Association Among MCT1 rs1049434 Polymorphism, Athlete Status, and Physiological Parameters in Japanese Long-Distance Runners
Highlights
- We found that the MCT1 rs1049434 polymorphism is associated with athlete status in male Japanese long-distance runners.
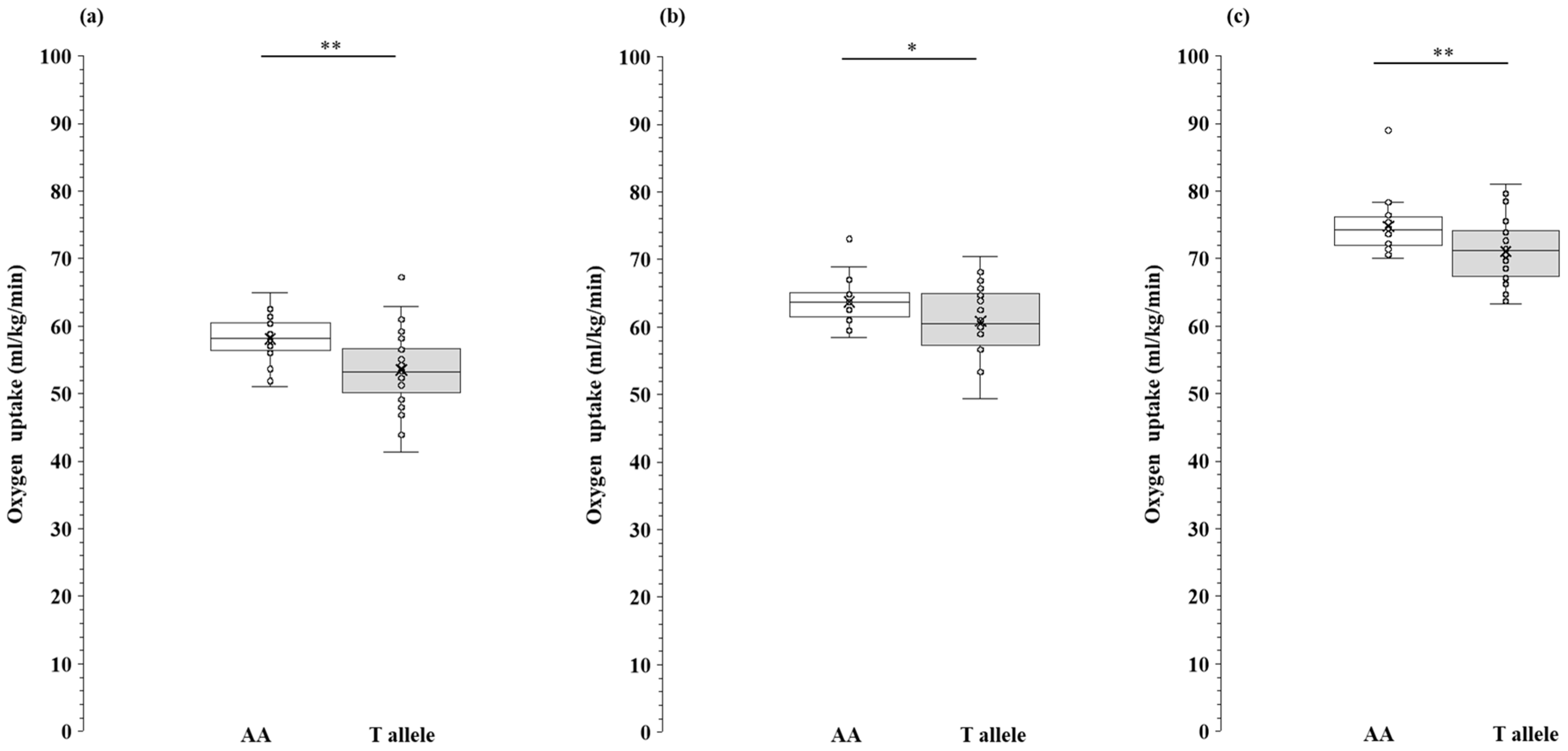
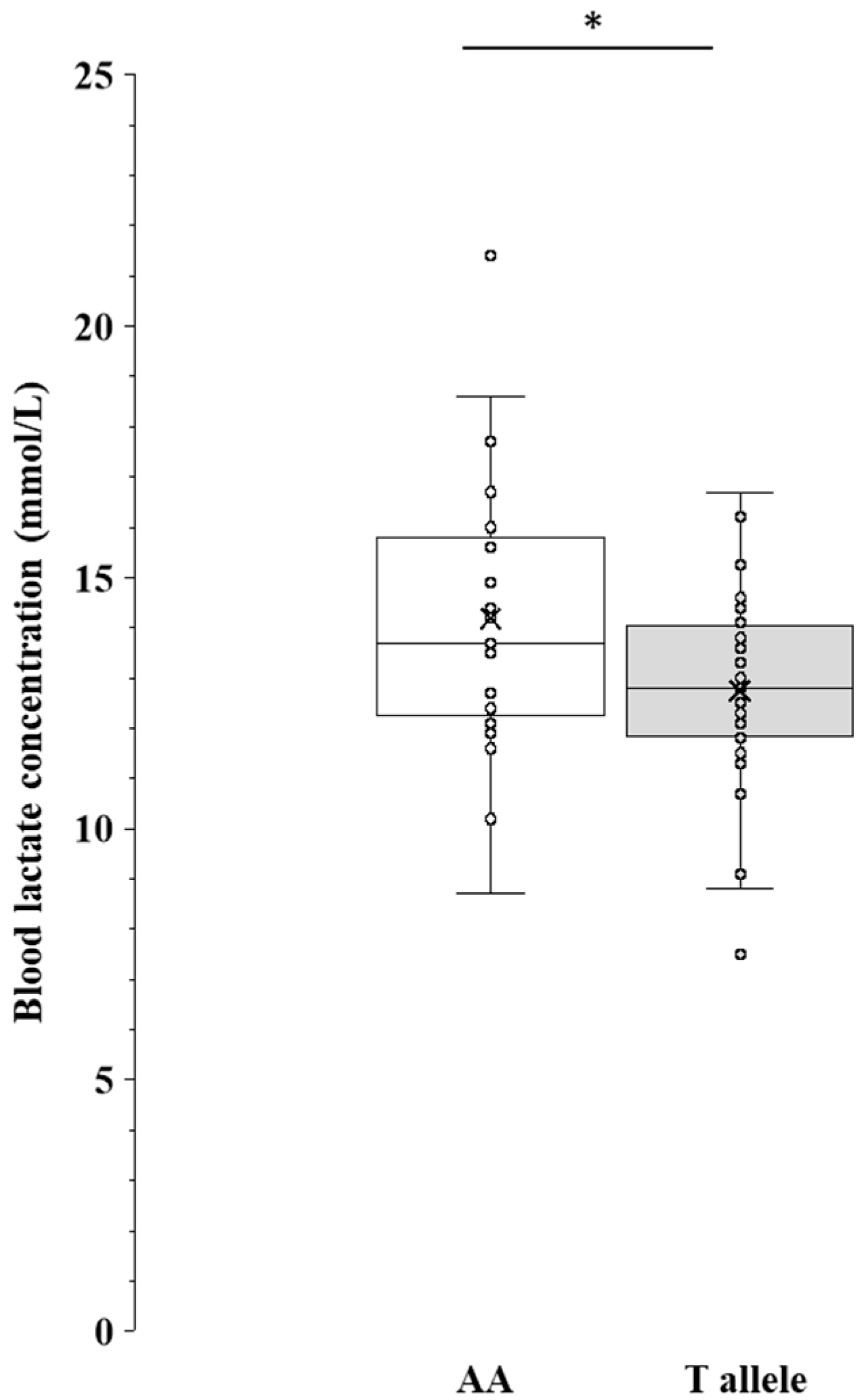
- We compared physiological parameters across the AA genotype and T allele of MCT1 rs1049434 and found that the AA genotype exhibited higher values in aerobic capacity (VO2max, VO2-LT, VO2-OBLA, BLamax).
