miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. HS Models
2.3. Transfection
2.4. Biological Analysis of Sequencing Data
2.5. Cell Counting Kit-8 (CCK-8) Detection of Cell Viability
2.6. EDU Assay to Detect Cell Proliferation Efficiency
2.7. RNA Extraction and Real-Time Fluorescence Quantitative PCR (RT-qPCR)
2.8. Western Blotting
2.9. Dual Luciferase Reporter Gene Assay
2.10. Results Statistical Analysis of Data
3. Results
3.1. miR-425-5p Is Associated with Breast Development
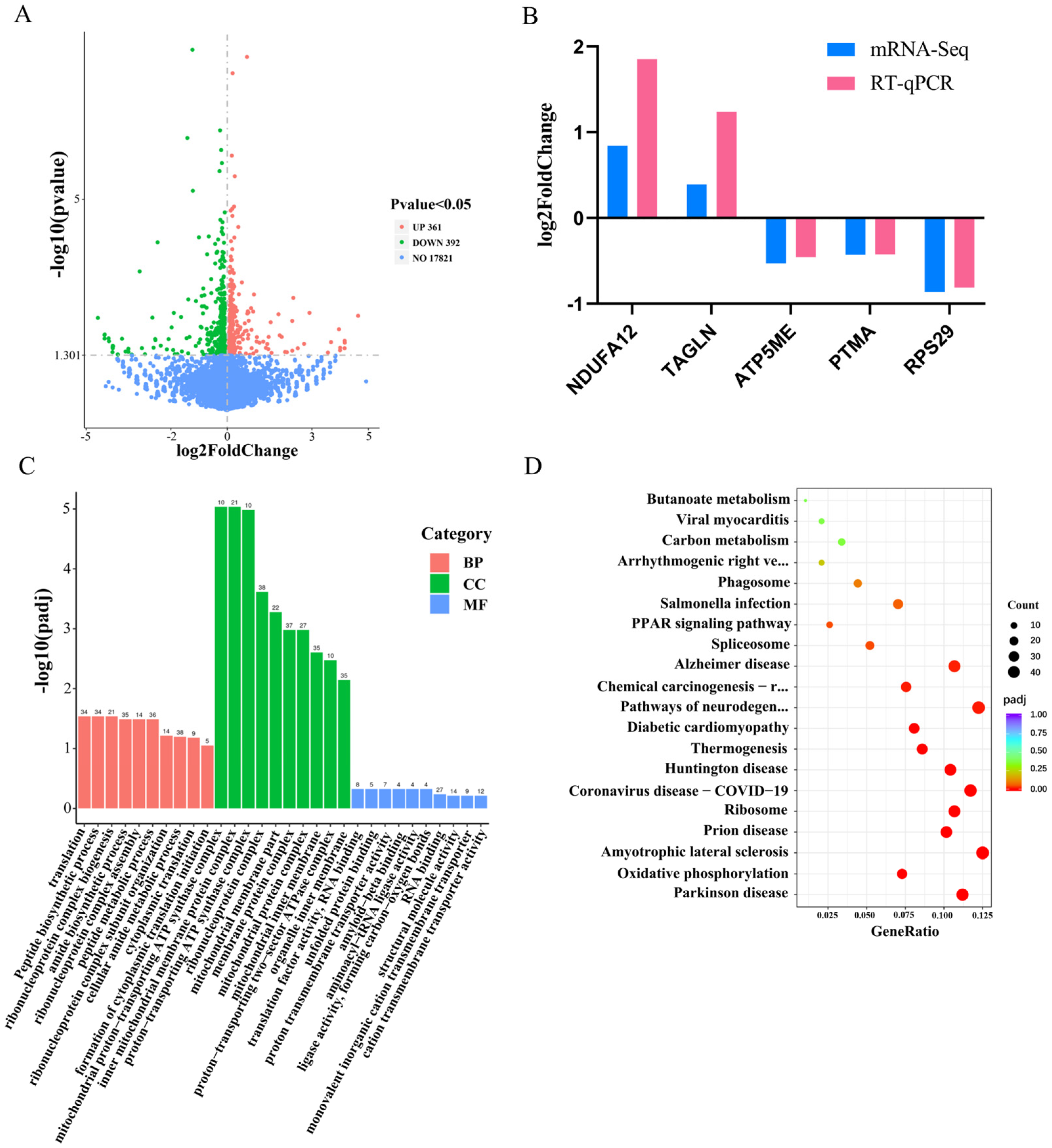
3.2. The Investigation of the Functionality by Differentially Expressed Genes in Response to miR-425-5p
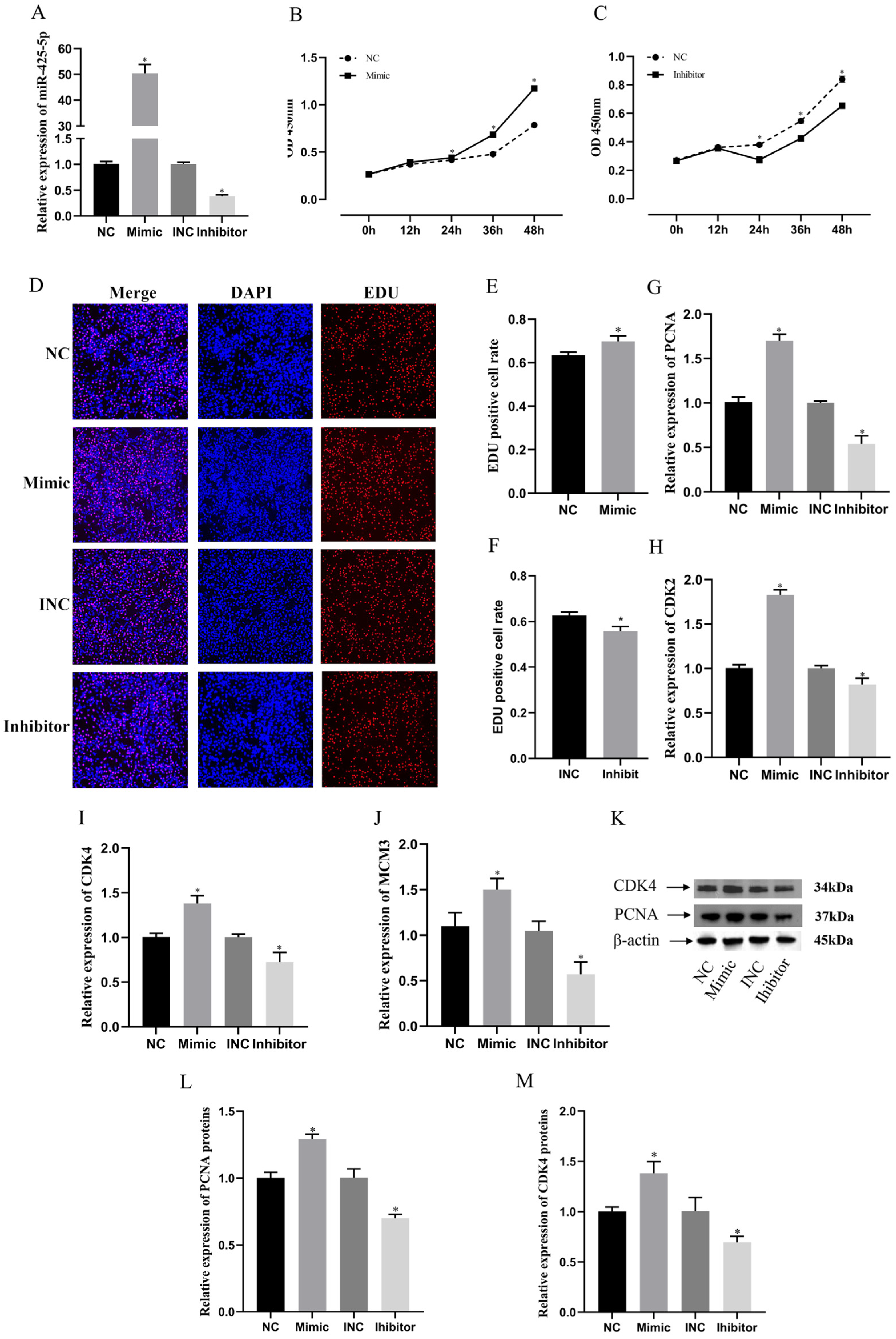
3.3. miR-425-5p Promotes Proliferation of BMECs
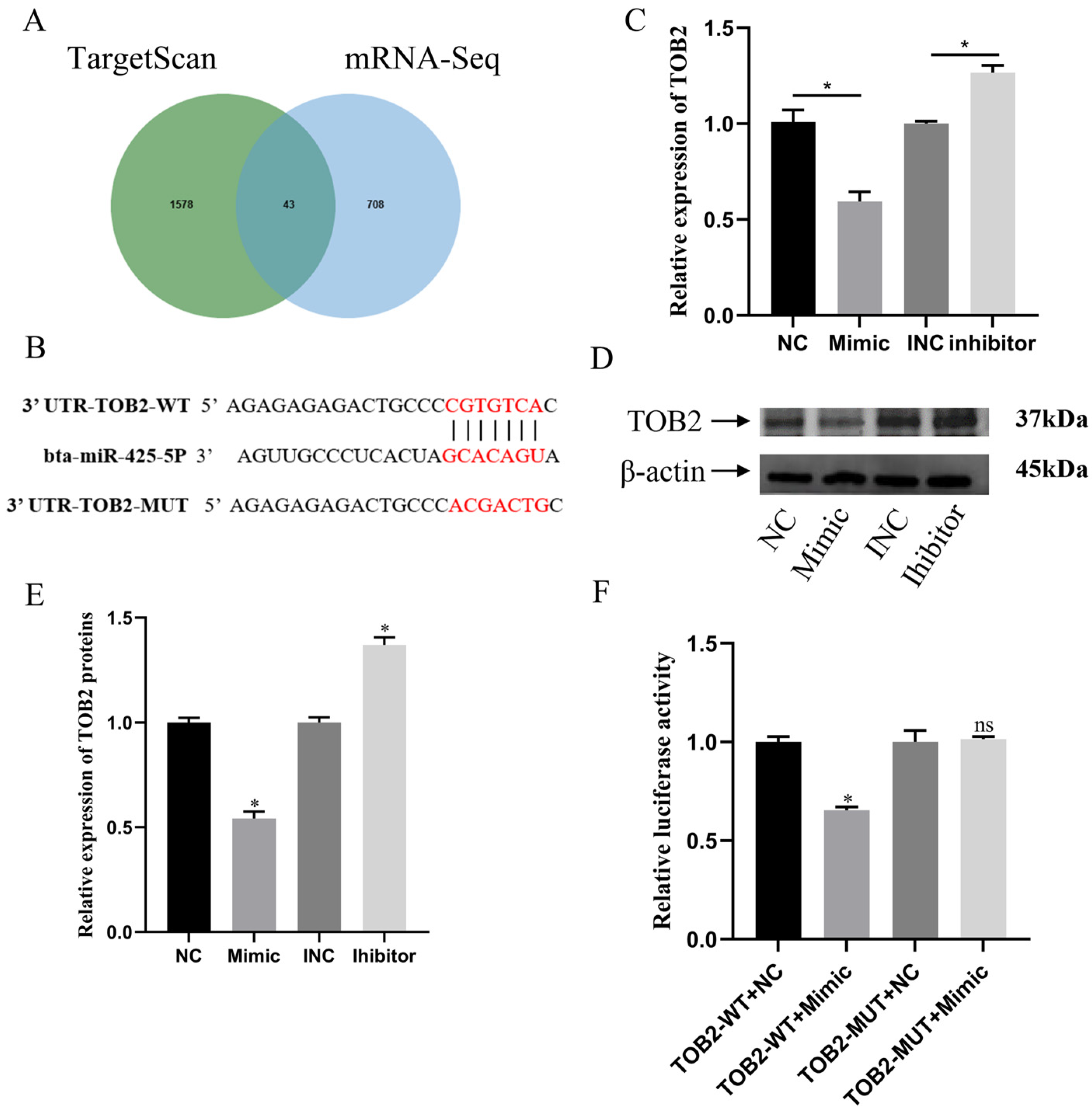
3.4. miR-425-5p Regulates BMECs by Targeting TOB2
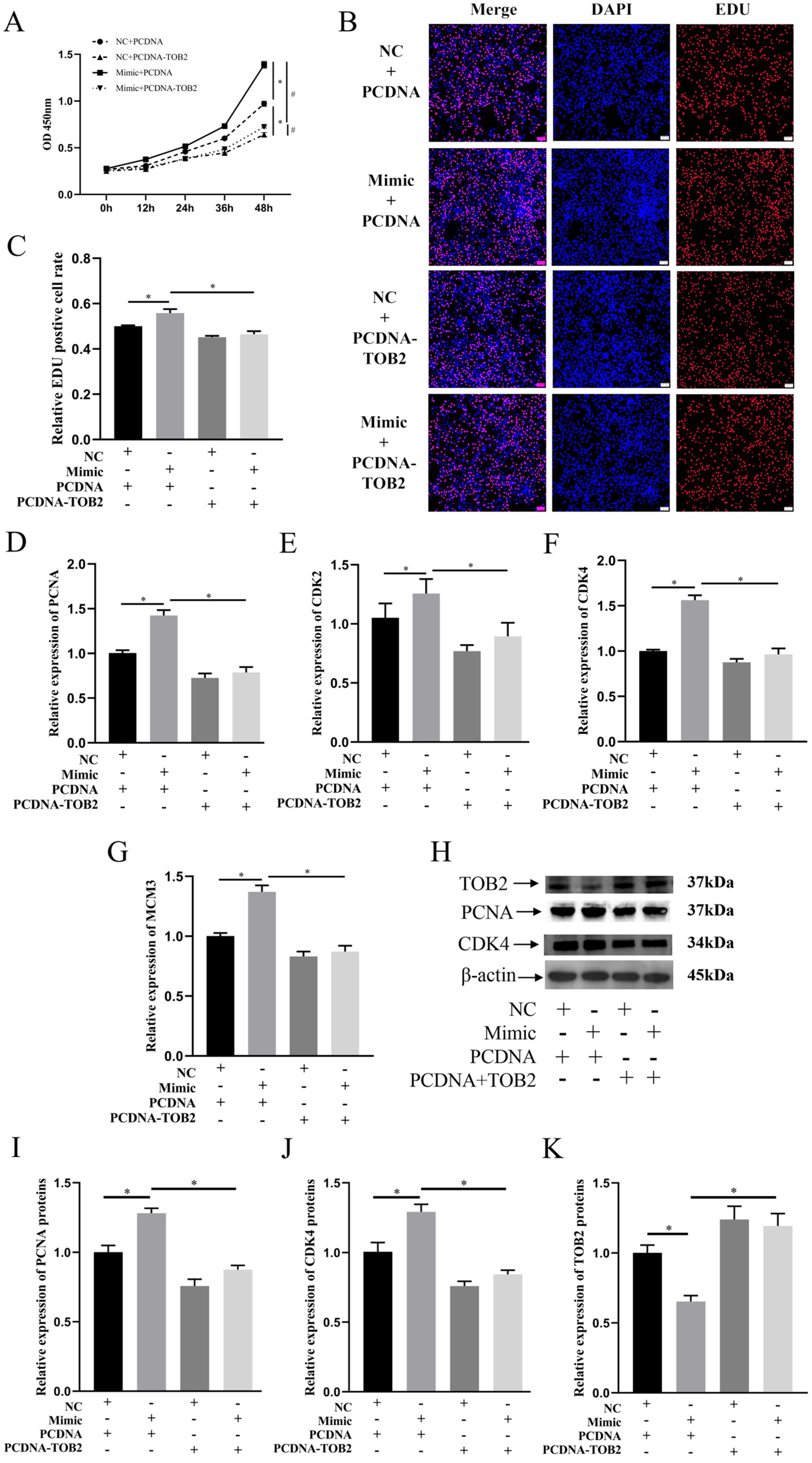
3.5. TOB2 Attenuated the Proliferation Effect of miR-425-5p on BMECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauner, G.; Barash, I. Cell hierarchy and lineage commitment in the bovine mammary gland. PLoS ONE 2012, 7, e3011. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zhang, Y.; Zhang, J.; Qi, S.; Zhang, Y.; Gao, M.-Q. Overexpression of lncRNA H19 changes basic characteristics and affects immune response of bovine mammary epithelial cells. PeerJ 2019, 7, 6715. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, Y.; Ju, X. Effect of heat stress on growth and production performance of livestock and poultry: Mechanism to prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef] [PubMed]
