FecB Was Associated with Litter Size and Follows Mendel’s Laws of Inheritance When It Transited to Next Generation in Suhu Meat Sheep Breeding Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Data Collection
2.3. FecB Genotyping
2.4. Statistical Analysis
3. Results
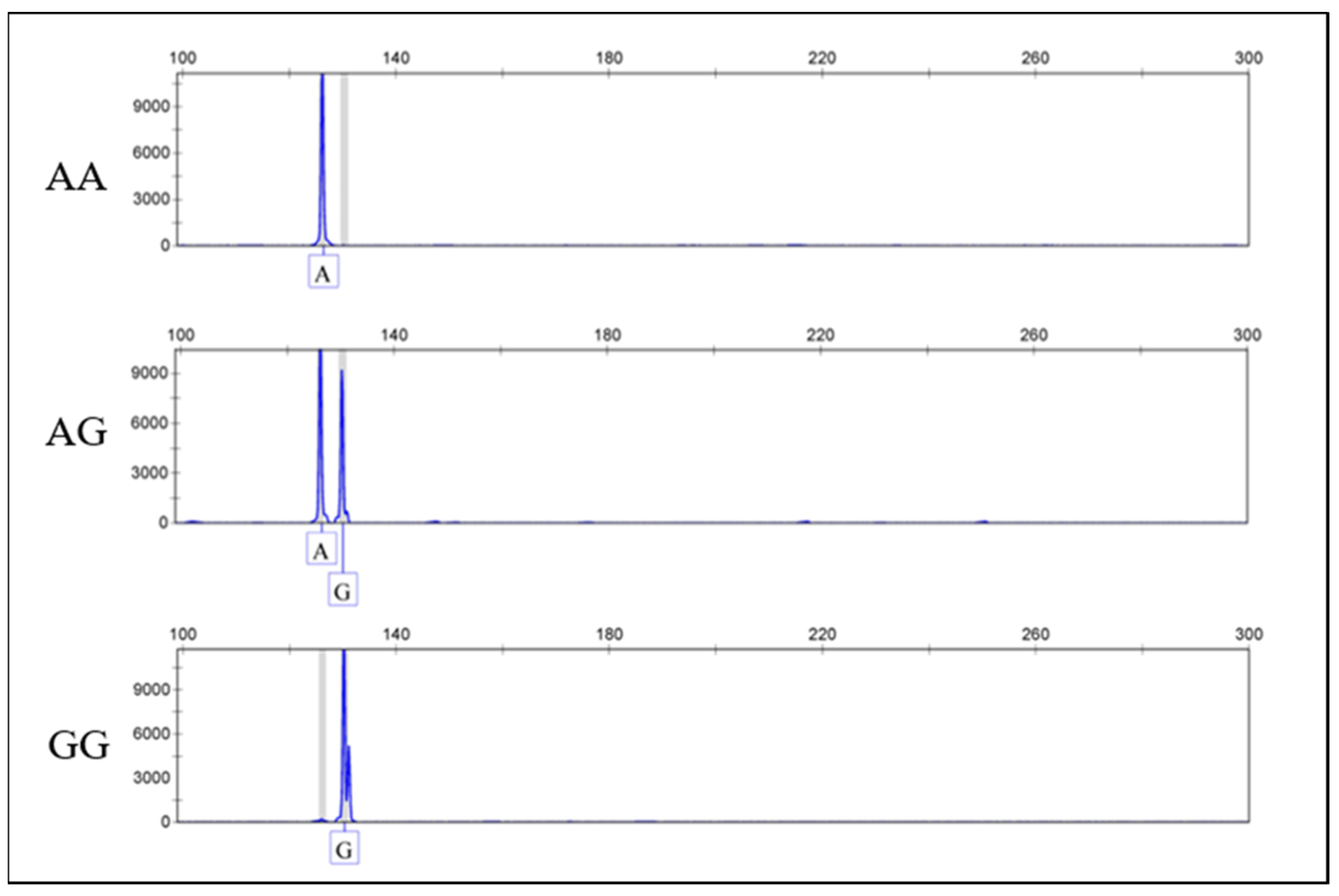
3.1. Genotyping Results
3.2. Effect of FecB on Litter Size and Lamb Survival Rate in the Suhu Meat Sheep Breeding Population
3.3. Analysis of Inheritance Patterns of FecB between Parents and Offspring in the Suhu Meat Sheep Breeding Population
3.4. Effect of FecB on Newborn and Weaning (2 Months of Age) Body Weight and Body Measurements of Suhu Meat Sheep Breeding Population.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, G.H.; Montgomery, G.W.; Allison, A.J.; Kelly, R.W.; Bray, A.R. Segregation of a major gene influencing fecundity in progeny of Booroola sheep. N. Z. J. Agr. Res. 1982, 25, 525–529. [Google Scholar] [CrossRef]
- Moghaddar, N.; Gore, K.P.; Daetwyler, H.D.; Hayes, B.J.; van der Werf, J.H. Accuracy of genotype imputation based on random and selected reference sets in purebred and crossbred sheep populations and its effect on accuracy of genomic prediction. Genet. Sel. Evol. 2015, 47, 97. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Pan, Z.Y.; Wang, X.Y.; Hu, W.P.; Di, R.; Yao, Y.X.; Chu, M.X. Progress on major genes for high fecundity in ewes. Front. Agr. Sci. Eng. 2014, 1, 282–290. [Google Scholar] [CrossRef]
- Mulsant, P.; Lecerf, F.; Fabre, S.; Schibler, L.; Monget, P.; Lanneluc, I.; Pisselet, C.; Riquet, J.; Monniaux, D.; Callebaut, I.; et al. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino ewes. Proc. Natl. Acad. Sci. USA 2001, 98, 5104–5109. [Google Scholar] [CrossRef]
- Fabre, S.; Pierre, A.; Mulsant, P.; Bodin, L.; Di Pasquale, E.; Persani, L.; Monget, P.; Monniaux, D. Regulation of ovulation rate in mammals: Contribution of sheep genetic models. Reprod. Biol. Endocrinol. 2006, 4, 20. [Google Scholar] [CrossRef]
- Kumar, S.; Rajput, P.K.; Bahire, S.V.; Jyotsana, B.; Kumar, V.; Kumar, D. Differential expression of BMP/SMAD signaling and ovarian-associated genes in the granulosa cells of FecB introgressed GMM sheep. Syst. Biol. Reprod. Med. 2020, 66, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; He, X.; Liu, Q.; Di, R.; Hu, W.; Cao, X.; Zhang, X.; Zhang, J.; Chu, M.X. Effects of FecB Mutation on