Abstract
This study compared the growth, carcass properties, fatty acid profile, lipid-producing enzyme activity, and expression pattern of genes involved in fat metabolism in Nanyang and Landrace pigs. In the study, 32 Nanyang (22.16 ± 0.59 kg) and 32 Landrace barrows (21.37 ± 0.57 kg) were selected and divided into two groups, each with eight pens and four pigs per pen. The trial period lasted 90 days. The findings showed that the Nanyang pigs had lower average daily weight gain and lean percentage and higher average backfat thickness and lipogenic enzyme activities, including for acetyl-CoA carboxylase, glucose-6-phosphate dehydrogenase, malic enzyme, and fatty acid synthase, than the Landrace pigs. A total of 14 long-chain fatty acids were detected using HPLC-MS, in which it was found that the levels of C14:0, C18:1n-9, C20:1n-9, C20:4n-6, and MUFA were up-regulated and C18:2n-6, C18:3n-3, PUFA n6, n3/n6, and total PUFA were down-regulated in the Nanyang pigs. Moreover, the mRNA levels for genes involved in fat metabolism, ME1, FAS, and LPL, were higher and the expression of SREBP1 mRNA was lower in the Nanyang pigs. Our results suggest genetic differences between the pig breeds in terms of growth, carcass traits, lipogenic enzyme activities, fatty acid profile, and the mRNA expression of genes involved in fat metabolism in subcutaneous fat tissue, which may provide a basis for high-quality pork production. Further studies are needed to investigate the regulation of lipid metabolism.
1. Introduction
In China, with the increase in living standards, high-quality pork is highly favored by the consumer market. However, the current market supply of high-quality pork cannot meet the needs of consumers. Fat deposition is a vital factor affecting pork quality and is related to meat appearance, flavor, juiciness, tenderness, and processing characteristics, as well as human health [1]. It is well known that cardiovascular disease is closely related to dietary fat intake and fatty acid composition [2]. Animal body fat, including subcutaneous, abdominal, intermuscular, and intramuscular fat, is distributed in different parts of the body. Therefore, exploring the molecular mechanism of fat deposition has great significance for high-quality pork production. In addition to nutritional levels, age, weight, gender, and environment, fat deposition is influenced by the breeds of the pigs [3,4,5]. Local pigs are known to produce high-quality pork due to having a higher capacity for fat deposition than modern commercial breeds [6]. Zhao et al. [7] found that the terminal body weight (BW) and the hormone-sensitive lipase (HSL) activity in longissimus dorsi muscles were lower, and the backfat thickness, lean fat weight, and fatty acid synthase (FAS) activity in longissimus dorsi muscles were greater in Mashen pigs than in Large White pigs. The mRNA expression of CCAAT/enhancer-binding protein α (C/EBPα), C/EBPβ, and peroxisome proliferator-activated receptor γ (PPARγ) in longissimus dorsi muscles was increased in Mashen pigs compared to Large White pigs. There was no difference in the sterol regulatory element-binding protein 1 (SREBP1) mRNA levels in longissimus lumborum muscles between these two breeds. Another study evaluated the effects of genetics on the fat amount and fatty acid composition in Iberian and Landrace × Large White pigs and found that the swine genetic type had an important influence on the fatty acid profile of the outer and inner backfat layers. The monounsaturated fatty acid (MUFA) level in Iberian pigs at 115 kg in the two layers was greater than that in Landrace × Large White pigs [8]. Zhang et al. [9] found that Bamei pigs had a greater capacity for fat deposition than Landrace pigs, owing to the function of pre-adipocytes; the adipogenesis induced by glucose was greater in Bamei pigs than in Landrace pigs. Although there have been several studies on fat deposition in different varieties of pigs, a deeper understanding of the underlying hereditary effects of fat deposition in pigs can be achieved through further in-depth study.