- West, J.W.; Mullinix, B.G.; Bernard, J.K. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J. Dairy Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ammer, S.; Lambertz, C.; von Soosten, D.; Zimmer, K.; Meyer, U.; Dänicke, S.; Gauly, M. Impact of diet composition and temperature-humidity index on water and dry matter intake of high-yielding dairy cows. J. Anim. Physiol. Anim. Nutr. 2017, 102, 103–113. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I.; Hoogenboom, G. Genetic Component of Heat Stress in Dairy Cattle, Development of Heat Index Function. J. Dairy Sci. 2000, 83, 2120–2125. [Google Scholar] [CrossRef]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat Stress: A Serious Disruptor of the Reproductive Physiology of Dairy Cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Ibáñez-Ventoso, C.; Vora, M.; Driscoll, M. Sequence relationships among C. elegans, D. melanogaster and human microRNAs highlight the extensive conservation of microRNAs in biology. PLoS ONE 2008, 3, e2818. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Reinhart, B.J.; Bartel, D.P.; Zamore, P.D. A biochemical framework for RNA silencing in plants. Genes Dev. 2003, 17, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Garibay, J.D.; Aquino-Jarquin, G. Transcriptional regulation mechanism mediated by miRNA-DNA•DNA triplex structure stabilized by Argonaute. