol. 2022, 10, 23. [Google Scholar] [CrossRef]
- Darnell, J.C.; Richter, J.D. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb. Perspect. Biol. 2012, 4, a012344. [Google Scholar] [CrossRef]
- Mirisis, A.A.; Carew, T.J. The ELAV family of RNA-binding proteins in synaptic plasticity and long-term memory. Neurobiol. Learn. Mem. 2019, 161, 143–148. [Google Scholar] [CrossRef]
- Fifková, E.; Van Harreveld, A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J. Neurocytol. 1977, 6, 211–230. [Google Scholar] [CrossRef]
- Desmond, N.L.; Levy, W.B. Synaptic interface surface area increases with long-term potentiation in the hippocampal dentate gyrus. Brain Res. 1988, 453, 308–314. [Google Scholar] [CrossRef]
- Weeks, A.C.; Ivanco, T.L.; Leboutillier, J.C.; Racine, R.J.; Petit, T.L. Sequential changes in the synaptic structural profile following long-term potentiation in the rat dentate gyrus: III. Long-term maintenance phase. Synapse 2001, 40, 74–84. [Google Scholar] [CrossRef]
- Mezey, S.; Doyère, V.; De Souza, I.; Harrison, E.; Cambon, K.; Kendal, C.E.; Davies, H.; Laroche, S.; Stewart, M.G. Long term synaptic morphometry changes after induction of long term potentiation and long term depression in the dentate gyrus of awake rats are not simply mirror phenomena. Eur. J. Neurosci. 2004, 19, 2310–2318. [Google Scholar] [CrossRef]
- Samavat, M.; Bartol, T.M.; Bromer, C.; Hubbard, D.D.; Hanka, D.C.; Kuwajima, M.; Mendenhall, J.M.; Parker, P.H.; Bowden, J.B.; Abraham, W.C.; et al. Long-Term Potentiation Produces a Sustained Expansion of Synaptic Information Storage Capacity in Adult Rat Hippocampus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bartol, T.M.; Ordyan, M.; Sejnowski, T.J.; Rangamani, P.; Kennedy, M.B. A spatial model of autophosphorylation of CaMKII in a glutamatergic spine suggests a network-driven kinetic mechanism for bistable changes in synaptic strength. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bonilla-Quintana, M.; Rangamani, P. Biophysical modeling of actin-mediated structural plasticity reveals mechanical adaptation in dendritic spines. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, G.; McLaughlin, D.W.; Peskin, C.S. A biochemical description of postsynaptic plasticity-with timescales ranging from milliseconds to seconds. Proc. Natl. Acad. Sci. USA 2024, 121, e2311709121. [Google Scholar] [CrossRef]
- Patton, M.H.; Thomas, K.T.; Bayazitov, I.T.; Newman, K.D.; Kurtz, N.B.; Robinson, C.G.; Ramirez, C.A.; Trevisan, A.J.; Bikoff, J.B.; Peters, S.T.; et al. Synaptic plasticity in human thalamocortical assembloids. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, S.; Zhang, T.; Song, J.; Dong, M.; Qian, Y.; Teng, J.; Wang, T.; He, C.; Shen, Y. Age-related differences in long-term potentiation-like plasticity and short-latency afferent inhibition and their association with cognitive function. Gen. Psychiatr. 2024, 37, e101181. [Google Scholar] [CrossRef]
- Szabo, A.; Dalmau, J.; Manley, G.; Rosenfeld, M.; Wong, E.; Henson, J.; Posner, J.B.; Furneaux, H.M. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell 1991, 67, 325–333. [Google Scholar] [CrossRef]
- Ma, W.J.; Chung, S.; Furneaux, H. The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 1997, 25, 3564–3569. [Google Scholar] [CrossRef]
- Joseph, B.; Orlian, M.; Furneaux, H. p21(waf1) mRNA contains a conserved element in its 3′-untranslated region that is bound by the Elav-like mRNA-stabilizing proteins. J. Biol. Chem. 1998, 273, 20511–20516. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.A.; Kaang, B.K. Regulation of mRNA stability by ARE-binding proteins in synaptic plasticity and memory. Neurobiol. Learn. Mem. 2015, 124, 28–33. [Google Scholar] [CrossRef]
- Deschenes-Furry, J.; Belanger, G.; Perrone-Bizzozero, N.; Jasmin, B.J. Post-transcriptional regulation of acetylcholinesterase mRNAs in nerve growth factor-treated PC12 cells by the RNA-binding protein HuD. J. Biol. Chem. 2003, 278, 5710–5717. [Google Scholar] [CrossRef]
- Wein, G.; Rossler, M.; Klug, R.; Herget, T. The 3′-UTR of the mRNA coding for the major protein kinase C substrate MARCKS contains a novel CU-rich element interacting with the mRNA stabilizing factors HuD and HuR. Eur. J. Biochem. 2003, 270, 350–365. [Google Scholar] [CrossRef]
- Jung, M.; Lee, E.K. RNA-Binding Protein HuD as a Versatile Factor in Neuronal and Non-Neuronal Systems. Biology 2021, 10, 361. [Google Scholar] [CrossRef]
- Bolognani, F.; Tanner, D.C.; Merhege, M.; Deschênes-Furry, J.; Jasmin, B.; Perrone-Bizzozero, N.I. In vivo post-transcriptional regulation of GAP-43 mRNA by overexpression of the RNA-binding protein HuD. J. Neurochem. 2006, 96, 790–801. [Google Scholar] [CrossRef]
- Lim, C.S.; Alkon, D.L. Protein kinase C stimulates HuD-mediated mRNA stability and protein expression of neurotrophic factors and enhances dendritic maturation of hippocampal neurons in culture. Hippocampus 2012, 22, 2303–2319. [Google Scholar] [CrossRef]
- Fiore, R.; Schratt, G. MicroRNAs in vertebrate synapse development. Sci. World J. 2007, 7, 167–177. [Google Scholar] [CrossRef]
- Corbin, R.; Olsson-Carter, K.; Slack, F. The role of microRNAs in synaptic development and function. BMB Rep. 2009, 42, 131–135. [Google Scholar] [CrossRef]
- Schratt, G. MicroRNAs at the synapse. Nat. Rev. Neurosci. 2009, 10, 842–849. [Google Scholar] [CrossRef]
- Wang, W.; Kwon, E.J.; Tsai, L.H. MicroRNAs in learning, memory, and neurological diseases. Learn. Mem. 2012, 19, 359–368. [Google Scholar] [CrossRef]
- Capitano, F.; Camon, J.; Ferretti, V.; Licursi, V.; De Vito, F.; Rinaldi, A.; Vincenti, S.; Mannironi, C.; Fragapane, P.; Bozzoni, I.; et al. microRNAs Modulate Spatial Memory in the Hippocampus and in the Ventral Striatum in a Region-Specific Manner. Mol Neurobiol. 2016, 53, 4618–4630. [Google Scholar] [CrossRef]
- Busto, G.U.; Guven-Ozkan, T.; Davis, R.L. MicroRNA function in Drosophila memory formation. Curr. Opin. Neurobiol. 2017, 43, 15–24. [Google Scholar] [CrossRef]