The Nanyang pig is an ancient breed distributed in the Nanyang area of Henan Province in China. It is early maturing, fattens easily, and is a medium-sized meat fat dual-use-type breed. It is a Chinese local pig breed famous for its stable genetic characteristics, strong and robust physique, strong adaptability, disease resistance, and delicious meat. A previous study carried out proteome analyses on longissimus dorsi and backfat tissues from two groups of Nanyang pigs with different fat deposition efficiencies and identified 15 candidate genes determining lipid deposition, in which FASN, CAT, and SLC25A20 were the most prominent; the findings indicated that the Nanyang pig is an optimal animal model to investigate high meat quality and fast fat deposition mechanisms in pigs [10]. The Landrace pig is a common commercial variety known for its high growth speed and lean carcass. In this study, we posit that a better understanding of these two breeds’ differences would be helpful to clarify the distinction in fat deposition mechanisms in pigs of different genetic backgrounds and provide a basis for high-quality pork production. Therefore, we investigate the differences in the growth performance, carcass properties, fatty acid profile, lipid-producing enzyme activity, and expression of genes involved in fat metabolism between Nanyang and Landrace pigs.
2. Materials and Methods
2.1. Animals, Experimental Design, Diets, and Management
The pig-rearing procedure was approved by the Animal Protection and Utilization Committee of Henan Institute of Science and Technology (No. 2020HIST004, 02/04/2020, Xinxiang, China). A total of 64 barrows (32 Nanyang pigs, average body weight: 22.16 ± 0.59 kg, and 32 Landrace pigs, average body weight: 21.37 ± 0.57 kg) were selected from Xinda Husbandry Development Co., Ltd. (Luoyang, Henan Province, China). Each variety had 8 pens, with 4 pigs per pen. The pigs in the 16 pens were reared in a hog house and fed with the same commodity feed; the ingredients and nutrient contents are presented in Table 1. During the experiment, the pigs were free to feed and drink water ad libitum. Daily management was carried out in accordance with the company’s procedures. After 5 days of pre-testing, the trial period lasted 90 days. In the whole experiment, the feed consumed and the residual feed were measured daily, and then the initial BW, final BW, average daily weight gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G) of the pigs were determined.

Table 1.
Composition and nutritional content of basic diet.
2.2. Sample Collection
At the end of the experiment, 16 pigs (2 pigs per pen, close to the average weight of their breed group) in each breed were selected; after weighing, the animals were fasted for twelve hours, given free access to water, and then slaughtered after electrical stunning at a commercial abattoir according to Chinese standard industry procedures. After slaughter, each carcass was weighed, and then the average backfat depth at the 1st rib, 1st lumbar, and last lumbar vertebrae was calculated using a sliding caliper in the midline at the right carcass sides [11]. The loin muscle area was measured at the transversal surface of the longissimus lumborum between the last thoracic vertebra and first lumbar vertebra using a sliding caliper (width × thickness × 0.7, cm2). The lean meat percent was calculated according to the formula (lean weight ÷ carcass weight × 100%). Approximately 100 g of subcutaneous fat from the outer backfat layer at the 13th rib on the right side of the carcass was obtained immediately post mortem and kept at −80 °C for further testing of the fatty acid profile, lipid-producing enzyme activity, and expression of genes involved in fat metabolism [12].
2.3. Assay of Lipid-Producing Enzyme Activities in Subcutaneous Fat Tissue
The activities of acetyl-CoA carboxylase (ACC, EC6.4.1.2), malic enzyme (ME, EC 1.1.1.40), glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49), and FAS (EC 2.3.1.85) in the subcutaneous fat tissue were determined according to Gerfault et al. [13]. All of the enzyme assays were determined in triplicate. Then, 1 g of frozen subsample from the collected subcutaneous fat tissue was immediately homogenized in 0.25 M sucrose solution on ice, and then centrifuged for 30 min at 30,000× g and 4 °C. Next, the supernatants were collected to detect the lipogenic enzyme activities using the colorimetric method according to the producer’s manual (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). One unit of G6PDH and ME activity was determined as the enzymes production of 1 nmol nicotinamide adenine dinucleotide phosphate (NADPH) min−1·g sample−1, resulting from the reduction in NADP+. ACC reacts with acetyl-CoA and ATP: the products are malonyl-CoA, ADP, and inorganic phosphorus, and its activity was defined as the enzyme generating 1 nmol of PO43−·g sample−1 per hour as one unit. The FAS activity was defined as 1 nmol of NADPH oxidized to NADP+ min−1·g sample−1 as one unit.
