Large-Scale Cytochrome C Oxidase Subunit I Gene Data Analysis for the Development of a Multiplex Polymerase Chain Reaction Test Capable of Identifying Biting Midge Vector Species and Haplotypes (Diptera: Ceratopogonidae) of the Culicoides Subgenus Avaritia Fox, 1955
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Morphologic Examination
2.2. DNA Extraction
2.3. COI Amplification and Sequencing
2.4. Data Analysis and Primer Design
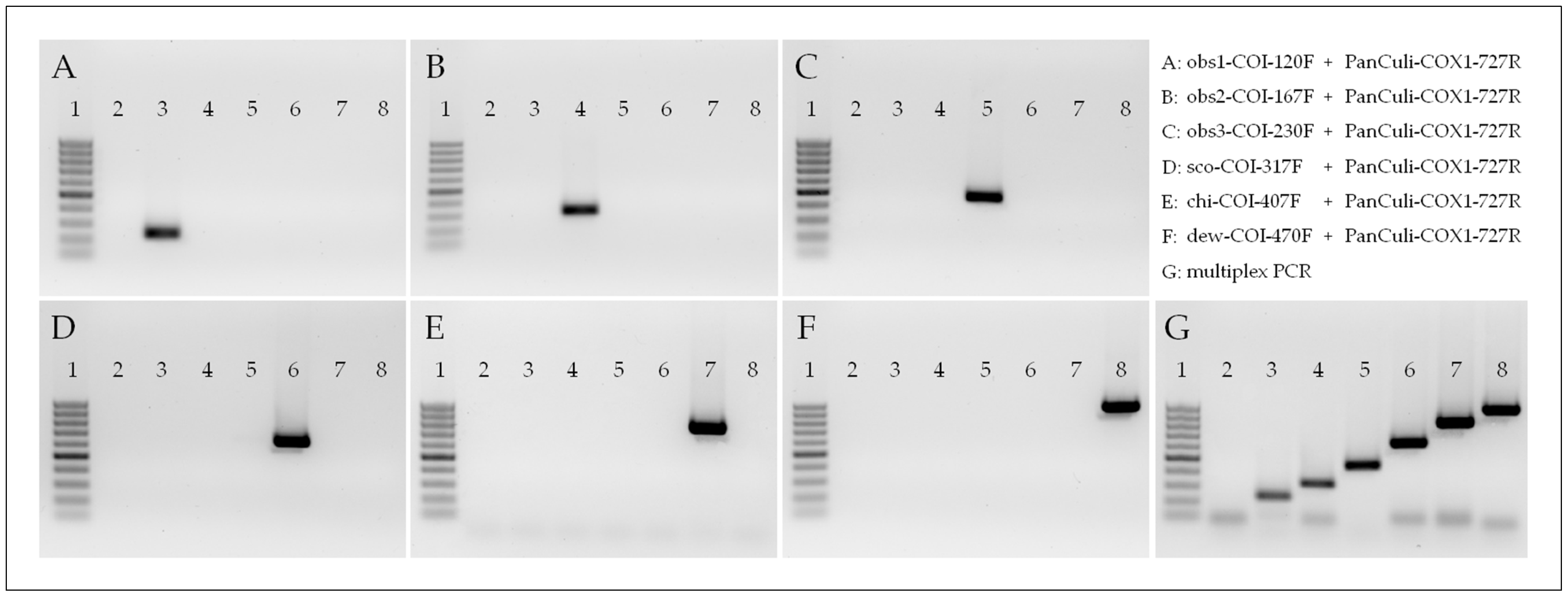
2.5. Performance of Multiplex PCR
3. Results
3.1. GenBank Search and Data Analysis
3.2. Primer Design and PCR Performance
3.3. Validation of the Multiplex PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; Gomulski, L.M.; Delécolle, J.-C.; Goffredo, M.; Gasperi, G. The taxonomy of Culicoides vector complexes—Unfinished business. Vet. Ital. 2004, 40, 151–159. [Google Scholar]
- Borkent, A. The biting midges, the Ceratopogonidae (Diptera). In Biology of Disease Vectors, 2nd ed.; Marquardt, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 113–126. [Google Scholar]
- Mellor, P.S.; Pitzolis, G. Observations on breeding sites and light-trap collections of Culicoides during an outbreak of bluetongue in Cyprus. Bull. Entomol. Res. 1979, 69, 229–234. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boned, J.; Hamblin, C.; Graham, S. Isolations of African horse sickness virus from vector insects made during the 1988 epizootic in Spain. Epidemiol. Infect. 1990, 105, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Walldorf, V.; Klimpel, S.; Jahn, B.; Jaeger, F.; Eschweiler, J.; Hoffmann, B.; Beer, M. First occurrence of Culicoides obsoletus-transmitted bluetongue virus epidemic in Central Europe. Parasitol. Res. 2007, 101, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; van Rijn, P.; Leijs, P.; Goffredo, M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet. Rec. 2007, 161, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.; van der Ven, I.J.K.; Meiswinkel, R.; Hölzel, D.R.; van Rijn, P.A. Culicoides chiopterus as a potential vector of bluetongue virus in Europe. Vet. Rec. 2008, 162, 422. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Bauer, B.; Bauer, C.; Bätza, H.J.; Beer, M.; Clausen, P.H.; Geier, M.; Gethmann, J.M.; Kiel, E.; Liebisch, G.; et al. Monitoring of putative vectors of bluetongue virus serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 1481–1484. [Google Scholar] [CrossRef]
- Vanbinst, T.; Vandenbussche, F.; Vandemeulebroucke, E.; de Leeuw, I.; Deblauwe, I.; de Deken, G.; Madder, M.; Haubruge, E.; Losson, B.; de Clercq, K. Bluetongue virus detection by real-time RT-PCR in Culicoides captured during the 2006 epizootic in Belgium and development of an internal control. Transbound. Emerg. Dis. 2009, 56, 170–177. [Google Scholar] [CrossRef]
- Romón, P.; Higuera, M.; Delécolle, J.-C.; Baldet, T.; Aduriz, G.; Goldarazena, A. Phenology and attraction of potential Culicoides vectors of bluetongue virus in Basque Country (northern Spain). Vet. Parasitol. 2012, 186, 415–424. [Google Scholar] [CrossRef]
- Goffredo, M.; Catalani, M.; Federici, V.; Portanti, O.; Marini, V.; Mancini, G.; Quaglia, M.; Santilli, A.; Teodori, L.; Savini, G. Vector species of Culicoides midges implicated in the 2012–2014 bluetongue epidemics in Italy. Vet. Ital. 2015, 51, 131–138. [Google Scholar] [CrossRef]
- Foxi, C.; Delrio, G.; Falchi, G.; Marche, M.G.; Satta, G.; Ruiu, L. Role of different Culicoides vectors (Diptera: Ceratopogonidae) in bluetongue virus transmission and overwintering in Sardinia (Italy). Parasit. Vectors 2016, 9, 440. [Google Scholar] [CrossRef]
- Foxi, C.; Meloni, G.; Puggioni, G.; Manunta, D.; Rocchigiani, A.; Vento, L.; Cabras, P.; Satta, G. Bluetongue virus detection in new Culicoides species in Sardinia, Italy. Vet. Rec. 2019, 184, 621. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Kristensen, B.; Kirkeby, C.; Rasmussen, T.B.; Belsham, G.J.; Bødker, R.; Bøtner, A. Culicoids as vectors of Schmallenberg virus. Emerg. Infect. Dis. 2012, 18, 1204–1206. [Google Scholar] [CrossRef]