- Barbato, C. MicroRNA-Mediated Silencing Pathways in the Nervous System and Neurological Diseases. Cells 2022, 11, 2375. [Google Scholar] [CrossRef]
- Parkins, E.V.; Burwinkel, J.M.; Ranatunga, R.; Yaser, S.; Hu, Y.C.; Tiwari, D.; Gross, C. Age-Dependent Regulation of Dendritic Spine Density and Protein Expression in Mir324 KO Mice. J. Mol. Neurosci. 2023, 73, 818–830. [Google Scholar] [CrossRef]
- Maiorano, N.A.; Mallamaci, A. The pro-differentiating role of miR-124: Indicating the road to become a neuron. RNA Biol. 2010, 7, 528–533. [Google Scholar] [CrossRef]
- Raj, B.; Blencowe, B.J. Alternative splicing in the mammalian nervous system: Recent insights into mechanisms and functional roles. Neuron 2015, 87, 14–27. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437–451. [Google Scholar] [CrossRef]
- Glanzer, J.; Miyashiro, K.Y.; Sul, J.Y.; Barrett, L.; Belt, B.; Haydon, P.; Eberwine, J. RNA splicing capability of live neuronal dendrites. Proc. Natl. Acad. Sci. USA 2005, 102, 16859–16864. [Google Scholar] [CrossRef]
- Zheng, J.; Redmond, L.; Xu, C.; Kuang, J.; Liao, W. Alternative splicing in the variable domain of CaMKII affects the level of F-actin association in developing neurons. Int. J. Clin. Exp. Pathol. 2014, 7, 2963–2975. [Google Scholar]
- Schreiner, D.; Nguyen, T.M.; Russo, G.; Heber, S.; Patrignani, A.; Ahrne, E.; Scheiffele, P. Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron 2014, 84, 386–398. [Google Scholar] [CrossRef]
- Sudhof, T.C. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 2017, 171, 745–769. [Google Scholar] [CrossRef]
- Gomez, A.M.; Traunmüller, L.; Scheiffele, P. Neurexins: Molecular codes for shaping neuronal synapses. Nat. Rev. Neurosci. 2021, 22, 137–151. [Google Scholar] [CrossRef]
- Lennox, A.L.; Mao, H.; Silver, D.L. RNA on the brain: Emerging layers of post-transcriptional regulation in cerebral cortex development. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e290. [Google Scholar] [CrossRef]
- Machida, K.; Shigeta, T.; Yamamoto, Y.; Ito, T.; Svitkin, Y.; Sonenberg, N.; Imataka, H. Dynamic interaction of poly(A)-binding protein with the ribosome. Sci. Rep. 2018, 8, 17435. [Google Scholar] [CrossRef]
- Stewart, M. Polyadenylation and nuclear export of mRNAs. J. Biol. Chem. 2019, 294, 2977–2987. [Google Scholar] [CrossRef]
- Eisen, T.J.; Eichhorn, S.W.; Subtelny, A.O.; Lin, K.S.; McGeary, S.E.; Gupta, S.; Bartel, D.P. The Dynamics of Cytoplasmic mRNA Metabolism. Mol. Cell. 2020, 77, 786–799. [Google Scholar] [CrossRef]
- Kiltschewskij, D.J.; Harrison, P.F.; Fitzsimmons, C.; Beilharz, T.H.; Cairns, M.J. Extension of mRNA poly(A) tails and 3′UTRs during neuronal differentiation exhibits variable association with post-transcriptional dynamics. Nucleic Acids Res. 2023, 51, 8181–8198. [Google Scholar] [CrossRef]
- Xia, Z.; Donehower, L.A.; Cooper, T.A.; Neilson, J.R.; Wheeler, D.A.; Wagner, E.J.; Li, W. Dynamic analyses of alternative polyadenylation from RNA-seq reveal a 3′-UTR landscape across seven tumour types. Nat. Commun. 2014, 5, 5274. [Google Scholar] [CrossRef]
- Miura, P.; Shenker, S.; Andreu-Agullo, C.; Westholm, J.O.; Lai, E.C. Widespread and extensive lengthening of 3′ UTRs in the mammalian brain. Genome Res. 2013, 23, 812–825. [Google Scholar] [CrossRef]
- Grassi, E.; Santoro, R.; Umbach, A.; Grosso, A.; Oliviero, S.; Neri, F.; Conti, L.; Ala, U.; Provero, P.; DiCunto, F.; et al. Choice of alternative polyadenylation sites, mediated by the RNA-binding protein Elavl3, plays a role in differentiation of inhibitory neuronal progenitors. Front. Cell Neurosci. 2018, 12, 518. [Google Scholar] [CrossRef]
- Xiang, K.; Bartel, D.P. The molecular basis of coupling between poly(A)-tail length and translational efficiency. eLife 2021, 10, e66493. [Google Scholar] [CrossRef]
- De Magistris, P. The Great Escape: mRNA Export through the Nuclear Pore Complex. Int. J. Mol. Sci. 2021, 22, 11767. [Google Scholar] [CrossRef]
- Gebauer, F.; Schwarzl, T.; Valcárcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet. 2021, 22, 185–198. [Google Scholar] [CrossRef]
- Hafner, A.S.; Donlin-Asp, P.G.; Leitch, B.; Herzog, E.; Schuman, E.M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 2019, 364, eaau3644. [Google Scholar] [CrossRef]
- Okano, H.J.; Darnell, R.B. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997, 17, 3024–3037. [Google Scholar] [CrossRef]
- Ustaoglu, P.; Gill, J.K.; Doubovetzky, N.; Haussmann, I.U.; Dix, T.C.; Arnold, R.; Devaud, J.M.; Soller, M. Dynamically expressed single ELAV/Hu orthologue elavl2 of bees is required for learning and memory. Commun. Biol. 2021, 4, 1234. [Google Scholar] [CrossRef]
- Fukuda, N.; Fukuda, T.; Percipalle, P.; Oda, K.; Takei, N.; Czaplinski, K.; Touhara, K.; Yoshihara, Y.; Sasaoka, T. Axonal mRNA binding of hnRNP A/B is crucial for axon targeting and maturation of olfactory sensory neurons. Cell Rep. 2023, 42, 112398. [Google Scholar] [CrossRef]
- Murn, J.; Zarnack, K.; Yang, Y.J.; Durak, O.; Murphy, E.A.; Cheloufi, S.; Gonzalez, D.M.; Teplova, M.; Curk, T.; Zuber, J.; et al. Control of a neuronal morphology program by an RNA-binding zinc finger protein, Unkempt. Genes Dev. 2015, 29, 501–512. [Google Scholar] [CrossRef]
- Vinsland, E.; Baskaran, P.; Mihaylov, S.R.; Hobbs, C.; Wood, H.; Bouybayoune, I.; Shah, K.; Houart, C.; Tee, A.R.; Murn, J.; et al. The zinc finger/RING domain protein Unkempt regulates cognitive flexibility. Sci. Rep. 2021, 11, 16299. [Google Scholar] [CrossRef]
- Baskaran, P.; Mihaylov, S.R.; Vinsland, E.; Shah, K.; Granat, L.; Ultanir, S.K.; Tee, A.R.; Murn, J.; Bateman, J.M. Phosphorylation of the novel mTOR substrate Unkempt regulates cellular morphogenesis. J. Biol. Chem. 2023, 299, 102788. [Google Scholar] [CrossRef]
- Harb, K.; Richter, M.; Neelagandan, N.; Magrinelli, E.; Harfoush, H.; Kuechler, K.; Henis, M.; Hermanns-Borgmeyer, I.; Calderon de Anda, F.; Duncan, K. Pum2 and TDP-43 refine area-specific cytoarchitecture post-mitotically and modulate translation of Sox5, Bcl11b, and Rorb mRNAs in developing mouse neocortex. eLife 2022, 11, e55199. [Google Scholar] [CrossRef]