2.4. Assay of Fatty Acid Profile in Subcutaneous Fat Tissue
Fatty acid samples from the subcutaneous fat tissue (0.05 g) were prepared according to Folch et al. [14], put into 10 mL centrifuge tubes, and obtained by mixing a solution of 5 mL chloroform/methanol (2:1 v/v) for two hours after homogenizing. Next, 5 mL of distilled water was added and mixed well, and the mixed solution was centrifuged for 5 min at 3000× g and 25 °C. Finally, the supernatants were discarded, and the lower solutions were dried with negative pressure. The methylation of fatty acids followed this procedure: 1 mL of n-hexane was added and swung for 25 min at 40 °C, and then 1 mL KOH-methanol solution (0.4 mol/L) was added; after standing for 25 min, the mixtures were shaken for 2 h. Then, 2 mL of deionized water was added and blended, and centrifugation for 5 min at 3000× g was carried out. The supernatant was collected and detected using high-performance liquid chromatography–mass spectrometry (HPLC-MS) for the analysis of the fatty acid profile. The HPLC-MS was performed on a SCIEX QTRAP 4500 mass spectrometer (Applied Biosystems, Framingham, MA, USA) coupled with an Agilent 1260 Infinity HPLC system (Agilent Technologies, Santa Clara, CA, USA). The mobile phases consisted of acetonitrile (A) and isopropanol (B). The procedure of gradient elution (0–10 min, 100% B; 11–30 min, 70% B; 31–120 min, 50% B) was performed at a flow rate of 0.2 mL/min; 10 μL of the sample was injected into the chromatographic system. Mass spectrometry was carried out under atmospheric pressure chemical ionization in positive ion mode (APCI+); spray voltage, 1.5 kV; capillary temperature, 300 °C; MS scanning range, 50−1200 amu; scanning rate, 50 amu/s. The result of the fatty acid analysis was described by g fatty acids/100 g of detected fatty acid methyl esters. All of the fatty acid assays were conducted in triplicate.
2.5. Assay of Quantitative Real-Time PCR
The isolation of total RNA from the subcutaneous fat tissues was performed with TRIzol (Invitrogen, Waltham, MA, USA) according to the producer’s manual. The quantity and quality of the obtained RNA were evaluated with a NanoPhotometer® spectrophotometer (Implen, Westlake Village, CA, USA; 1.8 < OD260/OD280 < 2.0). The cDNA synthesis was performed with AMV reverse transcriptase (Promega, Madison, WI, USA). The mRNAs of adipose triglyceride lipase (ATGL), SREBP1, leptin (LEP), malic enzyme 1 (ME1), FAS, hormone-sensitive lipase (HSL), and lipoprotein lipase (LPL) were selected for the detection of the expression profiles of genes involved in fat metabolism. The primer pairs were designed for quantitative real-time PCR (qRT-PCR), as presented in Table 2. The qRT-PCRs were performed with the QuantiFast SYBR® Green RT-PCR Kit (Qiagen, Hilden, Germany) according to the producer’s manual. The mRNA expression level of these genes was determined using the 2–△△Ct method [15], with β-actin as a housekeeping gene. The assays of the genes involved in fat metabolism were carried out in triplicate.

Table 2.
Design of primer pairs for genes involved in fat metabolism.