- de Regge, N.; Deblauwe, I.; de Deken, R.; Vantieghem, P.; Madder, M.; Geysen, D.; Smeets, F.; Losson, B.; van den Berg, T.; Cay, A.B. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transbound. Emerg. Dis. 2012, 59, 471–475. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Meiswinkel, R.; van Weezep, E.; van Sloet Oldruitenborgh-Oosterbaan, M.M.; Kooi, E.A. Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerg. Infect. Dis. 2013, 19, 106–109. [Google Scholar] [CrossRef]
- Goffredo, M.; Monaco, F.; Capelli, G.; Quaglia, M.; Federici, V.; Catalani, M.; Montarsi, F.; Polci, A.; Pinoni, C.; Calistri, P.; et al. Schmallenberg virus in Italy: A retrospective survey in Culicoides stored during the bluetongue Italian surveillance program. Prev. Vet. Med. 2013, 111, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Polak, M.P.; Grochowska, M.; Lechowski, L.; Związek, J.S.; Zmudziński, J.F. First report of Schmallenberg virus infection in cattle and midges in Poland. Transbound. Emerg. Dis. 2013, 60, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Lechowski, L.; Grochowska, M.; Żmudziński, J.F. Detection of the Schmallenberg virus in nulliparous Culicoides obsoletus/scoticus complex and C. punctatus—The possibility of transovarial virus transmission in the midge population and of a new vector. Vet. Microbiol. 2013, 166, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Balenghien, T.; Pagès, N.; Goffredo, M.; Carpenter, S.; Augot, D.; Jacquier, E.; Talavera, S.; Monaco, F.; Depaquit, J.; Grillet, C.; et al. The emergence of Schmallenberg virus across Culicoides communities and ecosystems in Europe. Prev. Vet. Med. 2014, 116, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.D.; Kirkeby, C.; Bødker, R.; Kristensen, B.; Rasmussen, T.B.; Belsham, G.J.; Bøtner, A. Rapid spread of Schmallenberg virus-infected biting midges (Culicoides spp.) across Denmark in 2012. Transbound. Emerg. Dis. 2014, 61, 12–16. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Meiswinkel, R.; van Weezep, E.; Kooi, E.A.; van der Poel, W.H.M. Schmallenberg virus in Culicoides biting midges in the Netherlands in 2012. Transbound. Emerg. Dis. 2015, 62, 339–342. [Google Scholar] [CrossRef]
- de Regge, N.; de Deken, R.; Fassotte, C.; Losson, B.; Deblauwe, I.; Madder, M.; Vantieghem, P.; Tomme, M.; Smeets, F.; Cay, A.B. Culicoides monitoring in Belgium in 2011: Analysis of spatiotemporal abundance, species diversity and Schmallenberg virus detection. Med. Vet. Entomol. 2015, 29, 263–275. [Google Scholar] [CrossRef]
- Pagès, N.; Talavera, S.; Verdún, M.; Pujol, N.; Valle, M.; Bensaid, A.; Pujols, J. Schmallenberg virus detection in Culicoides biting midges in Spain: First laboratory evidence for highly efficient infection of Culicoides of the Obsoletus Complex and Culicoides imicola. Transbound. Emerg. Dis. 2018, 65, e1–e6. [Google Scholar] [CrossRef]
- Ségard, A.; Gardès, L.; Jacquier, E.; Grillet, C.; Mathieu, B.; Rakotoarivony, I.; Setier-Rio, M.-L.; Chavernac, D.; Cêtre-Sossah, C.; Balenghien, T.; et al. Schmallenberg virus in Culicoides Latreille (Diptera: Ceratopogonidae) populations in France during 2011–2012 outbreak. Transbound. Emerg. Dis. 2018, 65, e94–e103. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Lunt, H.L.; Arav, D.; Venter, G.J.; Mellor, P.S. Oral susceptibility to bluetongue virus of Culicoides (Diptera: Ceratopogonidae) from the United Kingdom. J. Med. Entomol. 2006, 43, 73–78. [Google Scholar] [CrossRef]