- Liu, W.; Xie, H.; Liu, X.; Xu, S.; Cheng, S.; Wang, Z.; Xie, T.; Zhang, Z.C.; Han, J. PQBP1 regulates striatum development through balancing striatal progenitor proliferation and differentiation. Cell Rep. 2023, 42, 112277. [Google Scholar] [CrossRef]
- Jacko, M.; Weyn-Vanhentenryck, S.M.; Smerdon, J.W.; Yan, R.; Feng, H.; Williams, D.J.; Pai, J.; Xu, K.; Wichterle, H.; Zhang, C. Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron 2018, 97, 853–868.e6. [Google Scholar] [CrossRef]
- Leterrier, C. The axon initial segment: An updated viewpoint. J. Neurosci. 2018, 38, 2135–2145. [Google Scholar] [CrossRef]
- Szeto, R.A.; Tran, T.; Truong, J.; Negraes, P.D.; Trujillo, C.A. RNA processing in neurological tissue: Development, aging and disease. Semin. Cell. Dev. Biol. 2021, 114, 57–67. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mrnas linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef]
- Napoli, I.; Mercaldo, V.; Boyl, P.P.; Eleuteri, B.; Zalfa, F.; De Rubeis, S.; Di Marino, D.; Mohr, E.; Massimi, M.; Falconi, M.; et al. The fragile X syndrome protein represses activity-dependent translation through cyfip1, a new 4e-bp. Cell 2008, 134, 1042–1054. [Google Scholar] [CrossRef]
- Chen, E.; Sharma, M.R.; Shi, X.; Agrawal, R.K.; Joseph, S. Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell 2014, 54, 407–417. [Google Scholar] [CrossRef]
- Yu, J.; Woo, Y.; Kim, H.; An, S.; Park, S.K.; Jang, S.K. FMRP Enhances the Translation of 4EBP2 mRNA during Neuronal Differentiation. Int. J. Mol. Sci. 2023, 24, 16319. [Google Scholar] [CrossRef]
- Mao, S.; Li, H.; Sun, Q.; Zen, K.; Zhang, C.-Y.; Li, L. miR-17 regulates the proliferation and differentiation of the neural precursor cells during mouse corticogenesis. FEBS J. 2014, 281, 1144–1158. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chung, K.H.; Deo, M.; Thompson, R.C.; Turner, D.L. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp. Cell Res. 2008, 314, 2618–2633. [Google Scholar] [CrossRef]
- Wei, C.; Ren, L.; Li, K.; Lu, Z. The regulation of survival and differentiation of neural stem cells by miR-124 via modulating PAX3. Neurosci. Lett. 2018, 683, 19–26. [Google Scholar] [CrossRef]
- Hou, Q.; Ruan, H.; Gilbert, J.; Wang, G.; Ma, Q.; Yao, W.D.; Man, H.Y. MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat. Commun. 2015, 6, 10045. [Google Scholar] [CrossRef]
- Santos, M.C.; Tegge, A.N.; Correa, B.R.; Mahesula, S.; Kohnke, L.Q.; Qiao, M.; Ferreira, M.A.; Kokovay, E.; Penalva, L.O. miR-124, -128, and -137 Orchestrate Neural Differentiation by Acting on Overlapping Gene Sets Containing a Highly Connected Transcription Factor Network. Stem Cells 2016, 34, 220–232. [Google Scholar] [CrossRef]
- Fiorenza, A.; Barco, A. Role of Dicer and the miRNA system in neuronal plasticity and brain function. Neurobiol. Learn. Mem. 2016, 135, 3–12. [Google Scholar] [CrossRef]
- Ristori, E.; Lopez-Ramirez, M.A.; Narayanan, A.; Hill-Teran, G.; Moro, A.; Calvo, C.F.; Thomas, J.L.; Nicoli, S. A Dicer-miR-107 Interaction Regulates Biogenesis of Specific miRNAs Crucial for Neurogenesis. Dev. Cell 2015, 32, 546–560. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Han, X.; Yu, Y.; Ye, F.; Chen, Y.; Hoang, T.; Xu, X.; Mi, Q.S.; Xin, M.; et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 2010, 65, 612–626. [Google Scholar] [CrossRef]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef]
- Suster, I.; Feng, Y. Multifaceted Regulation of MicroRNA Biogenesis: Essential Roles and Functional Integration in Neuronal and Glial Development. Int. J. Mol. Sci. 2021, 22, 6765. [Google Scholar] [CrossRef]
- Yoon, K.J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.S.; Zhu, Y.; Zheng, L.; et al. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell 2017, 171, 877–889.e817. [Google Scholar] [CrossRef]
- Li, Y.; Xia, L.; Tan, K.; Ye, X.; Zuo, Z.; Li, M.; Xiao, R.; Wang, Z.; Liu, X.; Deng, M.; et al. N(6)-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat. Genet. 2020, 52, 870–877. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, S.M.; Yoon, K.J. Epitranscriptomic regulation of transcriptome plasticity in development and diseases of the brain. BMB Rep. 2020, 53, 551–564. [Google Scholar] [CrossRef]
- Thelen, M.P.; Kye, M.J. The Role of RNA Binding Proteins for Local mRNA Translation: Implications in Neurological Disorders. Front. Mol. Biosci. 2020, 6, 161. [Google Scholar] [CrossRef]
- Wang, B.; Pan, L.; Wei, M.; Wang, Q.; Liu, W.W.; Wang, N.; Jiang, X.Y.; Zhang, X.; Bao, L. FMRP-mediated axonal delivery of miR-181d regulates axon elongation by locally targeting Map1b and Calm1. Cell Rep. 2015, 13, 2794–2807. [Google Scholar] [CrossRef]
- El-Agamy, S.E.; Guillaud, L.; Kono, K.; Wu, Y.; Terenzio, M. FMRP Long-Range Transport and Degradation Are Mediated by Dynlrb1 in Sensory Neurons. Mol. Cell. Proteom. 2023, 22, 100653. [Google Scholar] [CrossRef]
- Antar, L.N.; Dictenberg, J.B.; Plociniak, M.; Afroz, R.; Bassell, G.J. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005, 4, 350–359. [Google Scholar] [CrossRef]
- Joo, Y.; Benavides, D.R. Local Protein Translation and RNA Processing of Synaptic Proteins in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 2811. [Google Scholar] [CrossRef]
- Oliver, R.J.; Kenton, J.A.; Stevens, W.; Perrone-Bizzozero, N.I.; Brigman, J.L. Overexpression of neuronal RNA-binding protein HuD increases reward induced reinstatement of an instrumental response. Neurosci. Lett. 2018, 683, 119–124. [Google Scholar] [CrossRef]
- Chan, J.N.; Sánchez-Vidaña, D.I.; Anoopkumar-Dukie, S.; Li, Y.; Benson Wui-Man, L. RNA-binding protein signaling in adult neurogenesis. Front. Cell Dev. Biol. 2022, 10, 982549. [Google Scholar] [CrossRef]
- Bredy, T.W.; Lin, Q.; Wei, W.; Baker-Andresen, D.; Mattick, J.S. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 2011, 96, 89–94. [Google Scholar] [CrossRef]