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2014, 1839, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ipsaro, J.J.; Joshua-Tor, L. From guide to target: Molecular insights into eukaryotic RNA-interference machinery. Nat. Struct. Mol. Biol. 2015, 22, 20–28. [Google Scholar] [CrossRef]
- Xu, W.; San Lucas, A.; Wang, Z.; Liu, Y. Identifying microRNA targets in different gene regions. BMC Bioinform. 2014, 15, S4. [Google Scholar] [CrossRef]
- Winkler, G.S. The mammalian anti-proliferative BTG/Tob protein family. J. Cell. Physiol. 2010, 222, 66–72. [Google Scholar] [CrossRef]
- Ge, Y.; Li, J.; Hao, Y.; Hu, Y.; Chen, D.; Wu, B.; Fang, F. MicroRNA-543 functions as an osteogenesis promoter in human periodontal ligament-derived stem cells by inhibiting transducer of ERBB2, 2. J. Periodontal Res. 2018, 53, 832–841. [Google Scholar] [CrossRef]
- Bao, N.; Zhang, P.; Zhu, Y.; Du, P.; Jin, G.; Wu, B.; Ding, T. miR-378a-3p promotes renal cell carcinoma proliferation, migration, and invasion by targeting TOB2. Clin. Transl. Oncol. 2023, 25, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Li, Y.; Pan, X.; Lai, Y.; He, T.; Lin, C.; Zhou, L.; Zhao, L.; Sun, S.; Ding, Y.; et al. Oncogenic miR-425-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Oncol. Lett. 2018, 16, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, J.; Zhang, Y.; Liu, J.; Ma, H.; Tang, Y. MiR-425-5p accelerated the proliferation, migration, and invasion of ovarian cancer cells via targeting AFF4. J. Ovarian Res. 