Exploration of Key Regulatory Factors in Mesenchymal Stem Cell Continuous Osteogenic Differentiation via Transcriptomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
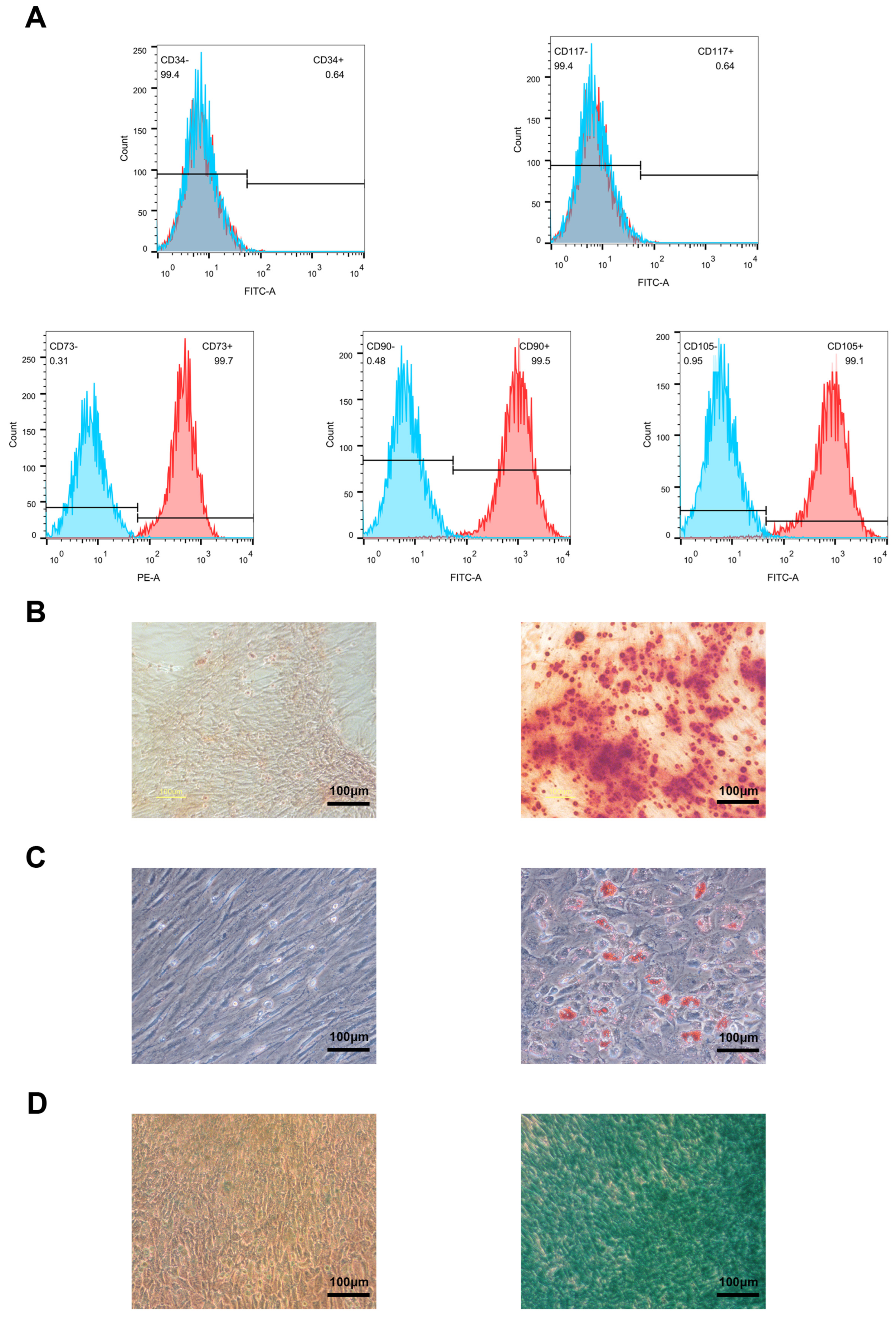
2.2. Flow Cytometry Analysis
2.3. Cell Differentiation
2.4. Lentivirus Transduction
2.5. Quantitative Real-Time PCR
2.6. Alizarin Red Staining (ARS)
2.7. Alcian Blue Staining
2.8. Oil Red O Staining
2.9. ALP Staining
2.10. Bioinformatics Analysis
2.10.1. Data Collection and Processing
2.10.2. Differential Expression Analysis
3. Results
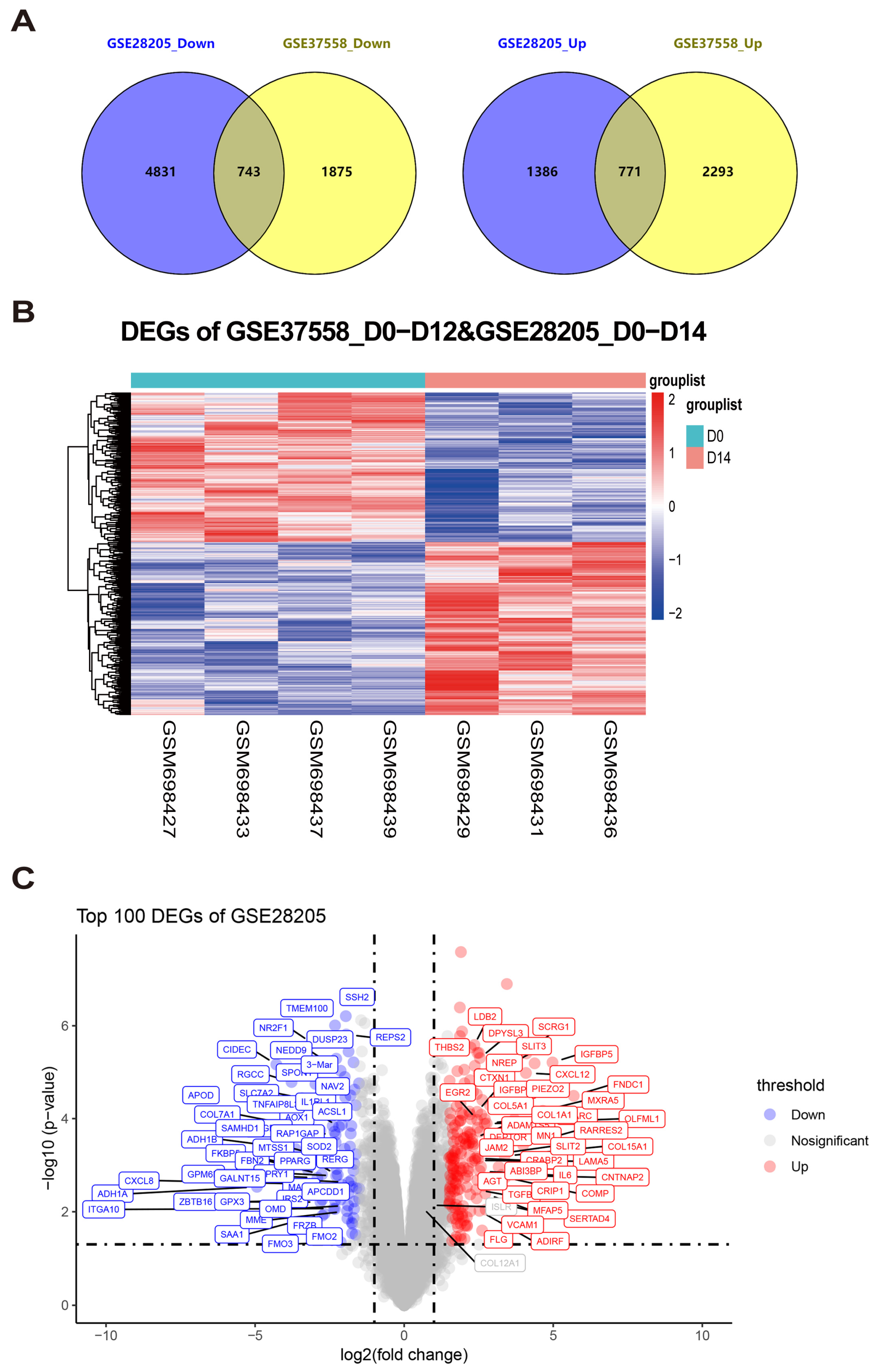
3.1. Integration Analysis of Microarray Datasets to Identify Differentially Expressed Genes in MSC Osteogenic Differentiation
3.2. Exploration of Continuously Differentially Expressed Genes During MSC Osteogenic Induction from GSE37558 Dataset
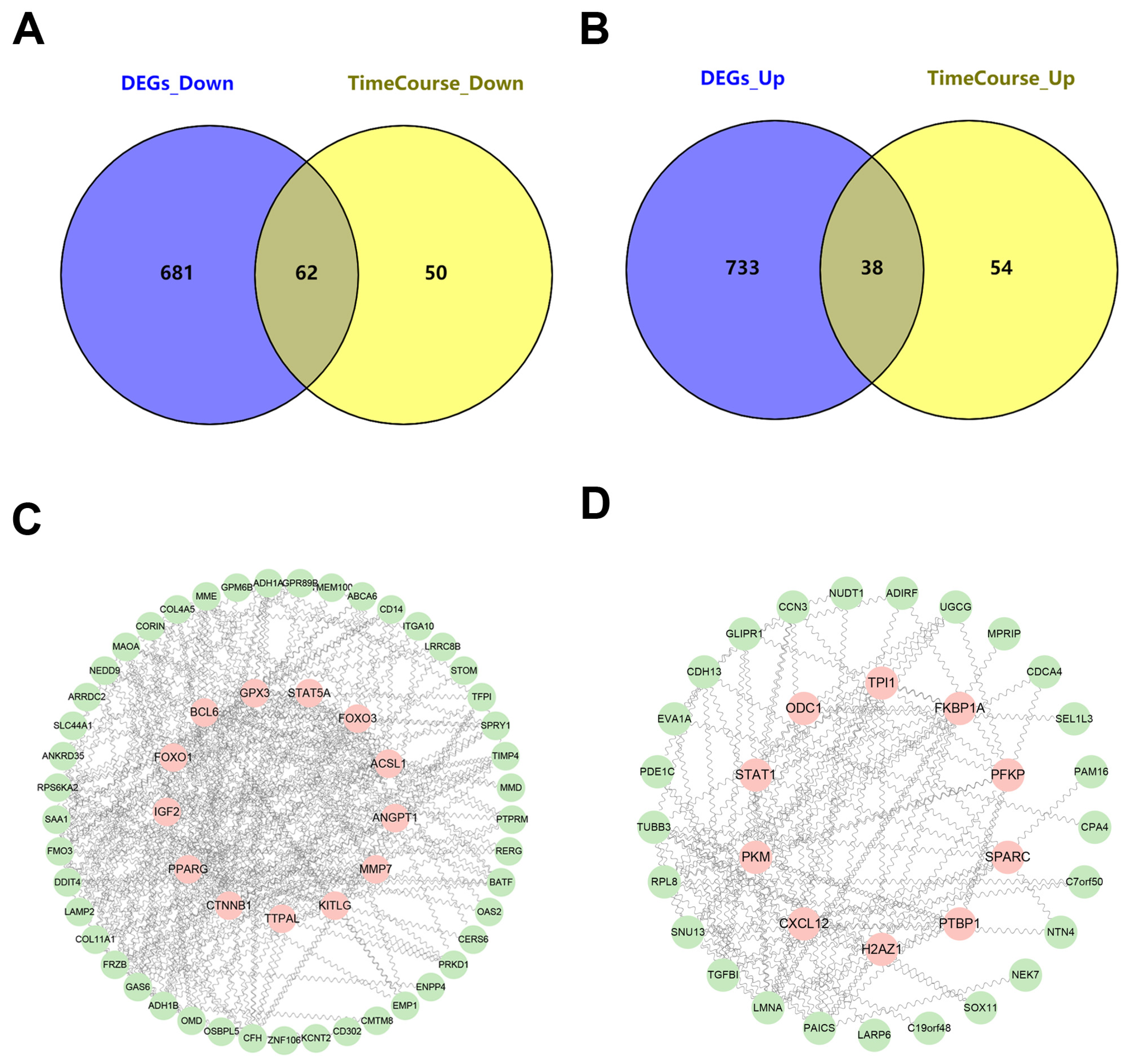
3.3. Integrated Time-Course Analysis of Differentially Expressed Genes During MSC Osteogenic Induction
3.4. Identification of Key Regulators Governing MSC Osteogenic Differentiation Through the HUMAN PROTEIN ATLAS Database
3.5. Isolation of Bone Mesenchymal Stem Cells and qRT–PCR Identification of Candidate Genes During Osteogenic Induction