- Carpenter, S.; McArthur, C.; Selby, R.; Ward, R.; Nolan, D.V.; Luntz, A.J.; Dallas, J.F.; Tripet, F.; Mellor, P.S. Experimental infection studies of UK Culicoides species midges with bluetongue virus serotypes 8 and 9. Vet. Rec. 2008, 163, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Delécolle, J.-C. Nouvelle Contribution à L’étude Systématique et Iconographique des Espèces du Genre Culicoides (Diptera: Ceratopogonidae) du Nord-Est de la France. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 1985. [Google Scholar]
- Campbell, J.A.; Pelham-Clinton, E.C. A taxonomic review of the British species of Culicoides Latreille (Diptera, Ceratopogonidae). Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1960, 67, 181–302. [Google Scholar] [CrossRef]
- Glukhova, V.M. Krovososuščie Mokrecy Rodov Culicoides i Forcipomyia (Ceratopogonidae); Nauka: Leningrad, Russia, 1989; ISBN 9785020257603. [Google Scholar]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the western Palaearctic region. Parasit. Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Zhang, X.; Phillips, R.A.; Gerry, A.C. Morphological and molecular identification of Culicoides (Diptera: Ceratopogonidae) species of the Southern California desert. J. Med. Entomol. 2022, 59, 1589–1600. [Google Scholar] [CrossRef]
- Mathieu, B. Les Espèces de Culicoides du Sous-Genre Avaritia (Diptera: Ceratopogonidae) dans le Monde: Revision Systématique et Taxonomique des Espèces d’Intéret dans la Transmission d´Orbivirus. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2011. [Google Scholar]
- Hajd-Henni, L.; Sauvage, F.; Ninio, C.; Depaquit, J.; Augot, D. Wing geometry as a tool for discrimination of Obsoletus Group (Diptera: Ceratopogonidae: Culicoides) in France. Infect. Genet. Evol. 2014, 21, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mignotte, A.; Garros, C.; Gardès, L.; Balenghien, T.; Duhayon, M.; Rakotoarivony, I.; Tabourin, L.; Poujol, L.; Mathieu, B.; Ibañez-Justicia, A.; et al. The tree that hides the forest: Cryptic diversity and phylogenetic relationships in the Palaearctic vector Obsoletus/Scoticus Complex (Diptera: Ceratopogonidae) at the European level. Parasit. Vectors 2020, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; De Bree, F.; Bossers-de Vries, R.; Elbers, A.R. An unrecognized species of the Culicoides obsoletus complex feeding on livestock in the Netherlands. Vet. Parasitol. 2015, 207, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Borkent, A.; Dominiak, P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa 2020, 4787, 1–377. [Google Scholar] [CrossRef]
- Goffredo, M.; Meiswinkel, R.; Federici, V.; Di Nicola, F.; Mancini, G.; Ippoliti, C.; Di Lorenzo, A.; Quaglia, M.; Santilli, A.; Conte, A.; et al. The ‘Culicoides obsoletus group’ in Italy: Relative abundance, geographic range, and role as vector for bluetongue virus. Vet. Ital. 2016, 52, 235–241. [Google Scholar] [CrossRef]
- Schwenkenbecher, J.M.; Mordue, A.J.; Piertney, S.B. Phylogenetic analysis indicates that Culicoides dewulfi should not be considered part of the Culicoides obsoletus complex. Bull. Entomol. Res. 2009, 99, 371–375. [Google Scholar] [CrossRef]
- Ander, M.; Troell, K.; Chirico, J. Barcoding of biting midges in the genus Culicoides: A tool for species determination. Med. Vet. Entomol. 2013, 27, 323–331. [Google Scholar] [CrossRef]
- Sarvašová, A.; Kočišová, A.; Halán, M.; Delécolle, J.-C.; Mathieu, B. Morphological and molecular analysis of the genus Culicoides (Diptera: Ceratopogonidae) in Slovakia with five new records. Zootaxa 2014, 3872, 541–560. [Google Scholar] [CrossRef]
- Augot, D.; Mathieu, B.; Hadj-Henni, L.; Barriel, V.; Zapata, M.S.; Smolis, S.; Augot, D.; Mathieu, B.; Hadj-Henni, L.; Barriel, V.; et al. Molecular phylogeny of 42 species of Culicoides (Diptera, Ceratopogonidae) from three continents. Parasite 2017, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, B.; Garros, C.; Balenghien, T.; Candolfi, E.; Delécolle, J.-C.; Cêtre-Sossah, C. A phylogenetic analysis of the biting midges belonging to Culicoides Latreille (Diptera: Ceratopogonidae) subgenus Avaritia using molecular data. Parasit. Vectors 2020, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.; Walldorf, V.; Klimpel, S.; Al-Quraishy, S.; Mehlhorn, H. The European vectors of Bluetongue virus: Are there species complexes, single species or races in Culicoides obsoletus and C. pulicaris detectable by sequencing ITS-1, ITS-2 and 18S-rDNA? Parasitol. Res. 2009, 105, 331–336. [Google Scholar] [CrossRef]