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.; Silva, A.; Cisilotto, J.; Rosolen, D.; Creczynski-Pasa, T.B. miR-425-5p as an exosomal biomarker for metastatic prostate cancer. Cell. Signal. 2021, 87, 110113. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiong, Y.; Peng, Y.; Gao, Y.; Qin, J.; Chu, G.; Pang, W.; Yang, G.S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. Int. J. Mol. Sci. 2017, 18, 2101. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.; Li, Y.; Li, M.; Jia, X.; Wang, J.; Lai, S. CDKN1BmiR-196a Promotes Proliferation of Mammary Epithelial Cells by Targeting. Animals 2023, 13, 3682. [Google Scholar] [CrossRef]
- Curtis, A.; Scharf, B.; Eichen, P.; Spiers, D.E. Relationships between ambient conditions, thermal status, and feed intake of cattle during summer heat stress with access to shade. J. Therm. Biol. 2017, 63, 104–111. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef]
- Cui, L.; Xue, R.; Zhang, X.; Chen, S.; Wan, Y.; Wu, W. Sleep deprivation inhibits proliferation of adult hippocampal neural progenitor cells by a mechanism involving IL-17 and p38 MAPK. Brain Res. 2019, 1714, 81–87. [Google Scholar] [CrossRef]
- Hu, S.; Cai, J.; Fang, H.; Chen, Z.; Zhang, J.; Cai, R. RPS14 promotes the development and progression of glioma via p53 signaling pathway. Exp. Cell Res. 2022, 423, 113451. [Google Scholar] [CrossRef]