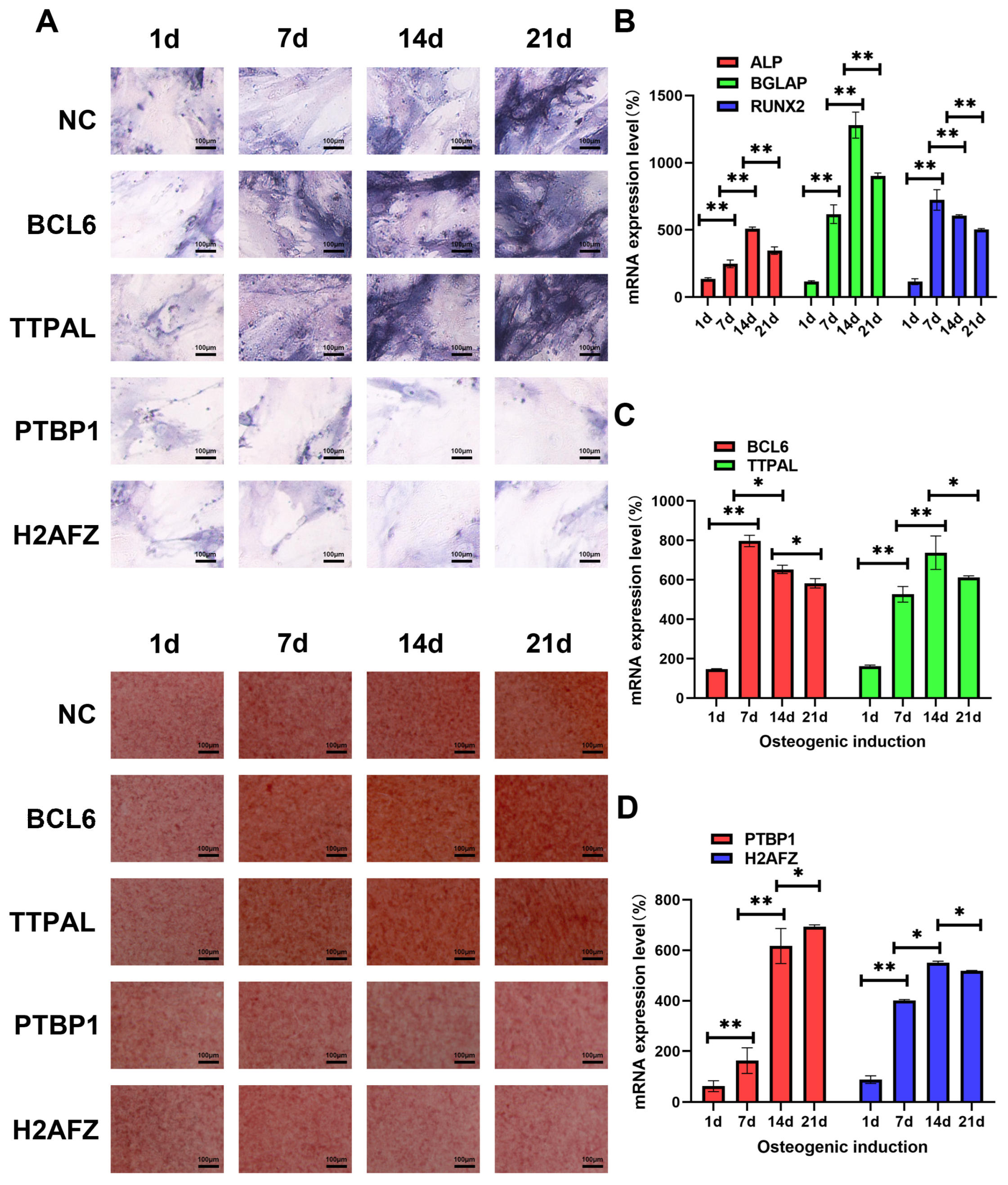
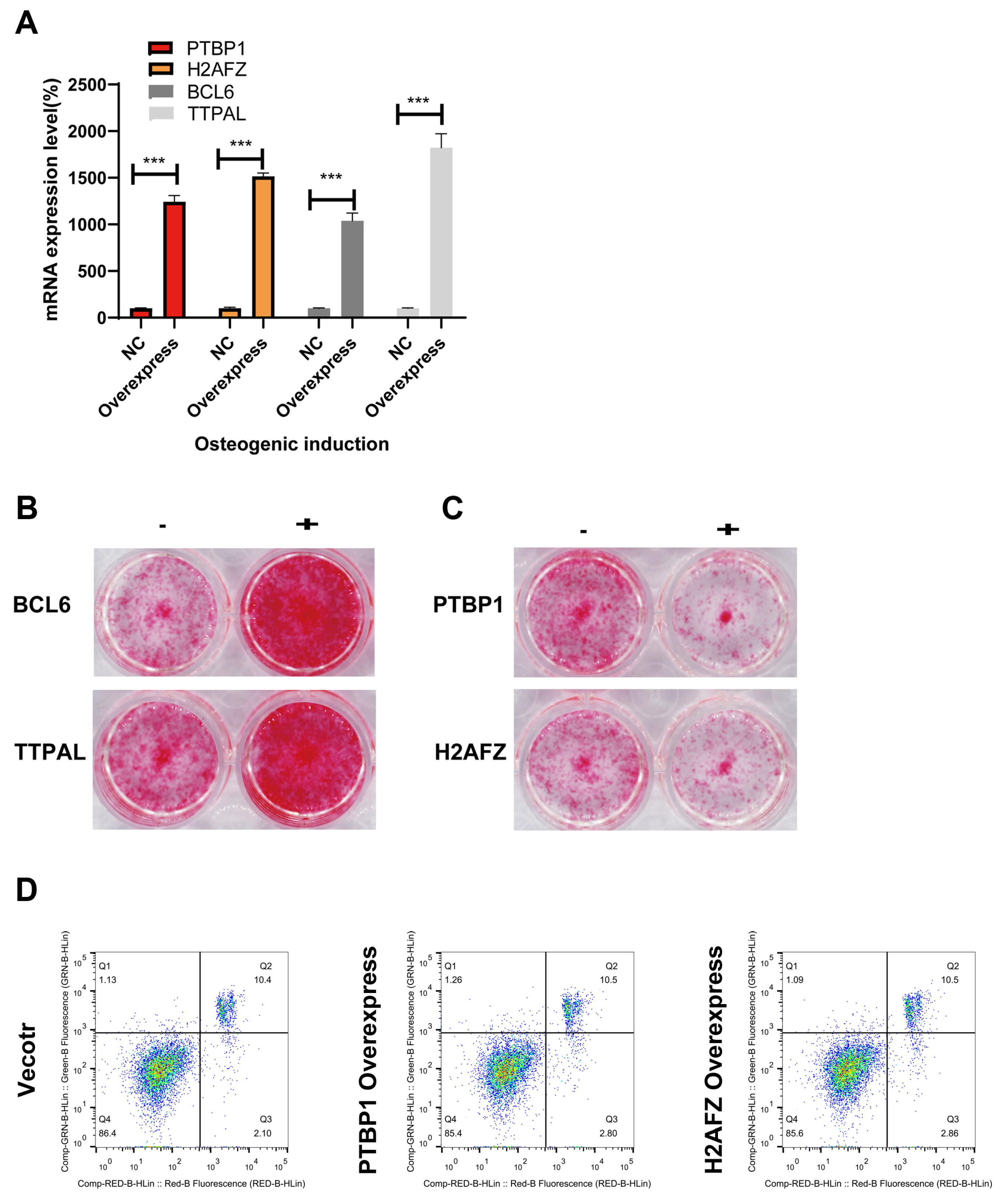
3.6. Identifying the Molecular Function of Osteogenic Regulators in MSCs via Lentiviral Overexpression of Candidate Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022, 80, 101684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, R.; Li, B.; Xin, Z.; Ling, Z.; Zhu, W.; Li, X.; Zhang, P.; Fu, Y.; Chen, J.; et al. LncRNA NEAT1 controls the lineage fates of BMSCs during skeletal aging by impairing mitochondrial function and pluripotency maintenance. Cell Death Differ. 2022, 29, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, N.; Shao, Q.; Zhu, D.; Feng, Y.; Wang, Y.; Yu, M.; Ren, L.; Chen, Q.; Yang, G. Light-controlled scaffold- and serum-free hard palatal-derived mesenchymal stem cell aggregates for bone regeneration. Bioeng. Transl. Med. 2023, 8, e10334. [Google Scholar] [CrossRef]
- Li, P.; Gong, Z.; Shultz, L.D.; Ren, G. Mesenchymal stem cells: From regeneration to cancer. Pharmacol. Ther. 2019, 200, 42–54. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.-J.; Fei, Y.-Y.; Han, H.