2.6. Statistical Analysis
All data were analyzed using an independent-group two-tailed t-test in SPSS 26.0 (IBM Corp., Armonk, NY, USA), and significant differences between breeds were indicated by p < 0.05.
3. Results
3.1. Growth Performance and Carcass Traits
The growth performance data of the Nanyang and Landrace pigs are presented in Table 3. There was no significant difference in the initial BW (0.79 kg; p = 0.172) or ADFI (114.9 g/d; p = 0.116) between the Nanyang and Landrace pigs. Compared to Landrace, the Nanyang pigs had a significantly lower final BW (20.04 kg; p < 0.001) and ADG (231.5 g/d; p < 0.001), and a significantly higher F/G (0.95; p = 0.001).

Table 3.
Impacts of genetic type on growth performance in Nanyang and Landrace pigs.
As presented in Table 4, the carcass weight (15.77 kg; p = 0.003), loin muscle area (18.07 cm2; p = 0.001), and lean percentage (10.99%; p = 0.005) in the Nanyang pigs were lower than those in the Landrace pigs. The average backfat thickness in the Nanyang pigs was higher than that in the Landrace pigs (6.76 mm; p = 0.003).

Table 4.
Effects of genetic type on carcass properties in Nanyang and Landrace pigs.
3.2. Assay of Lipid-Producing Enzyme Activities in Subcutaneous Fat Tissue
As presented in Table 5, the lipid-producing enzyme activities of ACC (6.39 U; p = 0.010), G6PDH (132.28 U; p = 0.001), ME (130.88 U; p = 0.002), and FAS (17.24 U; p = 0.001) were up-regulated in the Nanyang pigs compared to the Landrace pigs.

Table 5.
Effects of genetic type on the lipid-producing enzyme activities of subcutaneous fat tissue in Nanyang and Landrace pigs.
3.3. Assay of Fatty Acid Profile of Subcutaneous Fat Tissue
A total of 14 long-chain fatty acids were detected using HPLC-MS (Table 6). The levels of C14:0 (0.20 g; p = 0.047), C18:1n-9 (2.34 g; p = 0.011), C20:1n-9 (0.30 g; p = 0.040), C20:4n-6 (0.35 g; p = 0.023), and MUFA (3.14 g; p = 0.002) were greater in the Nanyang pigs than in the Landrace pigs, whereas the C18:2n-6 (5.14 g; p = 0.003), C18:3n-3 (0.08 g; p = 0.011), PUFA n6 (4.82 g; p < 0.001), PUFA n3/n6 (2.82 g; p < 0.001), and total PUFA (4.89 g; p = 0.005) levels were lower in the Nanyang pigs than in the Landrace pigs. The levels of PUFA n3 (0.09 g; p = 0.351) and saturated fatty acids (SFA, 1.75 g; p = 0.097) did not differ between the breeds.

Table 6.
Effects of genetic type on the fatty acid profile (g fatty acids/100 g of detected fatty acid methyl esters) of subcutaneous fat tissue in Nanyang and Landrace pigs.
3.4. Assay of Expression of mRNAs Involved in Fat Metabolism
As presented in Figure 1, the expression of LEP (1.00; p = 0.001), ME1 (0.72; p = 0.001), FAS (1.01; p = 0.002), and LPL (0.18; p = 0.005) was up-regulated in the Nanyang pigs compared to the Landrace pigs. SREBP1 expression was down-regulated in the Nanyang pigs compared to the Landrace pigs (0.34; p = 0.003) and the expression of ATGL (0.09; p = 0.058) and HSL (0.12; p = 0.065) did not differ between the breeds.
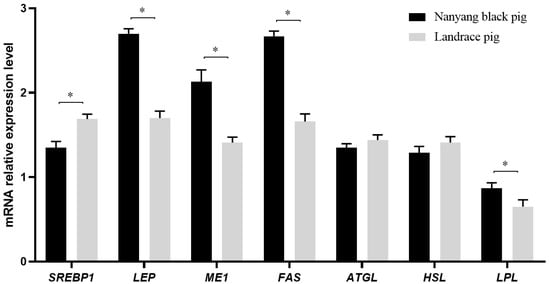
Figure 1.