- Lin, Q.; Wei, W.; Coelho, C.M.; Li, X.; Baker-Andresen, D.; Dudley, K.; Ratnu, V.S.; Boskovic, Z.; Kobor, M.S.; Sun, Y.E.; et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 2011, 14, 1115–1117. [Google Scholar] [CrossRef]
- Cohen, J.E.; Lee, P.R.; Fields, R.D. Systematic identification of 3′-UTR regulatory elements in activity-dependent mRNA stability in hippocampal neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130509. [Google Scholar] [CrossRef]
- Ryan, B.; Williams, J.M. Novel microRNA revealed by systematic analysis of the microRNA transcriptome in dentate gyrus granule cells. Neurosci. Lett. 2019, 707, 132280. [Google Scholar] [CrossRef]
- Rogelj, B. Brain-specific small nucleolar RNAs. J. Mol. Neurosci. 2006, 28, 103–109. [Google Scholar] [CrossRef]
- Sim, S.E.; Bakes, J.; Kaang, B.K. Neuronal activity-dependent regulation of MicroRNAs. Mol. Cells 2014, 37, 511–517. [Google Scholar] [CrossRef]
- Ho, V.M.; Dallalzadeh, L.O.; Karathanasis, N.; Keles, M.F.; Vangala, S.; Grogan, T.; Poirazi, P.; Martin, K.C. GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons. Mol. Cell. Neurosci. 2014, 61, 1–12. [Google Scholar] [CrossRef]
- Malmevik, J.; Petri, R.; Knauff, P.; Brattås, P.L.; Åkerblom, M.; Jakobsson, J. Distinct cognitive effects and underlying transcriptome changes upon inhibition of individual miRNAs in hippocampal neurons. Sci. Rep. 2016, 6, 19879. [Google Scholar] [CrossRef]
- Ahn, H.; Durang, X.; Shim, J.Y.; Park, G.; Jeon, J.-H.; Park, H.Y. Statistical modeling of mRNP transport in dendrites: A comparative analysis of β-actin and Arc mRNP dynamics. Traffic 2023, 24, 522–532. [Google Scholar] [CrossRef]
- Alecki, C.; Rizwan, J.; Le, P.; Jacob-Tomas, S.; Xu, S.; Minotti, S.; Wu, T.; Durham, H.; Yeo, G.W.; Vera, M. Localized synthesis of molecular chaperones sustains neuronal proteostasis. bioRxiv 2023. [Google Scholar] [CrossRef]
- D’Arcangelo, G. Apoer2: A reelin receptor to remember. Neuron 2005, 47, 471–473. [Google Scholar] [CrossRef]
- Gao, J.; Marosi, M.; Choi, J.; Achiro, J.M.; Kim, S.; Li, S.; Otis, K.; Martin, K.C.; Portera-Cailliau, C.; Tontonoz, P. The E3 ubiquitin ligase IDOL regulates synaptic ApoER2 levels and is important for plasticity and learning. eLife 2017, 6, e29178. [Google Scholar] [CrossRef]
- Ule, J.; Darnell, R.B. RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr. Opin. Neurobiol. 2006, 16, 102–110. [Google Scholar] [CrossRef]
- Denny, J.B. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr. Neuropharmacol. 2006, 4, 293–304. [Google Scholar] [CrossRef]
- Holaran, M.R. A Shift from a Pivotal to Supporting Role for the Growth-Associated Protein (GAP-43) in the Coordination of Axonal Structural and Functional Plasticity. Front. Cell. Neurosci. 2017, 11, 266. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Mendez, R.; Fernandez, M.; Richter, J.D. CPEB and translational control by cytoplasmic polyadenylation: Impact on synaptic plasticity, learning, and memory. Mol. Psychiatry 2023, 28, 2728–2736. [Google Scholar] [CrossRef]
- Kozlov, E.N.; Deev, R.V.; Tokmatcheva, E.V.; Tvorogova, A.; Kachaev, Z.M.; Gilmutdinov, R.A.; Zhukova, M.; Savvateeva-Popova, E.V.; Schedl, P.; Shidlovskii, Y.V. 3′UTR of mRNA Encoding CPEB Protein Orb2 Plays an Essential Role in Intracellular Transport in Neurons. Cells 2023, 12, 1717. [Google Scholar] [CrossRef]
- Ford, L.; Asok, A.; Tripp, A.D.; Parro, C.; Fitzpatrick, M.; de Solis, C.A.; Chen, P.Y.; Shafiian, N.; Fioriti, L.; Soni, R.K.; et al. CPEB3 low-complexity motif regulates local protein synthesis via protein-protein interactions in neuronal ribonucleoprotein granules. Proc. Natl. Acad. Sci. USA 2023, 120, e2114747120. [Google Scholar] [CrossRef]
- Capitano, F.; Camon, J.; Licursi, V.; Ferretti, V.; Maggi, L.; Scianni, M.; Del Vecchio, G.; Rinaldi, A.; Mannironi, C.; Limatola, C.; et al. MicroRNA-335-5p modulates spatial memory and hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2017, 139, 63–68. [Google Scholar] [CrossRef]
- Zhang, S.F.; Chen, J.C.; Zhang, J.; Xu, J.G. miR-181a involves in the hippocampus-dependent memory formation via targeting PRKAA1. Sci. Rep. 2017, 7, 8480. [Google Scholar] [CrossRef]
- Mladenova, D.; Barry, G.; Konen, L.M.; Pineda, S.S.; Guennewig, B.; Avesson, L.; Zinn, R.; Schonrock, N.; Bitar, M. Adar3 Is Involved in Learning and Memory in Mice. Front. Neurosci. 2018, 12, 243. [Google Scholar] [CrossRef]
- Warhaftig, G.; Sokolik, C.M.; Khermesh, K.; Lichtenstein, Y.; Barak, M.; Bareli, T.; Levanon, E.Y.; Yadid, G. RNA editing of the 5-HT2C receptor in the central nucleus of the amygdala is involved in resilience behavior. Transl. Psychiatry 2021, 11, 137. [Google Scholar] [CrossRef]
- Brito, D.V.C.; Gulmez Karaca, K.; Kupke, J.; Frank, L.; Oliveira, A.M.M. MeCP2 gates spatial learning-induced alternative splicing events in the mouse hippocampus. Mol. Brain. 2020, 13, 156. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhang, B.; Li, Y.; Long, Y.; Zhou, J.; Zou, X.; Zhang, M.; Hu, Y.; Chen, W.; et al. DeeReCT-APA: Prediction of Alternative Polyadenylation Site Usage Through Deep Learning. Genom. Proteom. Bioinform. 2022, 20, 483–495. [Google Scholar] [CrossRef]
- Luo, Z.; Su, W.; Lou, L.; Qiu, W.; Xiao, X.; Xu, Z. DLm6Am: A Deep-Learning-Based Tool for Identifying N6,2′-O-Dimethyladenosine Sites in RNA Sequences. Int. J. Mol. Sci. 2022, 23, 11026. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Huang, T.; Li, G.; Zhang, L.; Sun, Y. EMDLP: Ensemble multiscale deep learning model for RNA methylation site prediction. BMC Bioinform. 2022, 23, 221. [Google Scholar] [CrossRef]
- Lekk, I.; Cabrera-Cabrera, F.; Turconi, G.; Tuvikene, J.; Esvald, E.E.; Rähni, A.; Casserly, L.; Garton, D.R.; Andressoo, J.O.; Timmusk, T.; et al. Untranslated regions of brain-derived neurotrophic factor mRNA control its translatability and subcellular localization. J. Biol. Chem. 2023, 299, 102897. [Google Scholar] [CrossRef]