- Our findings suggest that the MCT1 rs1049434 polymorphism may offer a significant advantage to long-distance runners, particularly in the Eastern Asian region, and it is expected that future research combining it with other genetic polymorphisms and comparing populations from different regions will provide a more detailed understanding.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Genotyping
2.4. GXT
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Japanese Long-Distance Runners
3.2. The Distribution of T1470A Polymorphism in LD and CON Groups
3.3. Comparison of Genetic Model Frequencies Between LD and CON Groups
3.4. Comparison of Genetic Model Frequencies Between Athletic Levels in the LD and CON Groups
3.5. Comparison of Aerobic Capacity Between the AA Genotype and T Allele
3.6. Comparison of Maximal Blood Lactate Concentration Between the AA Genotype and T Allele
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Eydoux, N.; Py, G.; Lambert, K.; Dubouchaud, H.; Préfaut, C.; Mercier, J. Training does not protect against exhaustive exercise-induced lactate transport capacity alterations. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1045–E1052. [Google Scholar] [CrossRef] [PubMed]
- Juel, C. Current aspects of lactate exchange: Lactate/H+ transport in human skeletal muscle. Eur. J. Appl. Physiol. 2001, 86, 12–16. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactate transport and exchange during exercise. In Handbook of Physiology; Rowell, L.B., Shepherd, J.T., Eds.; Oxford University Press: Oxford, UK, 1996; Volume 12, pp. 614–648. [Google Scholar]
- Bonen, A.; McCullagh, K.J.A.; Putman, C.T.; Hultman, E.; Jones, N.L.; Heigenhauser, G.J.F. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am. J. Physiol. 1998, 274, E102–E107. [Google Scholar] [CrossRef]
- Halestrap, A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Dubouchaud, H.; Butterfield, G.E.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E571–E579. [Google Scholar] [CrossRef]
- Fishbein, W.N.; Merezhinskaya, N.; Foellmer, J.W. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve 2002, 26, 101–112. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- McCullagh, K.J.; Poole, R.C.; Halestrap, A.P.; O’Brien, M.; Bonen, A. Role of the lactate transporter (MCT1) in skeletal muscles. Am. J. Physiol. 1996, 271, E143–E150. [Google Scholar] [CrossRef]
- Wilson, M.C.; Jackson, V.N.; Heddle, C.; Price, N.T.; Pilegaard, H.; Juel, C.; Bonen, A.; Montgomery, I.; Hutter, O.F.; Halestrap, A.P. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 1998, 273, 15920–15926. [Google Scholar] [CrossRef]
- Thomas, C.; Bishop, D.; Moore-Morris, T.; Mercier, J. Effects of high-intensity training on MCT1, MCT4, and NBC expressions in rat skeletal muscles: Influence of chronic metabolic alkalosis. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E916–E922. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.R.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2008, 33, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Cermak, N.M.; Phillips, S.M.; Benton, C.R.; Bonen, A.; Gibala, M.J. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1970–R1976. [Google Scholar] [CrossRef]