- Wenk, C.E.; Kaufmann, C.; Schaffner, F.; Mathis, A. Molecular characterization of Swiss Ceratopogonidae (Diptera) and evaluation of real-time PCR assays for the identification of Culicoides biting midges. Vet. Parasitol. 2012, 184, 258–266. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Meiswinkel, R. Culicoides (Diptera: Ceratopogonidae) host preferences and biting rates in the Netherlands: Comparing cattle, sheep and the black-light suction trap. Vet. Parasitol. 2014, 205, 330–337. [Google Scholar] [CrossRef]
- Kirkeby, C.; Dominiak, P. Culicoides (Avaritia) gornostaevae Mirzaeva, 1984 (Diptera: Ceratopogonidae)—A possible vector species of the Obsoletus Group new to the European fauna. Parasit. Vectors 2014, 7, 445. [Google Scholar] [CrossRef]
- Cywinska, A.; Hunter, F.F.; Hebert, P.D. Identifying Canadian mosquito species through DNA barcodes. Med. Vet. Entomol. 2006, 20, 413–424. [Google Scholar] [CrossRef]
- Kumar, N.P.; Rajavel, A.R.; Natarajan, R.; Jambulingam, P. DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Azapurua, J.; De La Cruz, D.; Valderama, A.; Windsor, D. Lutzomyia sandfly diversity and rates of infection by Wolbachia and exotic Leishmania species on Barro Colorado Island, Panama. PLoS Negl. Trop. Dis. 2010, 4, e627. [Google Scholar] [CrossRef]
- Ács, Z.; Challis, R.J.; Bihari, P.; Blaxter, M.; Hayward, A.; Melika, G.; Csóka, G.; Pénzes, Z.; Pujade-Villar, J.; Nieves-Aldrey, J.L.; et al. Phylogeny and DNA barcoding of inquiline oak gallwasps (Hymenoptera: Cynipidae) of the Western Palaearctic. Mol. Phylogenet. Evol. 2010, 55, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Cywinska, A.; Hannan, M.A.; Kevan, P.G.; Roughley, R.E.; Iranpour, M.; Hunter, F.F. Evaluation of DNA barcoding and identification of new halomorphs in Canadian deerflies and horseflies. Med. Vet. Entomol. 2010, 24, 382–410. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.B.; Nielsen, S.A.; Skovgaard, H.; Kristensen, M. Molecular differentiation of Culicoides biting midges (Diptera: Ceratopogonidae) from the subgenus Culicoides Latreille in Denmark. Parasitol. Res. 2012, 110, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Bellis, G.; Dyce, A.; Gopurenko, D.; Yanase, T.; Garros, C.; Labuschagne, K.; Mitchell, A. Revision of the Culicoides (Avaritia) imicola complex Khamala & Kettle (Diptera: Ceratopogonidae) from the Australasian region. Zootaxa 2014, 3768, 401–427. [Google Scholar] [CrossRef]
- Debila, J. Characterisation of Selected Culicoides (Diptera: Ceratopogonidae) Populations in South Africa Using Genetic Markers. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2010. [Google Scholar]
- Linton, Y.M.; Mordue, A.J.; Cruickshank, R.H.; Meiswinkel, R.; Mellor, P.S.; Dallas, J.F. Phylogenetic analysis of the mitochondrial cytochrome oxidase subunit I gene of five species of the Culicoides imicola species complex. Med. Vet. Entomol. 2002, 16, 139–146. [Google Scholar] [CrossRef]
- Lunt, D.H.; Zhang, D.X.; Szymura, J.M.; Hewitt, G.M. The insect cytochrome oxidase I gene: Evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol. Biol. 1996, 5, 153–165. [Google Scholar] [CrossRef]