- Saletore, Y.; Meyer, K.; Korlach, J.; Vilfan, I.D.; Jaffrey, S.; Mason, C.E. The birth of the Epitranscriptome: Deciphering the function of RNA modifications. Genome Biol. 2012, 13, 175. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5′UTR M(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef]
- Weng, Y.L.; Wang, X.; An, R.; Cassin, J.; Vissers, C.; Liu, Y.; Liu, Y.; Xu, T.; Wang, X.; Wong, S.Z.; et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron 2018, 97, 313–325.e6. [Google Scholar] [CrossRef]
- Christofi, T.; Zaravinos, A. RNA editing in the forefront of epitranscriptomics and human health. J. Transl. Med. 2019, 17, 319. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, H.; Ming, G.L.; Weng, Y.L. Epigenetic and epitranscriptomic regulation of axon regeneration. Mol. Psychiatry 2023, 28, 1440–1450. [Google Scholar] [CrossRef]
- Ruffo, P.; De Amicis, F.; Giardina, E.; Conforti, F.L. Long-noncoding RNAs as epigenetic regulators in neurodegenerative diseases. Neural Regen. Res. 2023, 18, 1243–1248. [Google Scholar] [CrossRef]
- Shao, N.; Ye, T.; Xuan, W.; Zhang, M.; Chen, Q.; Liu, J.; Zhou, P.; Song, H.; Cai, B. The effects of N6-methyladenosine RNA methylation on the nervous system. Mol. Cell. Biochem. 2023, 478, 2657–2669. [Google Scholar] [CrossRef]
- Dermentzaki, G.; Lotti, F. New Insights on the Role of N 6-Methyladenosine RNA Methylation in the Physiology and Pathology of the Nervous System. Front. Mol. Biosci. 2020, 7, 555372. [Google Scholar] [CrossRef]
- Madugalle, S.U.; Liau, W.S.; Zhao, Q.; Li, X.; Gong, H.; Marshall, P.R.; Periyakaruppiah, A.; Zajaczkowski, E.L.; Leighton, L.J.; Ren, H.; et al. Synapse-Enriched m6A-Modified Malat1 Interacts with the Novel m6A Reader, DPYSL2, and Is Required for Fear-Extinction Memory. J. Neurosci. 2023, 43, 7084–7100. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Deth, R.C. Role of a redox-based methylation switch in mRNA life cycle (pre- and post-transcriptional maturation) and protein turnover: Implications in neurological disorders. Front. Neurosci. 2012, 6, 92. [Google Scholar] [CrossRef]
- Clark, K.D.; Lee, C.; Gillette, R.; Sweedler, J.V. Characterization of Neuronal RNA Modifications during Non-associative Learning in Aplysia Reveals Key Roles for tRNAs in Behavioral Sensitization. ACS Cent. Sci. 2021, 7, 1183–1190. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, J.H.; Marsal-García, L.; Amiri, M.; Yanagiya, A.; Sonenberg, N. The mRNA translation initiation factor eIF4G1 controls mitochondrial oxidative phosphorylation, axonal morphogenesis, and memory. Proc. Natl. Acad. Sci. USA 2023, 120, e2300008120. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, J.; Wang, Y.; Jiang, J.; Zhou, J.; Wang, X.; Zhang, D.; Xu, J. Mitochondrial protein prohibitin promotes learning memory recovery in mice following intracerebral hemorrhage via CAMKII/CRMP signaling pathway. Neurochem. Int. 2023, 171, 105637. [Google Scholar] [CrossRef]
- Nikolaienko, O.; Patil, S.; Eriksen, M.S.; Bramham, C.R. Arc protein: A flexible hub for synaptic plasticity and cognition. Semin. Cell Dev. Biol. 2017, 77, 33–42. [Google Scholar] [CrossRef]
- Okuno, H.; Minatohara, K.; Bito, H. Inverse synaptic tagging: An inactive synapse-specific mechanism to capture activity-induced Arc/arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin. Cell Dev. Biol. 2017, 77, 43–50. [Google Scholar] [CrossRef]
- Paolantoni, C.; Ricciardi, S.; De Paolis, V.; Okenwa, C.; Catalanotto, C.; Ciotti, M.T.; Cattaneo, A.; Cogoni, C.; Giorgi, C. Arc 3′ UTR Splicing Leads to Dual and Antagonistic Effects in Fine-Tuning Arc Expression Upon BDNF Signaling. Front. Mol. Neurosci. 2018, 11, 145. [Google Scholar] [CrossRef]
- Aparisi Rey, A.; Karaulanov, E.; Sharopov, S.; Arab, K.; Schäfer, A.; Gierl, M.; Guggenhuber, S.; Brandes, C.; Pennella, L.; Gruhn, W.H.; et al. Gadd45α modulates aversive learning through post-transcriptional regulation of memory-related mRNAs. EMBO Rep. 2019, 20, e46022. [Google Scholar] [CrossRef]
- Oz, G.; Kumar, A.; Rao, J.P.; Kodl, C.T.; Chow, L.; Eberly, L.E.; Seaquist, E.R. Human brain glycogen metabolism during and after hypoglycemia. Diabetes 2009, 58, 1978–1985. [Google Scholar] [CrossRef]
- Hirase, H.; Akther, S.; Wang, X.; Oe, Y. Glycogen distribution in mouse hippocampus. J. Neurosci. Res. 2019, 97, 923–932. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Brown, A.M.; Ransom, B.R. Astrocyte glycogen and brain energy metabolism. Glia 2007, 55, 1263–1271. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Anderson, D.G.; Hertz, L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 2006, 54, 214–222. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Cell-to-Cell Communication in Learning and Memory: From Neuro- and Glio-Transmission to Information Exchange Mediated by Extracellular Vesicles. Int. J. Mol. Sci. 2019, 21, 266. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Parpura, V.; Zorec, R. Gliotransmission: Exocytotic release from astrocytes. Brain Res. Rev. 2010, 63, 83–92. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Gala, D.S.; Titlow, J.S.; Teodoro, R.O.; Davis, I. Far from home: The role of glial mRNA localization in synaptic plasticity. RNA 2023, 29, 153–169. [Google Scholar] [CrossRef]
- Lim, D.; Tapella, L.; Dematteis, G.; Talmon, M.; Genazzani, A.A. Calcineurin Signalling in Astrocytes: From Pathology to Physiology and Control of Neuronal Functions. Neurochem. Res. 2023, 48, 1077–1090. [Google Scholar] [CrossRef]
- Court, F.A.; Hendriks, W.T.J.; MacGillavry, H.D.; Alvarez, J.; Van Minnen, J. Schwann cell to axon transfer of ribosomes: Toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 2008, 28, 11024–11029. [Google Scholar] [CrossRef]
- Twiss, J.L.; Fainzilber, M. Ribosomes in axons—Scrounging from the neighbors? Trends Cell Biol. 2009, 19, 236–243. [Google Scholar] [CrossRef]