- Thomas, C.; Perrey, S.; Lambert, K.; Hugon, G.; Mornet, D.; Mercier, J. Monocarboxylate transporters, blood lactate removal after supramaximal exercise, and fatigue indexes in humans. J. Appl. Physiol. (1985) 2005, 98, 804–809. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Fishbein, W.N.; Davis, J.I.; Foellmer, J.W. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 2000, 23, 90–97. [Google Scholar] [CrossRef]
- Sasaki, S.; Futagi, Y.; Kobayashi, M.; Ogura, J.; Iseki, K. Functional characterization of 5-oxoproline transport via SLC16A1/MCT1. J. Biol. Chem. 2015, 290, 2303–2311. [Google Scholar] [CrossRef]
- Sawczuk, M.; Banting, L.K.; Cięszczyk, P.; Maciejewska-Karłowska, A.; Zarębska, A.; Leońska-Duniec, A.; Jastrzębski, Z.; Bishop, D.J.; Eynon, N. MCT1 A1470T: A novel polymorphism for sprint performance? J. Sci. Med. Sport 2015, 18, 114–118. [Google Scholar] [CrossRef]
- Homma, H.; Saito, M.; Mochizuki, Y.; Shinogi, M.; Kobatake, N.; Okamoto, T.; Nishiyama, T.; Nakazato, K.; Kikuchi, N. Association between MCT1 T1470A polymorphism and athlete status in Japanese power-oriented athletes. Gazz. Med. Ital.-Arch. Sci. Med. 2023, 182, 43–48. [Google Scholar] [CrossRef]
- Kikuchi, N.; Fuku, N.; Matsumoto, R.; Matsumoto, S.; Murakami, H.; Miyachi, M.; Nakazato, K. The association between MCT1 T1470A polymorphism and power-oriented athletic performance. Int. J. Sports Med. 2017, 38, 76–80. [Google Scholar] [CrossRef]
- Ramírez de la Piscina-Viúdez, X.; Álvarez-Herms, J.; Bonilla, D.A.; Castañeda-Babarro, A.; Larruskain, J.; Díaz-Ramírez, J.; Ahmetov, I.I.; Martínez-Ascensión, A.; Kreider, R.B.; Odriozola-Martínez, A. Putative role of MCT1 rs1049434 polymorphism in high-intensity endurance performance: Concept and basis to understand possible individualization stimulus. Sports 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, J.P.L.F.; Bosnyák, E.; Semenova, E.A.; Szmodis, M.; Griff, A.; Móra, Á.; Almási, G.; Trájer, E.; Udvardy, A.; Kostryukova, E.S.; et al. The MCT1 gene Glu490Asp polymorphism (rs1049434) is associated with endurance athlete status, lower blood lactate accumulation and higher maximum oxygen uptake. Biol. Sport 2021, 38, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Fedotovskaya, O.N.; Mustafina, L.J.; Popov, D.V.; Vinogradova, O.L.; Ahmetov, I.I. A common polymorphism of the MCT1 gene and athletic performance. Int. J. Sports Physiol. Perform. 2014, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cupeiro, R.; Benito, P.J.; Maffulli, N.; Calderón, F.J.; González-Lamuño, D. MCT1 genetic polymorphism influence in high intensity circuit training: A pilot study. J. Sci. Med. Sport 2010, 13, 526–530. [Google Scholar] [CrossRef]
- Massidda, M.; Flore, L.; Kikuchi, N.; Scorcu, M.; Piras, F.; Cugia, P.; Cięszczyk, P.; Tocco, F.; Calò, C.M. Influence of the MCT1-T1470A polymorphism (rs1049434) on repeated sprint ability and blood lactate accumulation in elite football players: A pilot study. Eur. J. Appl. Physiol. 2021, 121, 3399–3408. [Google Scholar] [CrossRef]
- Higuchi, I.; Kimura, Y.; Kobayashi, M.; Narumi, K.; Furugen, A.; Miyoshi, H.; Nakamura, A.; Yamada, T.; Atsumi, T.; Iseki, K. Relationships between plasma lactate, plasma alanine, genetic variations in lactate transporters and type 2 diabetes in the Japanese population. Drug Metab. Pharmacokinet. 2020, 35, 131–138. [Google Scholar] [CrossRef]
- Paavolainen, L.; Häkkinen, K.; Hämäläinen, I.; Nummela, A.; Rusko, H. Explosive-strength training improves 5-km running time by improving running economy and muscle power. J. Appl. Physiol. (1985) 1999, 86, 1527–1533. [Google Scholar] [CrossRef]