-T.; Xu, J.; Cheng, L.; Li, X. Stem cell therapy in liver regeneration: Focus on mesenchymal stem cells and induced pluripotent stem cells. Pharmacol. Ther. 2022, 232, 108004. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Kim, P.; Park, J.; Lee, D.-J.; Mizuno, S.; Shinohara, M.; Hong, C.P.; Jeong, Y.; Yun, R.; Park, H.; Park, S.; et al. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Yuan, Q.; Cheng, Y.; Li, J.; Liu, Z.; Liu, Y.; Li, Y.; Su, T.; Wang, J.; Salvo, M.E.; et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell 2021, 28, 1057–1073.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yuan, Y.; Bai, L.; Yuan, L.; Li, L.; Liu, J.; Chen, Y.; Lu, Y.; Cheng, J.; Zhang, J. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021, 43, 101963. [Google Scholar] [CrossRef]
- Li, C.-J.; Xiao, Y.; Yang, M.; Su, T.; Sun, X.; Guo, Q.; Huang, Y.; Luo, X.-H. Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J. Clin. Investig. 2018, 128, 5251–5266. [Google Scholar] [CrossRef]
- Li, W.; Feng, W.; Su, X.; Luo, D.; Li, Z.; Zhou, Y.; Zhu, Y.; Zhang, M.; Chen, J.; Liu, B.; et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J. Clin. Investig. 2022, 132, e150051. [Google Scholar] [CrossRef]