- Sotelo, J.R.; Canclini, L.; Kun, A.; Sotelo-Silveira, J.R.; Calliari, A.; Cal, K.; Bresque, M.; Dipaolo, A.; Farias, J.; Mercer, J.A. Glia to axon RNA transfer. Dev. Neurobiol. 2014, 74, 292–302. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef]
- Montarolo, P.G.; Goelet, P.; Castellucci, V.F.; Morgan, J.; Kandel, E.R.; Schacher, S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 1986, 234, 1249–1254. [Google Scholar] [CrossRef]
- Otani, S.; Marshall, C.J.; Tate, W.P.; Goddgard, G.V.; Abraham, W.C. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience 1989, 28, 519–526. [Google Scholar] [CrossRef]
- Rao, A.; Steward, O. Evidence that protein constituents of postsynaptic membrane specilization are locally synthesized: Analilysis of proteins synthesized within synaptosomes. J. Neurosci. 1991, 11, 2881–2895. [Google Scholar] [CrossRef]
- Giuditta, A.; Chun, J.T.; Eyman, M.; Cefaliello, C.; Bruno, A.P.; Crispino, M. Local gene expression in axons and nerve endings: The glia-neuron unit. Physiol. Rev. 2008, 88, 515–555. [Google Scholar] [CrossRef]
- Giuditta, A.; Eyman, M.; Kaplan, B.B. Gene expression in the squid giant axon: Neurotransmitter modulation of RNA transfer from periaxonal glia to the axon. Biol. Bull. 2002, 203, 189–190. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Sonenberg, N.; Richter, J.D. Translational regulatory mechanisms in synaptic plasticity and memory storage. Prog. Mol. Biol. Transl. Sci. 2009, 90, 293–311. [Google Scholar] [CrossRef]
- McKinney, B.C.; Grossman, A.W.; Elisseou, N.M.; Greenough, W.T. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 136B, 98–102. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr. Res. 2015, 167, 42–56. [Google Scholar] [CrossRef]
- Zalfa, F.; Giorgi, M.; Primerano, B.; Moro, A.; Di Penta, A.; Reis, S.; Oostra, B.; Bagni, C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 2003, 112, 317–327. [Google Scholar] [CrossRef]
- Todd, P.K.; Mack, K.J.; Malter, J.S. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc. Natl. Acad. Sci. USA 2003, 100, 14374–14378. [Google Scholar] [CrossRef]
- Hou, L.; Antion, M.D.; Hu, D.; Spencer, C.M.; Paylor, R.E.; Klann, E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 2006, 51, 441–454. [Google Scholar] [CrossRef]
- Muddashetty, R.S.; Kelic, S.; Gross, C.; Xu, M.; Bassell, G.J. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007, 27, 5338–5348. [Google Scholar] [CrossRef]
- Narayanan, U.; Nalavadi, V.; Nakamoto, M.; Thomas, G.; Ceman, S.; Bassell, G.J.; Warren, S.T. S6K1 phosphorylates and regulates FMRP with the neuronal protein synthesis-dependent mTOR signaling cascade. J. Biol. Chem. 2008, 283, 18478–18482. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- Mazroui, R.; Huot, M.E.; Tremblay, S.; Filion, C.; Labelle, Y.; Khandjian, E.W. Trapping of messenger RNA by fragile mental retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 2002, 11, 3007–3017. [Google Scholar] [CrossRef]
- Plante, I.; Davidovic, L.; Ouellet, D.L.; Gobeil, L.A.; Tremblay, S.; Khandjian, E.W.; Provost, P. Dicer-derived micrornas are utilized by the fragile X mental retardation protein for assembly on target RNAs. J. Biomed. Biotechnol. 2006, 2006, 64347. [Google Scholar] [CrossRef]
- Bagni, C.; Greenough, W.T. From mRNP trafficking to spine dysmorphogenesis: The roots of fragile X syndrome. Nat. Neurosci. 2005, 6, 376–387. [Google Scholar] [CrossRef]
- Wu, L.; Wells, D.; Tay, J.; Mendis, D.; Abbott, M.A.; Barnitt, A.; Quinlan, E.; Heynen, A.; Fallon, J.R.; Richter, J.D. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of α-CaMKII mRNA at synapses. Neuron 1998, 21, 1129–1139. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Jung, M.Y.; Sarkissian, M.; Richter, J.D. Nmethyl-D-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and a CaMKII mRNA polyadenylation at synapses. EMBO J. 2002, 21, 2139–2148. [Google Scholar] [CrossRef]
- Shin, C.Y.; Kundel, M.; Wells, D.G. Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J. Neurosci. 2004, 24, 9425–9433. [Google Scholar] [CrossRef]
- Du, L.; Richter, J.D. Activity-dependent polyadenylation in neurons. RNA 2005, 11, 1340–1347. [Google Scholar] [CrossRef]
- Richter, J.D. CPEB: A life in translation. Trends Biochem. Sci. 2007, 32, 279–285. [Google Scholar] [CrossRef]
- Richter, J.D.; Klann, E. Making synaptic plasticity and memory last: Mechanisms of translational regulation. Genes Dev. 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Si, K.; Lindquist, S.; Kandel, E.R. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 2003, 115, 879–891. [Google Scholar] [CrossRef]
- Si, K.; Giustetto, M.; Etkin, A.; Hsu, R.; Janisiewicz, A.M.; Miniaci, M.C.; Kim, J.H.; Zhu, H.; Kandel, E.R. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell 2003, 115, 893–904. [Google Scholar] [CrossRef]
- Hervás, R.; Del Carmen Fernández-Ramírez, M.; Galera-Prat, A.; Suzuki, M.; Nagai, Y.; Bruix, M.; Menéndez, M.; Laurents, D.V.; Carrión-Vázquez, M. Divergent CPEB prion-like domains reveal different assembly mechanisms for a generic amyloid-like fold. BMC Biol. 2021, 19, 43. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory storage: A dialog between genes and synapses. Biosci. Rep. 2001, 21, 565–611. [Google Scholar] [CrossRef]
- Yap, E.L.; Greenberg, M.E. Activity-regulated transcription: Bridging the gap between neural activity and behavior. Neuron 2018, 100, 330–348. [Google Scholar] [CrossRef]
- Andres-Alonso, M.; Grochowska, K.M.; Gundelfinger, E.D.; Karpova, A.; Kreutz, M.R. Protein transport from pre- and postsynapse to the nucleus: Mechanisms and functional implications. Mol. Cell. Neurosci. 2023, 125, 103854. [Google Scholar] [CrossRef]
- Jordan, B.A.; Kreutz, M.R. Nucleocytoplasmic protein shuttling: The direct route in synapse-to-nucleus signaling. Trends Neurosci. 2009, 32, 392–401. [Google Scholar] [CrossRef]
- Herbst, W.A.; Deng, W.; Wohlschlegel, J.A.; Achiro, J.M.; Martin, K.C. Neuronal activity regulates the nuclear proteome to promote activity-dependent transcription. J. Cell Biol. 2021, 220, e202103087. [Google Scholar] [CrossRef]