Estrus, Ovulation, and Endocrine Characteristics in Small Tail Han Sheep. Front. Vet. Sci. 2021, 8, 709737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mishra, A.K.; Kolte, A.P.; Arora, A.L.; Singh, D.; Singh, V.K. Effects of the Booroola (FecB) genotypes on growth performance, ewe’s productivity efficiency and litter size in Garole x Malpura sheep. Anim. Reprod. Sci. 2008, 105, 319–331. [Google Scholar] [CrossRef]
- Davis, G.H.; Balakrishnan, L.; Ross, I.K.; Wilson, T.; Galloway, S.M.; Lumsden, B.M.; Hanrahan, J.P.; Mullen, M.; Mao, X.Z.; Wang, G.L.; et al. Investigation of the Booroola (FecB) and Inverdale (FecX(I)) mutations in 21 prolific breeds and strains of sheep sampled in 13 countries. Anim. Reprod. Sci. 2006, 92, 87–96. [Google Scholar] [CrossRef]
- Zuo, B.; Qian, H.; Wang, Z.; Wang, X.; Nisa, N.; Bayier, A.; Ying, S.; Hu, X.; Gong, C.; Guo, Z.; et al. A Study on BMPR-IB Genes of Bayanbulak Sheep. Asian Australas. J. Anim. Sci. 2013, 26, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ge, L.; Su, P.; Gu, Y.; Chen, W.; Cao, X.; Wang, S.; Lv, X.; Getachew, T.; Mwacharo, J.M.; et al. NCAPG Regulates Myogenesis in Sheep, and SNPs Located in Its Putative Promoter Region Are Associated with Growth and Development Traits. Animals 2023, 13, 3173. [Google Scholar] [CrossRef]
- Zhang, Y. Study on Production Performance and Multiplets of Hybrid Progeny of Dorper Sheep and Hu Sheep in Arid Area. Master’s Thesis, Xingjiang Agricultural University, Urumqi, China, 2021. [Google Scholar]
- McNatty, K.P.; Galloway, S.M.; Wilson, T.; Smith, P.; Hudson, N.L.; O’Connell, A.; Bibby, A.H.; Heath, D.A.; Davis, G.H.; Hanrahan, J.P.; et al. Physiological effects of major genes affecting ovulation rate in sheep. Genet. Sel. Evol. 2005, 37, S25–S38. [Google Scholar] [CrossRef]
- Isaacs, K.L.; McNatty, K.P.; Condell, L.; Shaw, L.; Heath, D.A.; Hudson, N.L.; Littlejohn, R.P.; McLeod, B.J. Plasma FSH, LH and immunoreactive inhibin concentrations in FecBB/FecBB and FecB+/FecB+ Booroola ewes and rams from birth to 12 months of age. J. Reprod. Fertil. 1995, 103, 89–97. [Google Scholar] [CrossRef]
- Goyal, S.; Aggarwal, J.; Dubey, P.K.; Mishra, B.P.; Ghalsasi, P.; Nimbkar, C.; Joshi, B.K.; Kataria, R.S. Expression Analysis of Genes Associated with Prolificacy in FecB Carrier and Noncarrier Indian Sheep. Anim. Biotechnol. 2017, 28, 220–227. [Google Scholar] [CrossRef]