- Sousa, C.V.; Sales, M.M.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. How much further for the sub-2-hour marathon? Open Access J. Sports Med. 2018, 9, 139–145. [Google Scholar] [CrossRef]
- Urhausen, A.; Coen, B.; Weiler, B.; Kindermann, W. Individual anaerobic threshold and maximum lactate steady state. Int. J. Sports Med. 1993, 14, 134–139. [Google Scholar] [CrossRef]
- Grant, S.; Craig, I.; Wilson, J.; Aitchison, T. The relationship between 3 km running performance and selected physiological variables. J. Sports Sci. 1997, 15, 403–410. [Google Scholar] [CrossRef]
- Midgley, A.W.; McNaughton, L.R.; Jones, A.M. Training to Enhance the Physiological Determinants of Long-Distance Running Performance: Can Valid Recommendations Be given to Runners and Coaches Based on Current Scientific Knowledge? Sports Med. 2007, 37, 857–880. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Doust, J.H. The validity of the lactate minimum test for determination of the maximal lactate steady state. Med. Sci. Sports Exerc. 1998, 30, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Llodio, I.; Gorostiaga, E.M.; Garcia-Tabar, I.; Granados, C.; Sánchez-Medina, L. Estimation of the maximal lactate steady state in endurance runners. Int. J. Sports Med. 2016, 37, 539–546. [Google Scholar] [CrossRef]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Kassem, E.; Eliakim, A. Genetic basis for the dominance of Israeli long-distance runners of Ethiopian origin. J. Strength Cond. Res. 2021, 35, 1885–1896. [Google Scholar] [CrossRef]
- World Athletics. Asian Records-10,000 Meters. Available online: https://worldathletics.org/records/all-time-toplists/middlelong/10000-metres/all/men/senior?regionType=area®ion=asia&page=2&bestResultsOnly=true&firstDay=1900-01-01&lastDay=2024-11-17&maxResultsByCountry=all&eventId=10229610&ageCategory=senior (accessed on 8 November 2024).
- World Athletics. 10,000 Meters Asian Season Record in 2024. Available online: https://worldathletics.org/records/toplists/middlelong/10000-metres/all/men/senior/2024?regionType=area®ion=asia&page=1&bestResultsOnly=true&maxResultsByCountry=all&eventId=10229610&ageCategory=senior (accessed on 8 November 2024).
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Duncan, G.E.; Howley, E.T.; Johnson, B.N. Applicability of VO2max Criteria: Discontinuous versus Continuous Protocols. Med. Sci. Sports Exerc. 1997, 29, 273–278. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. Improved detection of lactate threshold during exercise using a log-log transformation. J. Appl. Physiol. (1985) 1985, 59, 1936–1940. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Science; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Chen, B.; Cole, J.W.; Grond-Ginsbach, C. Departure from Hardy Weinberg Equilibrium and Genotyping Error. Front. Genet. 2017, 8, 167. [Google Scholar] [CrossRef]
- Saito, M.; Ginszt, M.; Massidda, M.; Cie¸szczyk, P.; Okamoto, T.; Majcher, P.; Nakazato, K.; Kikuchi, N. Association between MCT1T1470A Polymorphism and Climbing Status in Polish and Japanese Climbers. Biol. Sport 2021, 38, 229–234. [Google Scholar] [CrossRef]