- Dobler, S.; Farrell, B.D. Host use evolution in Chrysochus milkweed beetles: Evidence from behaviour, population genetics and phylogeny. Mol. Ecol. 1999, 8, 1297–1307. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Dähn, O.; Werner, D.; Mathieu, B.; Kampen, H. Development of conventional multiplex PCR assays for the identification of 21 West Palaearctic biting midge taxa (Diptera: Ceratopogonidae) belonging to the Culicoides subgenus Culicoides, including recently discovered species and genetic variants. Diversity 2023, 15, 699. [Google Scholar] [CrossRef]
- Leprince, D.J.; Higgins, J.A.; Church, G.E.; Issel, C.J.; McManus, J.M.; Foil, L.D. Body size of Culicoides variipennis (Diptera: Ceratopogonidae) in relation to bloodmeal size estimates and the ingestion of Onchocerca cervicalis (Nematoda: Filarioidea) microfiliariae. J. Am. Mosq. Control Assoc. 1989, 5, 100–103. [Google Scholar]
- Gomulski, L.M.; Meiswinkel, R.; Delécolle, J.C.; Goffredo, M.; Gasperi, G. Phylogenetic relationships of the subgenus Avaritia Fox, 1955 including Culicoides obsoletus (Diptera, Ceratopogonidae) in Italy based on internal transcribed spacer 2 ribosomal DNA sequences. Syst. Entomol. 2005, 30, 619–631. [Google Scholar] [CrossRef]
- Pagès, N.; Sarto i Monteys, V. Differentiation of Culicoides obsoletus and Culicoides scoticus (Diptera: Ceratopogonidae) based on mitochondrial cytochrome oxidase subunit I. J. Med. Entomol. 2005, 42, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, B.; Perrin, A.; Baldet, T.; Delécolle, J.-C.; Albina, E.; Cêtre-Sossah, C. Molecular identification of western European species of Obsoletus Complex (Diptera: Ceratopogonidae) by an internal transcribed spacer-1 rDNA multiplex polymerase chain reaction assay. J. Med. Entomol. 2007, 44, 1019–1025. [Google Scholar] [CrossRef]
- Nolan, D.V.; Carpenter, S.; Barber, J.; Mellor, P.S.; Dallas, J.F.; Mordue Luntz, A.J.; Piertney, S.B. Rapid diagnostic PCR assays for members of the Culicoides obsoletus and Culicoides pulicaris species complexes, implicated vectors of bluetongue virus in Europe. Vet. Microbiol. 2007, 124, 82–94. [Google Scholar] [CrossRef]
- Schwenkenbecher, J.M.; Mordue, A.J.; Switek, K.; Piertney, S.B. Discrimination of Culicoides midge larvae using multiplex polymerase chain reaction assays based on DNA sequence variation at the mitochondrial cytochrome c oxidase I gene. J. Med. Entomol. 2009, 46, 610–614. [Google Scholar] [CrossRef]
- Monaco, F.; Benedetto, L.; Di Marcello, V.; Lelli, R.; Goffredo, M. Development and preliminary evaluation of a real-time polymerase chain reaction for the identification of Culicoides obsoletus sensu strictu, C. scoticus and C. montanus in the Obsoletus Complex in Italy. Vet. Ital. 2010, 46, 215–220. [Google Scholar] [CrossRef]
- Mathieu, B.; Delecolle, J.-C.; Garros, C.; Balenghien, T.; Setier-Rio, M.-L.; Candolfi, E.; Cêtre-Sossah, C. Simultaneous quantification of the relative abundance of species complex members: Application to Culicoides obsoletus and Culicoides scoticus (Diptera: Ceratopogonidae), potential vectors of bluetongue virus. Vet. Parasitol. 2011, 182, 297–306. [Google Scholar] [CrossRef]
- Lehmann, K.; Werner, D.; Hoffmann, B.; Kampen, H. PCR identification of culicoid biting midges (Diptera, Ceratopogonidae) of the Obsoletus Complex including putative vectors of bluetongue and Schmallenberg viruses. Parasit. Vectors 2012, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.-O.; Beer, M.; Hoffmann, B. Putative novel serotypes ‘33’ and ‘35’ in clinically healthy small ruminants in Mongolia expand the group of atypical BTV. Viruses 2021, 13, 42. [Google Scholar] [CrossRef]
- Henegariu, O.; Heerema, N.A.; Dlouhy, S.R.; Vance, G.H.; Vogt, P.H. Multiplex PCR: Critical parameters and step-by-step protocol. Biotechniques 1997, 23, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.R.; Graham, A. PCR; Spektrum Akademischer Verlag: Heidelberg, Germany, 1994; ISBN 3-86025-236-4. [Google Scholar]