- Melgarejo da Rosa, M.; Yuanxiang, P.; Brambilla, R.; Kreutz, M.R.; Karpova, A. Synaptic GluN2B/CaMKII-α signaling induces synapto-nuclear transport of ERK and Jacob. Front. Mol. Neurosci. 2016, 9, 66. [Google Scholar] [CrossRef]
- Peixoto, L.L.; Wimmer, M.E.; Poplawski, S.G.; Tudor, J.C.; Kenworthy, C.A.; Liu, S.; Mizuno, K.; Garcia, B.A.; Zhang, N.R.; Giese, K.; et al. Memory acquisition and retrieval impact different epigenetic processes that regulate gene expression. BMC Genom. 2015, 16 (Suppl. S5), S5. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. H1.0 Linker Histone as an Epigenetic Regulator of Cell Proliferation and Differentiation. Genes 2018, 9, 310. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Schirò, G.; Di Liegro, I. Involvement of the H3.3 Histone Variant in the Epigenetic Regulation of Gene Expression in the Nervous System, in Both Physiological and Pathological Conditions. Int. J. Mol. Sci. 2023, 24, 11028. [Google Scholar] [CrossRef]
- Saladino, P.; Di Liegro, C.M.; Proia, P.; Sala, A.; Schiera, G.; Lo Cicero, A.; Di Liegro, I. RNA-binding activity of the rat calmodulin-binding PEP-19 protein and of the long PEP-19 isoform. Int. J. Mol. Med. 2012, 29, 141–145. [Google Scholar] [CrossRef][Green Version]
- Crenshaw, E.; Leung, B.P.; Kwok, C.K.; Sharoni, M.; Olson, K.; Sebastian, N.P.; Ansaloni, S.; Schweitzer-Stenner, R.; Akins, M.R.; Bevilacqua, P.C.; et al. Amyloid Precursor Protein Translation Is Regulated by a 3′UTR Guanine Quadruplex. PLoS ONE 2015, 10, e0143160. [Google Scholar] [CrossRef]
- Okamoto, K.; Mizuno, Y.; Fujita, Y. Bunina bodies in amyotrophic lateral sclerosis. Neuropathology 2008, 28, 109–115. [Google Scholar] [CrossRef]
- Lu, J.-X.; Wang, Y.; Zhang, Y.J.; Shen, M.F.; Li, H.Y.; Yu, Z.Q.; Chen, G. Axonal mRNA localization and local translation in neurodegenerative disease. Neural Regen. Res. 2021, 16, 1950–1957. [Google Scholar] [CrossRef]
- Nagano, S.; Jinno, J.; Abdelhamid, R.F.; Jin, Y.; Shibata, M.; Watanabe, S.; Hirokawa, S.; Nishizawa, M.; Sakimura, K.; Onodera, O.; et al. TDP-43 transports ribosomal protein mRNA to regulate axonal local translation in neuronal axons. Acta Neuropathol. 2020, 140, 695–713. [Google Scholar] [CrossRef]
- Coyne, A.N.; Siddegowda, B.B.; Estes, P.S.; Johannesmeyer, J.; Kovalik, T.; Daniel, S.G.; Pearson, A.; Bowser, R.; Zarnescu, D.C. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. J. Neurosci. 2014, 34, 15962–15974. [Google Scholar] [CrossRef]
- Tzeplaeff, L.; Seguin, J.; Le Gras, S.; Megat, S.; Cosquer, B.; Plassard, D.; Dieterlé, S.; Paiva, I.; Picchiarelli, G.; Decraene, C.; et al. Mutant FUS induces chromatin reorganization in the hippocampus and alters memory processes. Prog. Neurobiol. 2023, 227, 102483. [Google Scholar] [CrossRef]
- Ghosh, A.; Mizuno, K.; Tiwari, S.S.; Proitsi, P.; Gomez Perez-Nievas, B.; Glennon, E.; Martinez-Nunez, R.T.; Giese, K.P. Alzheimer’s disease-related dysregulation of mRNA translation causes key pathological features with ageing. Transl. Psychiatry 2020, 10, 192. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Guo, C.; Yalamanchili, H.K.; Abreha, M.; Al-Ouran, R.; Li, Y.; Dammer, E.B.; Lah, J.J.; Levey, A.I.; Bennett, D.A.; et al. Tau-Mediated Disruption of the Spliceosome Triggers Cryptic RNA Splicing and Neurodegeneration in Alzheimer’s Disease. Cell Rep. 2019, 29, 301–316.e10. [Google Scholar] [CrossRef]
- Langstrom, N.S.; Anderson, J.P.; Lindroos, H.G.; Winblad, B.; Wallace, W.C. Alzheimer’s disease-associated reduction of polysomal mRNA translation. Brain Res. Mol. Brain Res. 1989, 5, 259–269. [Google Scholar] [CrossRef]
- Pietrzak, M.; Rempala, G.; Nelson, P.T.; Zheng, J.J.; Hetman, M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS ONE 2011, 6, e22585. [Google Scholar] [CrossRef]
- Montalbano, M.; McAllen, S.; Puangmalai, N.; Sengupta, U.; Bhatt, N.; Johnson, O.D.; Kharas, M.G.; Kayed, R. RNA-binding proteins Musashi and tau soluble aggregates initiate nuclear dysfunction. Nat. Commun. 2020, 11, 4305. [Google Scholar] [CrossRef]
- Huang, H.; Camats-Perna, J.; Medeiros, R.; Anggono, V.; Widagdo, J. Altered Expression of the m6A Methyltransferase METTL3 in Alzheimer’s Disease. eNeuro 2020, 7, ENEURO.0125-20.2020. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, Q.; Zhao, X.; Zheng, F.; Xiao, J.; Ge, F.; Wang, D.; Gao, X. The Landscape of m6A Regulators in Multiple Brain Regions of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 5184–5198. [Google Scholar] [CrossRef]
- Pupak, A.; Singh, A.; Sancho-Balsells, A.; Alcalá-Vida, R.; Espina, M.; Giralt, A.; Martí, E.; Ørom, U.A.V.; Ginés, S.; Brito, V. Altered m6A RNA methylation contributes to hippocampal memory deficits in Huntington’s disease mice. Cell. Mol. Life Sci. 2022, 79, 416. [Google Scholar] [CrossRef]
- Ai, J.; Sun, L.H.; Che, H.; Zhang, R.; Zhang, T.Z.; Wu, W.C.; Su, X.L.; Chen, X.; Yang, G.; Li, K.; et al. MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J. Neurosci. 2013, 33, 3989–4001. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, R.; Jiang, L.; Zhou, H.; Wang, Q.; Ma, Y.; Zhang, D.; Qin, Y.; Tian, P.; Zhang, N.; et al. Administration of miR-195 Inhibitor Enhances Memory Function Through Improving Synaptic Degradation and Mitochondrial Dysfunction of the Hippocampal Neurons in SAMP8 Mice. J. Alzheimer’s Dis. 2022, 85, 1495–1509. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, C.J.; Prieto, G.A.; Martini, A.C.; Forner, S.; Trujillo-Estrada, L.; LaFerla, F.M.; Baglietto-Vargas, D.; Cotman, C.W.; Kitazawa, M. miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13118. [Google Scholar] [CrossRef]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Aβ(1-42)-induced model of Alzheimer’s disease. Aging Cell 2020, 19, e13046. [Google Scholar] [CrossRef]