Expression levels of mRNAs involved in fat metabolism in subcutaneous fat tissue in Nanyang and Landrace pigs. The mRNA expressions of SREBP1, LEP, ME1, FAS, ATGL, HSL, and LPL in the subcutaneous fat tissue of Nanyang pigs and Landrace pigs were detected using qRT-PCR. The values with an asterisk (*) mean significant differences (p < 0.05). ATGL, adipose triglyceride lipase; FAS, fatty acid synthase; HSL, hormone-sensitive lipase; LEP, leptin; LPL, lipoprotein lipase; ME1, malic enzyme 1; SREBP1, sterol regulatory element-binding transcription factor 1.
4. Discussion
We found that the Nanyang pigs had lower final BW, ADG, carcass weight, loin muscle area, and lean percentage compared with the Landrace pigs, but had greater F/G and average backfat thickness. These findings are consistent with those in previous research [9,16,17,18,19] in which native pig breeds (Iberian, Creole, Bamei, Iberian, Meishan) displayed a lower tendency for growth and better capacity for fat deposition than modern pig breeds (Landrace, Large White, Duroc). Miao et al. [20] studied Jinhua and Landrace pigs and found a lower carcass lean percentage and greater carcass fat frequency in Jinhua pigs than in Landrace pigs. Gispert et al. [21] studied five breeds of pigs (Large White, Landrace, Duroc, Pietrain, and Meishan) and discovered that pigs from the Chinese local breed, Meishan, had lower growth potential and carcass lean meat. Similar results were found for the growth and carcass traits of pigs with breed-specific patterns when Iberian [17], Creole [16], Alentejana [22], and Bamei [9] pigs were compared with modern pig breeds.
Lipogenesis is principally carried out in the fat tissue of pigs and depends on the activities of enzymes [1,23,24]. In this research, we identified that the Nanyang pigs had higher lipogenic enzyme activities, such as ACC, G6PDH, ME, and FAS activity, than those of the Landrace pigs. ACC is an important rate-limiting enzyme for malonyl coenzyme A synthesis, which is the first phase of adipose biosynthesis, and is also an enzyme that effectively inhibits mitochondrial fatty acid oxidation through the regulation of carnitine palmitoyltransferase-1 (CPT-1) [25]. FAS is the core enzyme needed for the transformation of carbohydrates into fatty acids, and it catalyzes the transformation of acetyl coenzyme A and malonyl coenzyme A into fatty acids in the presence of NADPH [26]. ME and G6PDH are important enzymes required to provide NADPH for the reductive biosynthesis of fatty acids [27,28]. Freire et al. found that the ACC (three- and nine-fold), ME (six- and five-fold), and G6PDH (four- and five-fold) increased significantly more in the dorsal subcutaneous and perirenal fat of Alentejano piglets than in Large White piglets [29]. Another study showed that the function of lipid-producing enzymes (FAS, ME, and G6PDH) was greater in the subcutaneous backfat tissue of Iberian pigs than that in Landrace × Large White pigs [30]. The breed-specific differences in the key lipogenic enzymes in this study are similar to those found in previous studies [26,27,28,29,30].