- Garros, C.; Balenghien, T.; Carpenter, S.; Delécolle, J.-C.; Meiswinkel, R.; Pédarrieu, A.; Rakotoarivony, I.; Gardès, L.; Golding, N.; Barber, J.; et al. Towards the PCR-based identification of Palaearctic Culicoides biting midges (Diptera: Ceratopogonidae): Results from an international ring trial targeting four species of the subgenus Avaritia. Parasit. Vectors 2014, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Bellis, G. Studies on the Taxonomy of Australasian Species of Culicoides Latreille (Diptera: Ceratopogonidae). Ph.D. Thesis, University of Queensland, Brisbane, QLD, Australia, 2013. [Google Scholar]
- Hurst, G.D.; Jiggins, F.M. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: The effects of inherited symbionts. Proc. Biol. Sci. 2005, 272, 1525–1534. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA Barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C. DNA barcoding: Promise and pitfalls. PLoS Biol. 2004, 2, 1529–1531. [Google Scholar] [CrossRef]
- Hickerson, M.J.; Meyer, C.P.; Moritz, C. DNA barcoding will often fail to discover new animal species over broad parameter space. Syst. Biol. 2006, 55, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Hill, R.I.; Willmott, K.R.; Dasmahapatra, K.K.; Brower, A.V.; Mallet, J.; Jiggins, C.D. Limited performance of DNA barcoding in a diverse community of tropical butterflies. Proc. R. Soc. B Biol. Sci. 2007, 274, 2881–2889. [Google Scholar] [CrossRef]
- Roe, A.D.; Sperling, F.A.H. Patterns of evolution of mitochondrial cytochrome c oxidase I and II DNA and implications for DNA barcoding. Mol. Phylogenetics Evol. 2007, 44, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, T.L.; Dawson, R.D.; Magalon, H.; Baudry, E. DNA barcoding cannot reliably identify species of the blowfly genus Protocalliphora (Diptera: Calliphoridae). Proc. R. Soc. B Biol. Sci. 2007, 274, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Wiemers, M.; Fiedler, K. Does the DNA barcoding gap exist?—A case study in blue butterflies (Lepidoptera: Lycaenidae). Front. Zool. 2007, 4, 8. [Google Scholar] [CrossRef]
- Rach, J.; DeSalle, R.; Sarkar, I.N.; Schierwater, B.; Hadrys, H. Character-based DNA barcoding allows discrimination of genera, species and populations in Odonata. Proc. R. Soc. B Biol. Sci. 2008, 275, 237–247. [Google Scholar] [CrossRef]
- Schmidt, B.C.; Sperling, F.A.H. Widespread decoupling of mtDNA variation and species integrity in Grammia tiger moths (Lepidoptera: Noctuidae). Syst. Entomol. 2008, 33, 613–634. [Google Scholar] [CrossRef]
- Zhang, A.B.; Sikes, D.S.; Muster, C.; Li, S.Q. Inferring species membership using DNA sequences with back-propagation neural networks. Syst. Biol. 2008, 57, 202–215. [Google Scholar] [CrossRef]
- Baker, A.J.; Tavares, E.S.; Elbourne, R.F. Countering criticisms of single mitochondrial DNA gene barcoding in birds. Mol. Ecol. Resour. 2009, 9, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, A.J.; Kesanakurti, P.R.; Burgess, K.S.; Percy, D.M.; Graham, S.W.; Barrett, S.C.; Newmaster, S.G.; Hajibabaei, M.; Husband, B.C. Are plant species inherently harder to discriminate than animal species using DNA barcoding markers? Mol. Ecol. Resour. 2009, 9, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Gibbs, J.; Sheffield, C.; Hanner, R. DNA barcoding and the mediocrity of morphology. Mol. Ecol. Resour. 2009, 9, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Frézal, L.; Leblois, R. Four years of DNA barcoding: Current advances and prospects. Infect. Genet. Evol. 2008, 8, 727–736. [Google Scholar] [CrossRef]
- Harrup, L.E.; Bellis, G.A.; Balenghien, T.; Garros, C. Culicoides Latreille (Diptera: Ceratopogonidae) taxonomy: Current challenges and future directions. Infect. Genet. Evol. 2015, 30, 249–266. [Google Scholar] [CrossRef]
- Bellis, G.; Heung-Chul, K.; Myung-Soon, K.; Klein, T.A.; Dong-Kyu, L.; Gopurenko, D. Three species of Culicoides Latreille (Diptera: Ceratopogonidae) newly recorded from the Republic of Korea. Zootaxa 2013, 3718, 171–182. [Google Scholar] [CrossRef]