- Ding, H.; Huang, Z.; Chen, M.; Wang, C.; Chen, X.; Chen, J.; Zhang, J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Park. Relat. Disord. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Sarachana, T.; Zhou, R.; Chen, G.; Manji, H.K.; Hu, V.W. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010, 2, 23. [Google Scholar] [CrossRef]
- Fregeac, J.; Colleaux, L.; Nguyen, L.S. The emerging roles of MicroRNAs in autism spectrum disorders. Neurosci. Biobehav. Rev. 2016, 71, 729–738. [Google Scholar] [CrossRef]
- Luoni, A.; Riva, M.A. MicroRNAs and psychiatric disorders: From aetiology to treatment. Pharmacol. Ther. 2016, 167, 13–27. [Google Scholar] [CrossRef]
- Srivastav, S.; Walitza, S.; Grünblatt, E. Emerging role of miRNA in attention deficit hyperactivity disorder: A systematic review. Atten. Defic. Hyperact. Disord. 2018, 10, 49–63. [Google Scholar] [CrossRef]
- Muñoz-Llanos, M.; García-Pérez, M.A.; Xu, X.; Tejos-Bravo, M.; Vidal, E.A.; Moyano, T.C.; Gutiérrez, R.A.; Aguayo, F.I.; Pacheco, A.; García-Rojo, G.; et al. MicroRNA Profiling and Bioinformatics Target Analysis in Dorsal Hippocampus of Chronically Stressed Rats: Relevance to Depression Pathophysiology. Front. Mol. Neurosci. 2018, 11, 251. [Google Scholar] [CrossRef]
- Griggs, E.M.; Young, E.J.; Rumbaugh, G.; Miller, C.A. MicroRNA-182 regulates amygdala-dependent memory formation. J. Neurosci. 2013, 33, 1734–1740. [Google Scholar] [CrossRef]
- Joilin, G.; Guévremont, D.; Ryan, B.; Claudianos, C.; Cristino, A.S.; Abraham, W.C.; Williams, J.M. Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front. Mol. Neurosci. 2014, 7, 98. [Google Scholar] [CrossRef]
- Woldemichael, B.T.; Jawaid, A.; Kremer, E.A.; Gaur, N.; Krol, J.; Marchais, A.; Mansuy, I.M. The microRNA cluster miR-183/96/182 contributes to long-term memory in a protein phosphatase 1-dependent manner. Nat. Commun. 2016, 7, 12594. [Google Scholar] [CrossRef]
- Dangla-Valls, A.; Molinuevo, J.L.; Altirriba, J.; Sánchez-Valle, R.; Alcolea, D.; Fortea, J.; Rami, L.; Balasa, M.; Muñoz-García, C.; Ezquerra, M.; et al. CSF microRNA Profiling in Alzheimer’s Disease: A Screening and Validation Study. Mol. Neurobiol. 2017, 54, 6647–6654. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.I.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef]
- Murphy, C.P.; Singewald, N. Potential of microRNAs as novel targets in the alleviation of pathological fear. Genes Brain Behav. 2018, 17, e12427. [Google Scholar] [CrossRef]
- Toyama, K.; Spin, J.M.; Deng, A.C.; Huang, T.T.; Wei, K.; Wagenhäuser, M.U.; Yoshino, T.; Nguyen, H.; Mulorz, J.; Kundu, S.; et al. MicroRNA-Mediated Therapy Modulating Blood-Brain Barrier Disruption Improves Vascular Cognitive Impairment. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1392–1406. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Si, Y.; Wu, N.; Cao, H.; Mei, B.; Meng, B. miR-125b promotes tau phosphorylation by targeting the neural cell adhesion molecule in neuropathological progression. Neurobiol. Aging 2019, 73, 41–49. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Chiricosta, L.; Boccardi, V.; Mecocci, P.; Bramanti, P.; Mazzon, E. MicroRNAs Modulate the Pathogenesis of Alzheimer’s Disease: An In Silico Analysis in the Human Brain. Genes 2020, 11, 983. [Google Scholar] [CrossRef]
- Abuelezz, N.Z.; Nasr, F.E.; AbdulKader, M.A.; Bassiouny, A.R.; Zaky, A. MicroRNAs as Potential Orchestrators of Alzheimer’s Disease-Related Pathologies: Insights on Current Status and Future Possibilities. Front. Aging Neurosci. 2021, 13, 743573. [Google Scholar] [CrossRef]
- Siedlecki-Wullich, D.; Miñano-Molina, A.J.; Rodríguez-Álvarez, J. microRNAs as Early Biomarkers of Alzheimer’s Disease: A Synaptic Perspective. Cells 2021, 10, 113. [Google Scholar] [CrossRef]
- Bandakinda, M.; Mishra, A. Insights into role of microRNA in Alzheimer’s disease: From contemporary research to bedside perspective. Int. J. Biol. Macromol. 2023, 253, 126561. [Google Scholar] [CrossRef]
- Liu, J.J.; Long, Y.F.; Xu, P.; Guo, H.D.; Cui, G.H. Pathogenesis of miR-155 on nonmodifiable and modifiable risk factors in Alzheimer’s disease. Alzheimer’s Res. Ther. 2023, 15, 122. [Google Scholar] [CrossRef]
- Lyons, L.C.; Vanrobaeys, Y.; Abel, T. Sleep and memory: The impact of sleep deprivation on transcription, translational control, and protein synthesis in the brain. J. Neurochem. 2023, 166, 24–46. [Google Scholar] [CrossRef]
- Cao, W.; Oldstone, M.B.; De La Torre, J.C. Viral persistent infection affects both transcriptional and posttranscriptional regulation of neuron-specific molecule GAP43. Virology 1997, 230, 147–154. [Google Scholar] [CrossRef]
- Hirano, M.; Muto, M.; Sakai, M.; Kondo, H.; Kobayashi, S.; Kariwa, H.; Yoshii, K. Dendritic transport of tick-borne flavivirus RNA by neuronal granules affects development of neurological disease. Proc. Natl. Acad. Sci. USA 2017, 114, 9960–9965. [Google Scholar] [CrossRef]
- Iselin, L.; Palmalux, N.; Kamel, W.; Simmonds, P.; Mohammed, S.; Castello, A. Uncovering viral RNA-host cell interactions on a proteome-wide scale. Trends Biochem. Sci. 2022, 47, 23–38. [Google Scholar] [CrossRef]
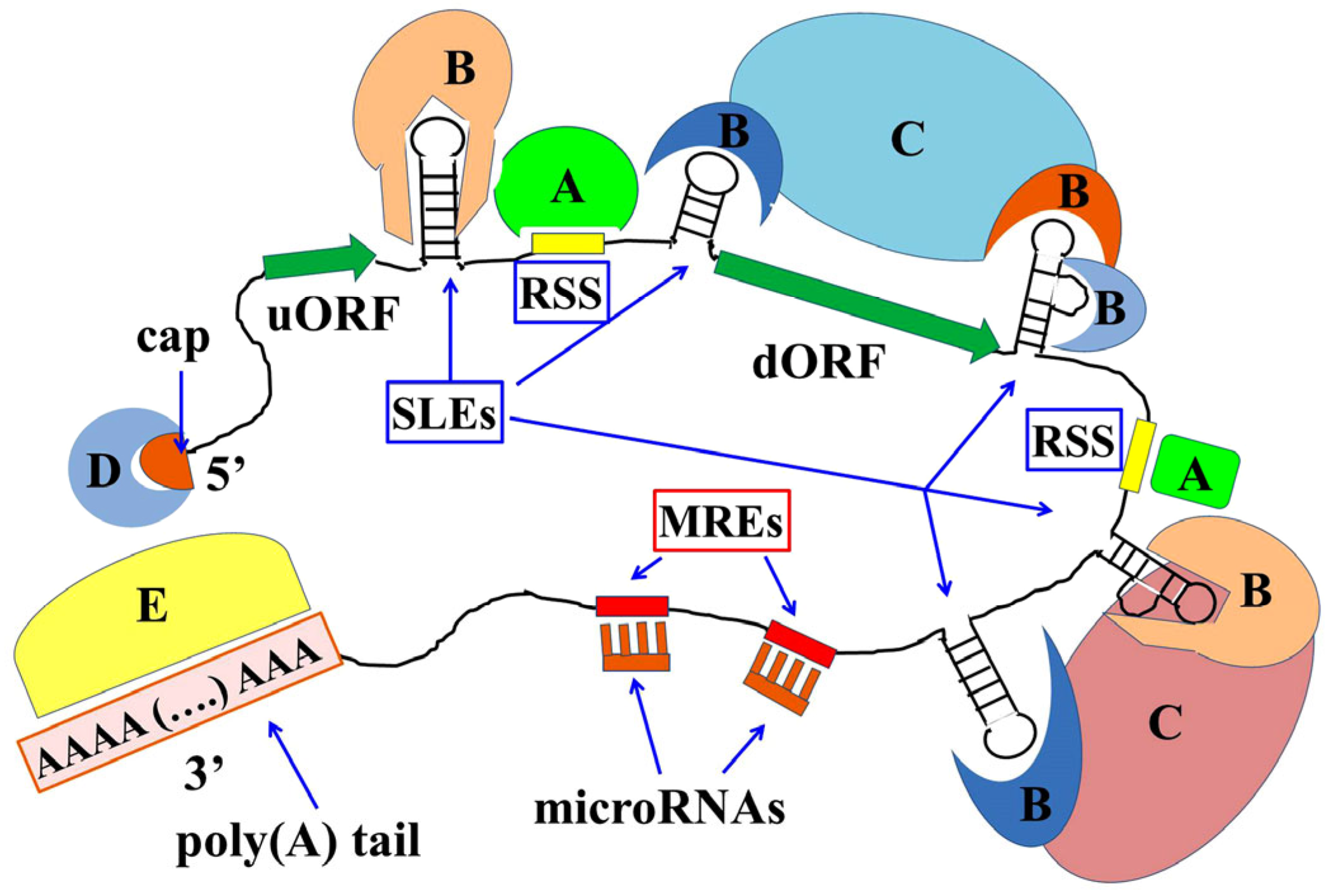
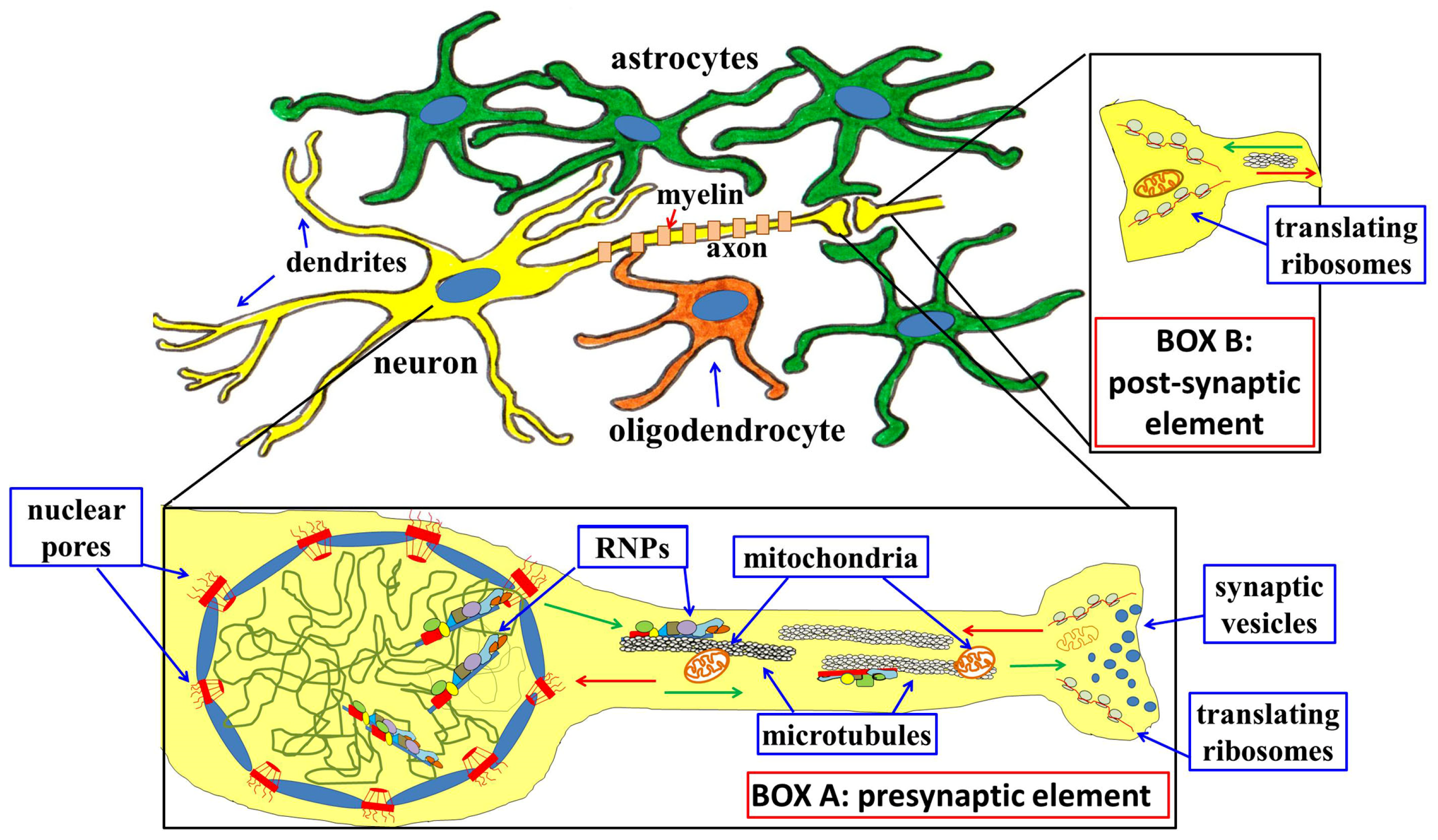
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Liegro, C.M.; Schiera, G.; Schirò, G.; Di Liegro, I. Role of Post-Transcriptional Regulation in Learning and Memory in Mammals. Genes 2024, 15, 337. https://doi.org/10.3390/genes15030337
Di Liegro CM, Schiera G, Schirò G, Di Liegro I. Role of Post-Transcriptional Regulation in Learning and Memory in Mammals. Genes. 2024; 15(3):337. https://doi.org/10.3390/genes15030337
Chicago/Turabian StyleDi Liegro, Carlo Maria, Gabriella Schiera, Giuseppe Schirò, and Italia Di Liegro. 2024. "Role of Post-Transcriptional Regulation in Learning and Memory in Mammals" Genes 15, no. 3: 337. https://doi.org/10.3390/genes15030337
APA StyleDi Liegro, C. M., Schiera, G., Schirò, G., & Di Liegro, I. (2024). Role of Post-Transcriptional Regulation in Learning and Memory in Mammals. Genes, 15(3), 337. https://doi.org/10.3390/genes15030337