- Li, N.; Xiao, Y.; Wang, H.; Zhong, Y.; Yang, H.; Huang, K. Insulin desensitization and cell senescence induced by heat stress in pig testicular cell model. Anim. Biotechnol. 2023, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.; Stiening, C.; Pollard, B.; VanBaale, M.; Baumgard, L.; Gentry, P.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006, 84, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.O.; Song, N.-H.; Park, K.-J.; Park, D.-H.; Kim, S.-H.; Chae, Y.-S.; Chung, Y.-G.; Chi, S.-G.; Kang, S.-H. Romo1 is associated with ROS production and cellular growth in human gliomas. J. Neuro-Oncol. 2014, 121, 73–81. [Google Scholar] [CrossRef]
- Ran, T.; Ke, S.; Song, X.; Ma, T.; Xu, Y.; Wang, M. WIPI1 promotes osteosarcoma cell proliferation by inhibiting CDKN1A. Gene 2021, 782, 145537. [Google Scholar] [CrossRef]
- Wu, F.; Ji, A.; Zhang, Z.; Li, J.; Li, P. miR-491-5p inhibits the proliferation and migration of A549 cells by FOXP4. Exp. Ther. Med. 2021, 21, 622. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Liu, Y.; Zhu, C.; Ling, Y.; Liu, C.; Li, L.; Liu, Y.; He, X.; Cao, J.; et al. Effects of resveratrol on DLD and NDUFB9 decrease in frozen semen of Mongolian sheep. Cryobiology 2023, 114, 104791. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Elzo, M.A.; Dang, S.; Jia, X.; Lai, S. Genetic diversity of ATP8 and ATP6 genes is associated with high-altitude adaptation in yak. Mitochondrial DNA Part A 2017, 114, 104791. [Google Scholar] [CrossRef]