- Rot, A.; Meiswinkel, R.; Fleker, M.; Blum, S.E.; Behar, A. Towards modernizing the taxonomy of Mediterranean Culicoides using classical morphology, mtDNA barcoding, and MALDI-TOF MS protein profiling. Acta Trop. 2020, 211, 105628. [Google Scholar] [CrossRef]
- Sites, J.W., Jr.; Crandall, K.A. Testing species boundaries in biodiversity studies. Conserv. Biol. 1997, 11, 1289–1297. [Google Scholar] [CrossRef]
- Balakrishnan, R. Species concepts, species boundaries and species identification: A view from the tropics. Syst. Biol. 2007, 54, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.G.; Larson, E.L. Hybridization, introgression, and the nature of species boundaries. J. Hered. 2014, 105, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-D.; Gao, X.-F.; Harris, A.J. Species boundaries and parapatric speciation in the complex of alpine shrubs, Rosa sericea (Rosaceae), based on population genetics and ecological tolerances. Front. Plant Sci. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Cappai, S.; Loi, F.; Pinna, L.; Ruiu, A.; Puggioni, G.; Guercio, A.; Purpari, G.; Vicari, D.; Sghaier, S.; et al. Epizootic hemorrhagic disease virus serotype 8, Italy, 2022. Emerg. Infect. Dis. 2023, 29, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Paslaru, A.; Torgerson, P.R.; Veronesi, E.; Mathis, A. Vector competence of Culicoides biting midges from Switzerland for African horse sickness virus and epizootic haemorrhagic disease virus. Schweiz. Arch. Tierheilkd. 2022, 164, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Kampen, H.; Heuser, E.; Zeiske, S.; Hoffmann, B.; Höper, D.; Holsteg, M.; Sick, F.; Ziegler, S.; Wernike, K.; et al. Emergence of bluetongue virus serotype 3 in western Germany, October 2023, and ad-hoc monitoring in Culicoides biting midges. Emerg. Infect Dis. 2024. Available online: https://www.biorxiv.org/content/10.1101/2024.02.26.582175v1 (accessed on 21 February 2024).

| Species/Haplotype | Primer Code | Primer Sequence (5′ → 3′) | Modification (Position) | Amplicon (bp) |
|---|---|---|---|---|
| C. obsoletus clade O1 | obs1-COI-120F 1 | CTATCACCATRCTCTTAACYGAC | Y-wobble (4), R-wobble (13) | 120 |
| C. obsoletus clade O2 | obs2-COI-167F | AATTACTGCTATTTTACTCCTRC | R-wobble (2) | 167 |
| C. obsoletus clade O3 | obs3-COI-230F | TATCAATATRCGATCATACGGG | R-wobble (13) | 230 |
| C. scoticus | sco-COI-317F 2 | AGGAGCCTCAGTTGACTTA | none | 317 |
| C. chiopterus | chi-COI-407F | CACCCTACTATTARTAAGTAGC | R-wobble (9) | 407 |
| C. dewulfi | dew-COI-470F | AGCGACCGACATAGCATTC | C > A (15) 3 | 470 |
| Species/Haplotype | GenBank Accession No. | Specimens Tested [n] | New mPCR | Reference mPCR 1 | ||
|---|---|---|---|---|---|---|
| Positive [n] | Sensitivity [%] | Positive [n] | Sensitivity [%] | |||
| C. obsoletus clade O1 | OQ789075, OQ941500-537 | 39 | 39 | 100 | 39 | 100 |
| C. obsoletus clade O2 | OQ789076, OQ941538-560, PP110209-212 | 28 | 28 | 100 | 15 | 53.6 |
| C. obsoletus clade O3 | OQ789077, OQ941561-562 | 3 | 3 | 100 | 2 | 66.7 |
| C. scoticus clade 1 | OQ941563-572 | 10 | 10 | 100 | 8 | 80.0 |
| C. chiopterus | KJ624070, MK760108, MK760110, OQ789068, OQ941573 | 5 | 5 | 100 | 5 | 100 |
| C. dewulfi | MK760112-114, OQ789069, OQ941574-576 | 7 | 7 | 100 | 7 | 100 |
| Total | 92 | 92 | 100 | 76 | 82.6 | |
| Subgenus | Species/Haplotype | GenBank Accession No. | New mPCR | Reference mPCR |
|---|---|---|---|---|
| Unplaced | C. pallidicornis 1 | OQ789078 | + (sco) | - |
| Avaritia Fox, 1955 | C. imicola 1 | OQ789072 | - | - |
| C. montanus 1 | OQ789074 | + (O1) | + (obs) | |
| C. scoticus clade 2 1 | OQ789084 | + (sco) | - | |
| C. sanguisuga 1 | MK760238 | - | + (obs) | |
| C. sinanoensis 1 | MK760244 | - | + (obs) | |
| Beltranmyia Vargas, 1953 | C. salinarius 1 | OQ789083 | + (sco) | + (dew) |
| Culicoides Latreille, 1809 | C. boyi 2 | n.a. | - | - |