- Gasser, B.; Dössegger, A.; Giraud, M.N.; Flück, M. T-allele carriers of mono carboxylate transporter one gene polymorphism rs1049434 demonstrate altered substrate metabolization during exhaustive exercise. Genes 2024, 15, 918. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.P.C.; Patrick, F. Heredity and segregation of mtDNA. In The Human Mitochondrial Genome—From Basic Biology to Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 87–107. [Google Scholar]
- Eynon, N.; Ruiz, J.R.; Femia, P.; Pushkarev, V.P.; Cieszczyk, P.; Maciejewska-Karlowska, A.; Sawczuk, M.; Dyatlov, D.A.; Lekontsev, E.V.; Kulikov, L.M.; et al. The actn3 r577x polymorphism across three groups of elite male european athletes. PLoS ONE 2012, 7, e43132. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Kobatake, N.; Sekimoto, Y.; Saito, M.; Mochizuki, Y.; Okamoto, T.; Nakazato, K.; Nishiyama, T.; Kikuchi, N. Ciliary Neurotrophic Factor Receptor rs41274853 Polymorphism Is Associated with Weightlifting Performance in Japanese Weightlifters. J. Strength Cond. Res. 2020, 34, 3037–3041. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, M.; Onori, M.E.; Canu, G.; Moretti, G.; Minucci, A.; Baroni, S.; Mordente, A.; Urbani, A.; Galvani, C. The relationship between ACE, ACTN3 and MCT1 genetic polymorphisms and athletic performance in elite rugby union players: A preliminary study. Genes 2022, 13, 969. [Google Scholar] [CrossRef]
- Prince, F.P.; Hikida, R.S.; Hagerman, F.C. Human muscle fiber types in powerlifters, distance runners and untrained subjects. Pflugers Arch. 1976, 363, 19–26. [Google Scholar] [CrossRef]
- Fridén, J.; Sjöström, M.; Ekblom, B. Muscle fibre type characteristics in endurance trained and untrained individuals. Eur. J. Appl. Physiol. Occup. Physiol. 1984, 52, 266–271. [Google Scholar] [CrossRef]
- Bergh, U.; Thorstensson, A.; Sjödin, B.; Hulten, B.; Piehl, K.; Karlsson, J. Maximal oxygen uptake and muscle fiber types in trained and untrained humans. Med. Sci. Sports 1978, 10, 151–154. [Google Scholar]
- Ivy, J.L.; Withers, R.T.; Van Handel, P.J.; Elger, D.H.; Costill, D.L. Muscle respiratory capacity and fiber type as determinants of the lactate threshold. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 523–527. [Google Scholar] [CrossRef]
- Sjödin, B.; Jacobs, I.; Svedenhag, J. Changes in onset of blood lactate accumulation (OBLA) and muscle enzymes after training at OBLA. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 45–57. [Google Scholar] [CrossRef]
- Favier, R.J.; Constable, S.H.; Chen, M.; Holloszy, J.O. Endurance exercise training reduces lactate production. J. Appl. Physiol. (1985) 1986, 61, 885–889. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Guo, Y.J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Bishop, D.J.; Lambert, K.; Mercier, J.; Brooks, G.A. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: Current status. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, K.J.; Juel, C.; O’Brien, M.; Bonen, A. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Mol. Cell. Biochem. 1996, 156, 51–57. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, K.J.; Poole, R.C.; Halestrap, A.P.; Tipton, K.F.; O’Brien, M.; Bonen, A. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am. J. Physiol. 1997, 273, E239–E246. [Google Scholar] [CrossRef]
| MCT1 rs1049434 | n (158) | Age (yrs) | Career (yrs) | 10,000 m PBR (MM:SS) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | 66 | 26.7 | ± | 10.2 | 12.6 | ± | 6.4 | 29:07 | ± | 1:35 |
| AT | 84 | 25.8 | ± | 9.6 | 11.7 | ± | 6.5 | 29:04 | ± | 1:10 |