Our study revealed significant differences between the Nanyang and Landrace pigs in terms of MUFAs and PUFAs. There were significantly more MUFAs in the Nanyang pigs than in the Landrace pigs, mainly because of the addition of oleic acid (C18:1 n-9). The Nanyang pigs had significantly lower PUFA levels compared to the Landrace pigs, mainly due to the variations in linoleic acid (C18:2 n-6). These findings are in agreement with those in Barea’s research, which reported that Iberian barrows had higher MUFA levels and lower SFA levels than Landrace × Large White barrows in the subcutaneous outer fat both at 50 and 115 kg of body weight [8]. The MUFA content was greater in Iberian pigs at 115 kg body weight in both the outer and inner backfat, and PUFAs and C18:2 n-6 were higher in both layers (along with body weight) in Landrace × Large White barrows [8]. Another study found a higher content of MUFAs and lower PUFA levels in Pulawska pigs than those in Polish Landrace pigs, demonstrating that the genetic type had a vital function in the fatty acid profile [31]. The PUFA n-6/n-3 ratio is a valuable indicator for evaluating the nutritional quality of meat. A previous study showed that a low PUFA n-6/n-3 ratio (4:1) diet increased piglets’ weaning survival rate and weight gain, and improved the piglets’ health [32]. In this paper, interestingly, we found that the Nanyang pigs had a lower PUFA n-6/n-3 ratio (6.90:1) in subcutaneous backfat tissue than the Landrace pigs (9.72:1), which may mean that the former has higher nutritive value. However, no significant differences in the PUFA n-3 or SFAs between the Nanyang and Landrace pigs were detected in this study.
We found that the contents of LEP, ME1, FAS, and LPL mRNA were significantly up-regulated and the SREBP1 mRNA contents were significantly down-regulated in the Nanyang pigs compared to the Landrace pigs, with no significant difference in the content of ATGL and HSL mRNA being observed between them. Benítez et al. found that LEP, ME1, and FAS had higher expression and ATGL lower expression in the subcutaneous adipose tissue from Iberian pigs compared to Duroc pigs, showing that the Iberian pigs had a more stable expression of lipogenic genes [19]. Xing et al. identified the differential expression of genes in backfat tissue between Songliao and Landrace pigs using high-throughput sequencing, and found that the ACC increased significantly and LEP decreased significantly in Songliao pigs compared to Landrace pigs [33]. In another study, the SREBP-1c, FAS, and ACC1 (on d 10) mRNA expression levels were increased in the subcutaneous fat of Bamei pigs compared to in Landrace pigs [9]. In this paper, genes involved in fat metabolism were identified, and they may explain the phenotypic differences observed between Nanyang and Landrace pigs.
5. Conclusions
In the present paper, we demonstrated the phenotypic differences between Nanyang and Landrace pigs in terms of growth, carcass traits, fatty acid profile, lipogenic enzyme activities, and expression of mRNAs involved in fat metabolism in subcutaneous fat tissue, which might explain the differences in fat deposition linked to different breeds of pigs. This paper provides references for the future breeding of native breeds and for high-quality pork production. Further studies to elucidate the molecular mechanisms of lipid metabolism, so as to reduce the fatness of Nanyang pigs, have practical implications.
Author Contributions
Conceptualization, J.Z. and Z.M.; methodology, S.M. and H.W.; validation, Z.S. and L.H.; formal analysis, J.Z. and Z.M.; investigation, J.Z. and Z.M.; data curation, S.M. and H.W.; writing—original draft preparation, J.Z. and Z.M.; writing—review and editing, J.Z., C.Z., Z.S. and L.H.; visualization, C.Z.; supervision, Z.M.; project administration, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Program for Innovative Research Team (in Science and Technology) at the University of Henan Province (Henan Province, China; 22IRTSTHN026), the Provincial Key Technology Research and Development Program of Henan (232102110079), and the Key Scientific Research Projects of Colleges and Universities in Henan Province (Henan Province, China; 23B230003).