- Ji, X.; Cao, Z.; Hao, Q.; He, M.; Cang, M.; Yu, H.; Ma, Q.; Li, X.; Bao, S.; Wang, J.; et al. Effects of New Mutations in BMPRIB, GDF9, BMP15, LEPR, and B4GALNT2 Genes on Litter Size in Sheep. Vet. Sci. 2023, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Liu, G.; Jiang, X. Effect of BMPRIB gene on litter size of sheep in China: A meta-analysis. Anim. Reprod. Sci. 2019, 210, 106175. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Nanekarani, S.; Hosseini, S.D. Mutation in BMPR-IB gene is associated with litter size in Iranian Kalehkoohi sheep. Anim. Reprod. Sci. 2014, 147, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.X.; Liu, Z.H.; Jiao, C.L.; He, Y.Q.; Fang, L.; Ye, S.C.; Chen, G.H.; Wang, J.Y. Mutations in BMPR-IB and BMP-15 genes are associated with litter size in Small Tailed Han sheep (Ovis aries). J. Anim. Sci. 2007, 85, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Gootwine, E.; Reicher, S.; Rozov, A. Prolificacy and lamb survival at birth in Awassi and Assaf sheep carrying the FecB (Booroola) mutation. Anim. Reprod. Sci. 2008, 108, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Sodiq, A.; Yuwono, P.; Santosa, S.A. Litter size and lamb survivability of Batur sheep in upland areas of Banjarnegara Regency, Indonesia. Anim. Prod. 2011, 13, 166–172. [Google Scholar]
- Baird, D.T.; Campbell, B.K. Follicle selection in sheep with breed differences in ovulation rate. Mol. Cell Endocrinol. 1998, 145, 89–95. [Google Scholar] [CrossRef] [PubMed]
- GUO, X.F. Study on Molecular Mechanism of FecB Gene for Fecundity in Small Tail Han Sheep. Ph.D. Thesis, China Agricultural University, Beijing, China, 2018. [Google Scholar]
- Gootwine, E.; Rozov, A.; Bor, A.; Reicher, S. Carrying the FecB (Booroola) mutation is associated with lower birth weight and slower post-weaning growth rate for lambs, as well as a lighter mature bodyweight for ewes. Reprod. Fertil. Dev. 2006, 18, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Margawati, E.T.; Putra, W.P.B.; Rizki, M.; Soetrisno, E.; Raadsma, H.W. Detection of carrier Booroola (FecB) allele in BMPR1B gene of MEGA (Merino × Garut) sheep and its association with growth traits. J. Genet. Eng. Biotechnol. 2023, 21, 19. [Google Scholar] [CrossRef]
- Guan, F.; Liu, S.R.; Shi, G.Q.; Yang, L.G. Polymorphism of FecB gene in nine sheep breeds or strains and its effects on litter size, lamb growth and development. Anim. Reprod. Sci. 2007, 99, 44–52. [Google Scholar] [CrossRef] [PubMed]