| TT | 8 | 24.5 | ± | 11.1 | 9.5 | ± | 5.6 | 30:03 | ± | 1:59 |
| A allele | 150 | 26.1 | ± | 9.9 | 12.1 | ± | 6.4 | 29:05 | ± | 1:21 |
| T allele | 92 | 25.6 | ± | 9.7 | 11.5 | ± | 6.4 | 29:08 | ± | 1:15 |
| n | Genotype Frequency, % (n) | HWE p-Value | Allele, % | |||||
|---|---|---|---|---|---|---|---|---|
| AA | AT | TT | A | T | ||||
| MCT1 T1470A polymorphism | ||||||||
| LD | 158 | 41.8 (66) | 53.2 (84) | 5.1 (8) | 0.016 * | 68.4 | 31.6 | |
| CON | 649 | 47.0 (305) | 42.7 (277) | 10.3 (67) | 0.941 | 68.3 | 31.7 | |
| Genetic Model | CON % (n) | LD % (n) | CON vs. LD | ||
|---|---|---|---|---|---|
| p-Value | OR | 95%CI | |||
| Codominant | |||||
| AA | 47.0 (305) | 41.8 (66) | 1.00 | ||
| AT | 42.7 (277) | 53.2 (84) | 0.07 | 1.40 | 0.98–2.01 |
| TT | 10.3 (67) | 5.1 (8) | 0.13 | 0.55 | 0.25–1.20 |
| Recessive | |||||
| AA | 47.0 (305) | 41.8 (66) | 0.24 | 0.89 | 0.57–1.15 |
| AT + TT | 53.0 (344) | 58.2 (92) | 1.00 | ||
| Dominant | |||||
| AA + AT | 89.7 (582) | 94.9 (150) | 0.04 * | 2.16 | 1.01–4.59 |
| TT | 10.3 (67) | 5.1 (8) | 1.00 | ||
| Genetic Model | CON % (n) | Sub-28 min % (n) | 28 min or Above % (n) | CON vs. Sub-28 min | CON vs. 28 min or Above | 28 min or Above vs. Sub-28 min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | ||||
| Codominant | ||||||||||||
| AA | 47.0 (305) | 64.7 (11) | 39.0 (55) | 1.00 | 1.00 | 1.00 | ||||||
| AT | 42.7 (277) | 29.4 (5) | 56.0 (79) | 0.22 | 0.50 | 0.17–1.46 | 0.02 * | 1.58 | 1.08–2.31 | 0.03 * | 0.32 | 0.10–0.96 |
| TT | 10.3 (67) | 5.9 (1) | 5.0 (7) | 0.70 | 0.41 | 0.05–3.26 | 0.19 | 0.57 | 0.25–1.33 | 1.00 | 0.71 | 0.08–6.40 |
| Recessive | ||||||||||||
| AA | 47.0 (305) | 64.7 (11) | 39.0 (55) | 0.15 | 2.07 | 0.76–5.66 | 0.08 | 0.72 | 0.50–1.05 | 0.04 * | 2.87 | 1.00–8.19 |
| AT + TT | 53.0 (344) | 35.3 (6) | 61.0 (86) | 1.00 | 1.00 | 1.00 | ||||||
| Dominant | ||||||||||||
| AA + AT | 89.7 (582) | 94.1 (16) | 95.0 (134) | 1.00 | 1.84 | 0.24–14.11 | 0.05 | 2.20 | 0.99–4.91 | 1.00 | 0.84 | 0.10–7.24 |
| TT | 10.3 (67) | 5.9 (1) | 5.0 (7) | 1.00 | 1.00 | 1.00 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, S.; Kobayashi, T.; Beppu, K.; Nojo, M.; Hoshina, K.; Kikuchi, N.; Okamoto, T.; Nakazato, K.; Hwang, I. Association Among MCT1 rs1049434 Polymorphism, Athlete Status, and Physiological Parameters in Japanese Long-Distance Runners. Genes 2024, 15, 1627. https://doi.org/10.3390/genes15121627
Seki S, Kobayashi T, Beppu K, Nojo M, Hoshina K, Kikuchi N, Okamoto T, Nakazato K, Hwang I. Association Among MCT1 rs1049434 Polymorphism, Athlete Status, and Physiological Parameters in Japanese Long-Distance Runners. Genes. 2024; 15(12):1627. https://doi.org/10.3390/genes15121627
Chicago/Turabian StyleSeki, Shotaro, Tetsuro Kobayashi, Kenji Beppu, Manabu Nojo, Kosaku Hoshina, Naoki Kikuchi, Takanobu Okamoto, Koichi Nakazato, and Inkwan Hwang. 2024. "Association Among MCT1 rs1049434 Polymorphism, Athlete Status, and Physiological Parameters in Japanese Long-Distance Runners" Genes 15, no. 12: 1627. https://doi.org/10.3390/genes15121627
APA StyleSeki, S., Kobayashi, T., Beppu, K., Nojo, M., Hoshina, K., Kikuchi, N., Okamoto, T., Nakazato, K., & Hwang, I. (2024). Association Among MCT1 rs1049434 Polymorphism, Athlete Status, and Physiological Parameters in Japanese Long-Distance Runners. Genes, 15(12), 1627. https://doi.org/10.3390/genes15121627