- Čunátová, K.; Reguera, D.P.; Vrbacký, M.; Fernández-Vizarra, E.; Ding, S.; Fearnley, I.M.; Zeviani, M.; Houštěk, J.; Mráček, T.; Pecina, P. Loss of COX4I1 Leads to Combined Respiratory Chain Deficiency and Impaired Mitochondrial Protein Synthesis. Cells 2021, 10, 369. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Z.; Cheng, S.; Yu, J.; Huang, C.; Feng, Q. miRNA-425-5p enhances lung cancer growth via the PTEN/PI3K/AKT signaling axis. BMC Pulm. Med. 2020, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef]
- Lei, M. The MCM complex: Its role in DNA replication and implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Bačević, K.; Lossaint, G.; Achour, T.; Georget, V.; Fisher, D.; Dulić, V. Cdk2 strengthens the intra-S checkpoint and counteracts cell cycle exit induced by DNA damage. Sci. Rep. 2017, 7, 13429. [Google Scholar] [CrossRef]
- Goel, S.; Bergholz, J.; Zhao, J.J. Targeting CDK4 and CDK6 in cancer. Nat. Rev. Cancer 2022, 22, 356–372. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yuan, S.; Pan, Y.; Hua, Y.; Liu, J.Y. MiR-375 promotes human periodontal ligament stem cells proliferation and osteogenic differentiation by targeting transducer of ERBB2, 2. Arch. Oral Biol. 2020, 117, 104818. [Google Scholar] [CrossRef]
- Ikematsu, N.; Yoshida, Y.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Onda, M.; Hirai, M.; Fujimoto, J.; Yamamoto, T. Tob2, a novel anti-proliferative Tob/BTG1 family member, associates with a component of the CCR4 transcriptional regulatory complex capable of binding cyclin-dependent kinases. Oncogene 1999, 18, 7432–7441. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Strouz, K.; Huang, K.; Shyu, A.B. Tob2 phosphorylation regulates global mRNA turnover to reshape transcriptome and impact cell proliferation. RNA 2020, 26, 1143–1159. [Google Scholar] [CrossRef]