| Reagent | 10 μL System | Temperature | Time | Cycle |
|---|---|---|---|---|
| 2 × Buffer B | 5 µL | 95 ℃ | 5 min | |
| Primer Mix | 2 µL | 95 ℃ | 30 s | 35 cycles |
| 2G Fast Taq | 0.2 µL | 56 ℃ | 30 s | |
| DNA | 1.0 µL | 72 ℃ | 30 s | |
| ddH2O | to 10 µL | 60 ℃ | 30 min |
| Time | Genotype Frequency | Allele Frequency | PIC | He | Ne | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | A | G | |||||
| 2022/1/21 | 0.25 (97) | 0.50 (190) | 0.25 (95) | 0.503 | 0.497 | 0.375 | 0.500 | 2.000 | 0.99 |
| 2022/11/10 | 0.21 (173) | 0.52 (429) | 0.27 (223) | 0.470 | 0.530 | 0.374 | 0.498 | 1.993 | 0.45 |
| 2023/2/26 | 0.12 (122) | 0.55 (567) | 0.33 (347) | 0.391 | 0.609 | 0.363 | 0.476 | 1.910 | 0.00 |
| Genotype | Litter Size | Lamb Survival Rate/% | ||
|---|---|---|---|---|
| Primiparous Ewes | Parturient Ewes | Average Litter Size | ||
| AA | / | 1.14 ± 0.45 (28) | 1.14 ± 0.45 C (28) | 100 Aa (19/19) |
| AG | 1.98 ± 0.72 (42) | 1.96 ± 0.67 (170) | 1.96 ± 0.68 B (212) | 94.6 Ab (334/356) |
| GG | 2.41 ± 1 (150) | 2.37 ± 0.81 (83) | 2.40 ± 0.93 A (233) | 90.8 B (436/480) |
| Mating Type | Number of Offspring | Genotype | Quantitative Ratio | Actual Ratio | Theoretical Ratio | p-Value |
|---|---|---|---|---|---|---|
| AA × AA | 4 | AA | / | / | / | / |
| AA × AG | 45 | AA:AG | 17:28 | 1:1.65 | 1:1 | 0.101 |
| AA × GG | 123 | AG | / | / | / | / |
| AG × AG | 136 | AA:AG:GG | 35:75:26 | 1:2.14:0.74 | 1:2:1 | 0.268 |
| AG × GG | 271 | AG:GG | 157:135 | 1:0.86 | 1:1 | 0.198 |
| GG × GG | 64 | GG | / | / | / | / |
| Sex | Genotype | Body Weight | Body Height | Body Length | Chest Circumference | Chest Depth | Chest Width | Shin Circumference |
|---|---|---|---|---|---|---|---|---|
| Male (312) | AA | 4.26 ± 0.14 A | 39.31 ± 0.5 A | 31.49 ± 0.45 A | 36.04 ± 0.5 A | 15.32 ± 0.27 Aa | 10.55 ± 0.26 A | 6.25 ± 0.13 Aa |
| AG | 3.55 ± 0.07 B | 37.58 ± 0.25 B | 30.07 ± 0.22 B | 34.41 ± 0.25 B | 14.57 ± 0.13 Ab | 10.14 ± 0.13 A | 5.96 ± 0.07 Ab | |
| GG | 3.44 ± 0.09 B | 36.81 ± 0.32 B | 28.62 ± 0.29 C | 33.17 ± 0.32 C | 13.7 ± 0.17 B | 9.23 ± 0.17 B | 5.59 ± 0.08 B | |
| Female (569) | AA | 4.14 ± 0.08 A | 38.39 ± 0.39 Aa | 31.57 ± 0.29 A | 36.66 ± 1.31 | 15.83 ± 0.22 A | 10.2 ± 0.16 A | 5.98 ± 0.26 |
| AG | 3.63 ± 0.04 B | 37.31 ± 0.22 b | 30.59 ± 0.16 B | 35.93 ± 0.74 | 15.08 ± 0.12 B | 10.23 ± 0.09 A | 6.04 ± 0.15 a | |