| C. bysta 2 | n.a. | - | - | |
| C. cryptipulicaris 2 | n.a. | - | - | |
| C. delta 1 | OQ789035 | - | - | |
| C. fagineus haplotype F1 2 | n.a. | + (sco) | - | |
| C. fagineus haplotype F2 1 | OQ789036 | + (sco) | - | |
| C. flavipulicaris 2 | n.a. | - | - | |
| C. grisescens haplotype G1 1 | OQ789037 | + (sco) | + (obs) | |
| C. grisescens haplotype G2 1 | OQ789038 | + (chi) | - | |
| C. kalix 2 | n.a. | - | - | |
| C. lupicaris haplotype L1 1 | OQ789039 | + (sco) | + (dew) | |
| C. lupicaris haplotype L2 1 | OQ789041 | - | - | |
| C. newsteadi s.s. 2 | n.a. | + (sco) | + (sco) | |
| C. newsteadi haplotype N1 1 | OQ789045 | - | - | |
| C. newsteadi haplotype N2 2 | n.a. | + (sco) | - | |
| C. newsteadi haplotype N3 1 | OQ789048 | - | - | |
| C. pulicaris s.s. 1 | OQ789058 | - | - | |
| C. punctatus 1 | OQ789064 | - | - | |
| C. selandicus 1 | OQ789052 | - | - | |
| C. subfagineus 2 | n.a. | + (dew) | - | |
| Monoculicoides Khalaf, 1954 | C. riethi 1 | OQ789081 | - | - |
| Wirthomyia Vargas, 1973 3 | C. riouxi1 | OQ789082 | - | - |
| Sensiculicoides Shevchenko, 1977 | C. alazanicus 1 | OQ789067 | + (O1) | + (obs)/+ (dew) |
| C. festivipennis 1 | OQ789070 | + (O2) | - | |
| C. griseidorsum 1 | OQ789071 | - | - | |
| C. kibunensis 1 | OQ789073 | - | + (obs) | |
| C. pictipennis 1 | OQ789079 | + (dew) | + (obs)/+ (dew) | |
| C. poperinghensis 1 | OQ789080 | - | - | |
| Silvaticulicoides Glukhova, 1977 | C. achrayi 1 | OQ789066 | - | + (obs)/+ (sco) |
| Genus/Species | GenBank Accession No. | New mPCR | Reference mPCR |
|---|---|---|---|
| Alluaudomyia spec. | PP110213 | + (sco) | + (obs) |
| C. stercorarius | PP110214 | + (O3) | + (>500 bp) |
| Chironomus lugubris | PP110215 | + (dew) | + (dew) |
| Clogmia albipunctata | PP110216 | - | + (obs) |
| Forcipomyia spec. | PP110217 | - | - |
| N. notatus | PP110218 | + (O1)/+ (dew) | + (obs)/+ (dew) |
| Nilotanypus dubius | PP110219 | - | - |
| Physiphora alceae | PP110220 | - | + (obs) |
| Psychoda cinerea | PP110221 | - | - |
| S. violacea | PP110222 | + (dew) | + (sco)/+ (dew) |
| Smittia spec. | PP110223 | + (dew) | + (dew) |
| Spelobia luteilabris | PP110224 | - | + (obs) |
| S. curvipes | PP110225 | + (sco) | + (obs)/+ (chi) |
| Tephrochlamys rufiventris | PP110226 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dähn, O.; Werner, D.; Mathieu, B.; Kampen, H. Large-Scale Cytochrome C Oxidase Subunit I Gene Data Analysis for the Development of a Multiplex Polymerase Chain Reaction Test Capable of Identifying Biting Midge Vector Species and Haplotypes (Diptera: Ceratopogonidae) of the Culicoides Subgenus Avaritia Fox, 1955. Genes 2024, 15, 323. https://doi.org/10.3390/genes15030323
Dähn O, Werner D, Mathieu B, Kampen H. Large-Scale Cytochrome C Oxidase Subunit I Gene Data Analysis for the Development of a Multiplex Polymerase Chain Reaction Test Capable of Identifying Biting Midge Vector Species and Haplotypes (Diptera: Ceratopogonidae) of the Culicoides Subgenus Avaritia Fox, 1955. Genes. 2024; 15(3):323. https://doi.org/10.3390/genes15030323
Chicago/Turabian StyleDähn, Oliver, Doreen Werner, Bruno Mathieu, and Helge Kampen. 2024. "Large-Scale Cytochrome C Oxidase Subunit I Gene Data Analysis for the Development of a Multiplex Polymerase Chain Reaction Test Capable of Identifying Biting Midge Vector Species and Haplotypes (Diptera: Ceratopogonidae) of the Culicoides Subgenus Avaritia Fox, 1955" Genes 15, no. 3: 323. https://doi.org/10.3390/genes15030323
APA StyleDähn, O., Werner, D., Mathieu, B., & Kampen, H. (2024). Large-Scale Cytochrome C Oxidase Subunit I Gene Data Analysis for the Development of a Multiplex Polymerase Chain Reaction Test Capable of Identifying Biting Midge Vector Species and Haplotypes (Diptera: Ceratopogonidae) of the Culicoides Subgenus Avaritia Fox, 1955. Genes, 15(3), 323. https://doi.org/10.3390/genes15030323