Institutional Review Board Statement
The animal protocol for this study followed the Animal Care and Use Statute of China and was approved by the Animal Protection and Utilization Committee of Henan Institute of Science and Technology (No. 2020HIST004, Xinxiang, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat deposition and fat effects on meat quality: A review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yang, D.D.; Liu, Z.L.; Zeng, Y.Q.; Chen, W. Expression of lipid metabolism genes provides new insights into intramuscular fat deposition in Laiwu pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, Q.; Sun, Y.; Pan, T.; Liu, S.; Miao, W.; Li, Y.; Zhou, L.; Xu, G. Lipidomic and transcriptomic analysis of the longissimus muscle of Luchuan and Duroc pigs. Front. Nutr. 2021, 8, 667622. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wei, P.; Khan, M.A.; Zhang, J.; Guo, L.; Liu, D.; Zhang, X.; Bai, Y.; Wang, S. Transcriptome analysis reveals differential gene expression in intramuscular adipose tissues of Jinhua and Landrace pigs. J. Vet. Med. Sci. 2018, 80, 953–959. [Google Scholar] [CrossRef]
- Poklukar, K.; Candek-Potokar, M.; Batorek Lukac, N.; Tomazin, U.; Skrlep, M. Lipid deposition and metabolism in local and modern pig breeds: A review. Animals 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, K.; Yang, Q.; Du, M.; Liu, X.; Cao, G. Enhanced adipogenesis in Mashen pigs compared with Large White pigs. Ital. J. Anim. Sci. 2017, 16, 217–225. [Google Scholar] [CrossRef]
- Barea, R.; Isabel, B.; Nieto, R.; Lopez-Bote, C.; Aguilera, J.F. Evolution of the fatty acid profile of subcutaneous back-fat adipose tissue in growing Iberian and Landrace × Large White pigs. Animal 2013, 7, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lu, J.X.; Chen, Y.; Zhao, Y.Q.; Guo, P.H.; Yang, J.T.; Zang, R.X. Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from Bamei and Landrace pigs. Biochem. Cell Biol. 2014, 92, 259–267. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhang, B.; Zhong, H.; Lu, Y.; Zhang, H. Candidate gene screening for lipid deposition using combined transcriptomic and proteomic data from Nanyang black pigs. BMC Genom. 2021, 22, 441. [Google Scholar] [CrossRef]
- Nong, Q.; Wang, L.; Zhou, Y.; Sun, Y.; Chen, W.; Xie, J.; Zhu, X.; Shan, T. Low dietary n-6/n-3 PUFA ratio regulates meat quality, reduces triglyceride content, and improves fatty acid composition of meat in Heigai pigs. Animals 2020, 10, 1543. [Google Scholar] [CrossRef]
- Song, B.; Di, S.; Cui, S.; Chen, N.; Wang, H.; Wang, X.; Gao, Q.; Tong, G.; Wang, H.; Huang, X.; et al. Distinct patterns of PPARgamma promoter usage, lipid degradation activity, and gene expression in subcutaneous adipose tissue of lean and obese swine. Int. J. Mol. Sci. 2018, 19, 3892. [Google Scholar] [CrossRef]
- Gerfault, V.; Louveau, I.; Mourot, J.; Le Dividich, J. Lipogenic enzyme activities in subcutaneous adipose tissue and skeletal muscle from neonatal pigs consuming maternal or formula milk. Reprod. Nutr. Dev. 2000, 40, 103–112. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D.; Mourot, J. A comparison of carcass and meat quality characteristics of Creole and Large White pigs slaughtered at 90kg BW. Meat Sci. 2007, 76, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Serra, X.; Gil, F.; Pérez-Enciso, M.; Oliver, M.A.; Vázquez, J.M.; Gispert, M.; Díaz, I.; Moreno, F.; Latorre, R.; Noguera, J.L. A comparison of carcass, meat quality and histochemical characteristics of Iberian (Guadyerbas line) and Landrace pigs. Livest. Prod. Sci. 1998, 56, 215–223. [Google Scholar] [CrossRef]