| GG | 3.25 ± 0.06 C | 36.99 ± 0.31 B | 29 ± 0.23 C | 33.41 ± 1.06 | 14.06 ± 0.18 C | 9.41 ± 0.13 B | 5.43 ± 0.21 b |
| Sex | Genotype | Body Weight | Body Height | Body Length | Chest Circumference | Chest Depth | Chest Width | Shin Circumference |
|---|---|---|---|---|---|---|---|---|
| Male (312) | AA | 20.87 ± 0.7 A | 52.66 ± 0.54 Aa | 55.12 ± 0.76 A | 61.82 ± 0.83 A | 24.29 ± 0.41 | 17.61 ± 0.4 a | 7.5 ± 0.13 A |
| AG | 18.11 ± 0.35 B | 51.21 ± 0.27 b | 53.21 ± 0.38 | 58.58 ± 0.42 B | 23.77 ± 0.2 | 17.05 ± 0.2 | 7.12 ± 0.06 B | |
| GG | 17.81 ± 0.45 B | 50.88 ± 0.35 B | 52 ± 0.49 B | 57.89 ± 0.53 B | 23.54 ± 0.26 | 16.53 ± 0.26 b | 7 ± 0.08 B | |
| Female (569) | AA | 19.11 ± 0.41 A | 49.74 ± 0.37 | 52.43 ± 0.48 A | 60.98 ± 0.56 Aa | 23.15 ± 0.9 A | 16.55 ± 0.25 | 7.02 ± 0.07 A |
| AG | 17.85 ± 0.23 B | 50.07 ± 0.21 | 52.74 ± 0.27 A | 59.56 ± 0.31 Ab | 24.13 ± 0.5 a | 16.9 ± 0.14 | 6.92 ± 0.04 A | |
| GG | 16.31 ± 0.34 C | 49.57 ± 0.3 | 50.75 ± 0.39 B | 57.43 ± 0.45 B | 22.91 ± 0.73 Bb | 15.79 ± 0.2 | 6.55 ± 0.05 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.; Gu, Y.; Wang, S.; Cao, X.; Lv, X.; Getachew, T.; Li, Y.; Song, Z.; Yuan, Z.; Sun, W. FecB Was Associated with Litter Size and Follows Mendel’s Laws of Inheritance When It Transited to Next Generation in Suhu Meat Sheep Breeding Population. Genes 2024, 15, 260. https://doi.org/10.3390/genes15030260
Su P, Gu Y, Wang S, Cao X, Lv X, Getachew T, Li Y, Song Z, Yuan Z, Sun W. FecB Was Associated with Litter Size and Follows Mendel’s Laws of Inheritance When It Transited to Next Generation in Suhu Meat Sheep Breeding Population. Genes. 2024; 15(3):260. https://doi.org/10.3390/genes15030260
Chicago/Turabian StyleSu, Pengwei, Yifei Gu, Shanhe Wang, Xiukai Cao, Xiaoyang Lv, Tesfaye Getachew, Yutao Li, Zhenghai Song, Zehu Yuan, and Wei Sun. 2024. "FecB Was Associated with Litter Size and Follows Mendel’s Laws of Inheritance When It Transited to Next Generation in Suhu Meat Sheep Breeding Population" Genes 15, no. 3: 260. https://doi.org/10.3390/genes15030260
APA StyleSu, P., Gu, Y., Wang, S., Cao, X., Lv, X., Getachew, T., Li, Y., Song, Z., Yuan, Z., & Sun, W. (2024). FecB Was Associated with Litter Size and Follows Mendel’s Laws of Inheritance When It Transited to Next Generation in Suhu Meat Sheep Breeding Population. Genes, 15(3), 260. https://doi.org/10.3390/genes15030260