- Ogami, K.; Hosoda, N.; Funakoshi, Y.; Hoshino, S. Antiproliferative protein Tob directly regulates c-myc proto-oncogene expression through cytoplasmic polyadenylation element-binding protein CPEB. Oncogene 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Chen, G.; Huang, G.; Lin, H.; Wu, X.; Tan, X.; Chen, Z. MicroRNA-425-5p modulates osteoporosis by targeting annexin A2. Immun. Ageing 2021, 18, 45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, G.; Xu, S.; Xia, S.; Sun, W.; Wang, J.; Chen, S.; Lai, S.; Jia, X. miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2. Genes 2024, 15, 174. https://doi.org/10.3390/genes15020174
Li Y, Chen G, Xu S, Xia S, Sun W, Wang J, Chen S, Lai S, Jia X. miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2. Genes. 2024; 15(2):174. https://doi.org/10.3390/genes15020174
Chicago/Turabian StyleLi, Yuchao, Guanhe Chen, Shuxiang Xu, Siqi Xia, Wenqiang Sun, Jie Wang, Shiyi Chen, Songjia Lai, and Xianbo Jia. 2024. "miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2" Genes 15, no. 2: 174. https://doi.org/10.3390/genes15020174
APA StyleLi, Y., Chen, G., Xu, S., Xia, S., Sun, W., Wang, J., Chen, S., Lai, S., & Jia, X. (2024). miR-425-5p Regulates Proliferation of Bovine Mammary Epithelial Cells by Targeting TOB2. Genes, 15(2), 174. https://doi.org/10.3390/genes15020174