- Kojima, M.; Nakajima, I.; Arakawa, A.; Mikawa, S.; Matsumoto, T.; Uenishi, H.; Nakamura, Y.; Taniguchi, M. Differences in gene expression profiles for subcutaneous adipose, liver, and skeletal muscle tissues between Meishan and Landrace pigs with different backfat thicknesses. PLoS ONE 2018, 13, e0204135. [Google Scholar] [CrossRef] [PubMed]
- Benitez, R.; Fernandez, A.; Isabel, B.; Nunez, Y.; De Mercado, E.; Gomez-Izquierdo, E.; Garcia-Casco, J.; Lopez-Bote, C.; Ovilo, C. Modulatory effects of breed, feeding status, and diet on adipogenic, lipogenic, and lipolytic gene expression in growing Iberian and Duroc pigs. Int. J. Mol. Sci. 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.G.; Wang, L.J.; Xu, Z.R.; Huang, J.F.; Wang, Y.R. Developmental changes of carcass composition, meat quality and organs in the Jinhua pig and Landrace. Animal 2009, 3, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Gispert, M.; Font, I.F.M.; Gil, M.; Velarde, A.; Diestre, A.; Carrion, D.; Sosnicki, A.A.; Plastow, G.S. Relationships between carcass quality parameters and genetic types. Meat Sci. 2007, 77, 397–404. [Google Scholar] [CrossRef]
- Madeira, M.S.; Pires, V.M.; Alfaia, C.M.; Costa, A.S.; Luxton, R.; Doran, O.; Bessa, R.J.; Prates, J.A. Differential effects of reduced protein diets on fatty acid composition and gene expression in muscle and subcutaneous adipose tissue of Alentejana purebred and Large White × Landrace × Pietrain crossbred pigs. Br. J. Nutr. 2013, 110, 216–229. [Google Scholar] [CrossRef]
- Song, C.H.; Oh, S.M.; Lee, S.; Choi, Y.; Kim, J.D.; Jang, A.; Kim, J. The ratio of dietary n-3 polyunsaturated fatty acids influences the fat composition and lipogenic enzyme activity in adipose tissue of growing pigs. Food Sci. Anim. Resour. 2020, 40, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, J.F.; Hernández-Matamoros, A.; González, E. Free-range and low-protein concentrated diets in Iberian pigs: Effect on plasma insulin and leptin concentration, lipogenic enzyme activity, and fatty acid composition of adipose tissue. Animals 2020, 10, 1917. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-coA carboxylases and diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Jones, S.F.; Infante, J.R. Molecular pathways: Fatty acid synthase. Clin. Cancer Res. 2015, 21, 5434–5438. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Simmen, F.A.; Alhallak, I.; Simmen, R.C.M. Malic enzyme 1 (ME1) in the biology of cancer: It is not just intermediary metabolism. J. Mol. Endocrinol. 2020, 65, R77–R90. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.P.; Mourot, J.; Cunha, L.F.; Almeida, J.A.; Aumaitre, A. Effect of the source of dietary fat on postweaning lipogenesis in lean and fat pigs. Ann. Nutr. Metab. 1998, 42, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Palma-Granados, P.; Seiquer, I.; Benitez, R.; Ovilo, C.; Nieto, R. Effects of lysine deficiency on carcass composition and activity and gene expression of lipogenic enzymes in muscles and backfat adipose tissue of fatty and lean piglets. Animal 2019, 13, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, A.; Tyra, M.; Babicz, M. Fatty acid profile of pork from a local and a commercial breed. Arch. Anim. Breed. 2015, 58, 379–385. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Agazzi, A.; Comi, M.; Bontempo, V.; Guido, I.; Panseri, S.; Sauerwein, H.; Eckersall, P.D.; Burchmore, R.; Savoini, G. Effects of low ω6:ω3 ratio in sow diet and seaweed supplement in piglet diet on performance, colostrum and milk fatty acid profiles, and oxidative status. Animals 2020, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Wang, K.; Ao, H.; Chen, S.; Tan, Z.; Wang, Y.; Xitong, Z.; Yang, T.; Zhang, F.; Liu, Y.; et al. Comparative adipose transcriptome analysis digs out genes related to fat deposition in two pig breeds. Sci. Rep. 2019, 9, 12925. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).