- Xing, W.; Godwin, C.; Pourteymoor, S.; Mohan, S. Conditional disruption of the osterix gene in chondrocytes during early postnatal growth impairs secondary ossification in the mouse tibial epiphysis. Bone Res. 2019, 7, 24. [Google Scholar] [CrossRef]
- Yoshida, G.; Kawabata, T.; Takamatsu, H.; Saita, S.; Nakamura, S.; Nishikawa, K.; Fujiwara, M.; Enokidani, Y.; Yamamuro, T.; Tabata, K. Degradation of the NOTCH intracellular domain by elevated autophagy in osteoblasts promotes osteoblast differentiation and alleviates osteoporosis. Autophagy 2022, 18, 2323–2332. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Hong, C.-G.; Hu, W.-B.; Chen, M.-L.; Duan, R.; Li, H.-M.; Yue, T.; Cao, J.; Wang, Z.-X.; Chen, C.-Y.; et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy 2021, 17, 2766–2782. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, D.; Zheng, Y.; Chen, Y.; Yu, M.; Cui, D.; Huang, M.; Su, X.; Sun, Y.; Chen, Y.; et al. MiR-9-1 controls osteoblastic regulation of lymphopoiesis. Leukemia 2023, 37, 2261–2275. [Google Scholar] [CrossRef]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.; Kim, H.K.; Yeom, S.H.; Woo, C.H.; Jung, Y.J.; Yun, Y.E.; Park, S.Y.; Han, J.; Kim, E.; et al. Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p. J. Extracell. Vesicles 2021, 10, e12152. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Heo, J.; Ju, H.; Shin, J.-W.; Kim, Y.; Lee, S.; Yu, H.Y.; Ryu, C.-M.; Yun, H.; Song, S.; et al. Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway. Sci. Adv. 2020, 6, eaba1334. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Theret, M.; Scott, W.; Hill, L.; Underhill, T.M.; Hinz, B.; Rossi, F.M. Multipotent stromal cells: One name, multiple identities. Cell Stem Cell 2021, 28, 1690–1707. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Meng, Y.; Ye, F.; Yang, X.; Li, R.; Jiang, J.; Zhao, Q.; Gao, L.; Han, Z.; Wei, L. AIF1 + CSF1R + MSCs, induced by TNF-α, act to generate an inflammatory microenvironment and promote hepatocarcinogenesis. Hepatology 2023, 78, 434–451. [Google Scholar] [CrossRef]
- Tsai, J.N.; Lee, H.; David, N.L.; Eastell, R.; Leder, B.Z. Combination denosumab and high dose teriparatide for postmenopausal osteoporosis (DATA-HD): A randomised, controlled phase 4 trial. Lancet Diabetes Endocrinol. 2019, 7, 767–775. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, P.; Liu, Y.; Wu, Y.; Chen, Y.; Guo, Y.; Zhang, S.; Zheng, X.; Zhou, L.; Liu, W.; et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat. Commun. 2020, 11, 5596. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Yu, Y.; Ni, Y.; Wei, Y.; Cheng, Y.; Han, L.; Xiao, L.; Ma, X.; Wei, H.; et al. Mitochondrial Transfer Regulates Cell Fate Through Metabolic Remodeling in Osteoporosis. Adv. Sci. 2023, 10, e2204871. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Z.; Zou, S.; Deng, D.; Lai, P.; Hu, H.; Yao, Y.; Hu, L.; Zhang, S.; Li, K.; et al. Vangl2 limits chaperone-mediated autophagy to balance osteogenic differentiation in mesenchymal stem cells. Dev. Cell 2021, 56, 2103–2120.e9. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-Wide Analysis of the Protein Phosphatase 2C Genes in Tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Nguyen, M.; Sabry, R.; Davis, O.S.; Favetta, L.A. Effects of BPA, BPS, and BPF on Oxidative Stress and Antioxidant Enzyme Expression in Bovine Oocytes and Spermatozoa. Genes 2022, 13, 142. [Google Scholar] [CrossRef]
- Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Zavala, E.I.; Thomas, J.T.; Sturk-Andreaggi, K.; Daniels-Higginbotham, J.; Meyers, K.K.; Barrit-Ross, S.; Aximu-Petri, A.; Richter, J.; Nickel, B.; Berg, G.E.; et al. Ancient DNA Methods Improve Forensic DNA Profiling of Korean War and World War II Unknowns. Genes 2022, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Eijken, M.; van de Peppel, J.; van Leeuwen, J.P. Calcifying vascular smooth muscle cells and osteoblasts: Independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genom. 2014, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Bruedigam, C.; Leeuwen, J.; Peppel, J.V. Gene Expression Analyses in Models of Rosiglitazone-Induced Physiological and Pathological Mineralization Identify Novel Targets to Improve Bone and Vascular Health. Cells 2023, 12, 2462. [Google Scholar] [CrossRef]
- Bozoky, B.; Szekely, L.; Ernberg, I.; Savchenko, A. AtlasGrabber: A software facilitating the high throughput analysis of the human protein atlas online database. BMC Bioinform. 2022, 23, 546. [Google Scholar] [CrossRef]
- Zhao, P.; Zhen, H.; Zhao, H.; Huang, Y.; Cao, B. Identification of hub genes and potential molecular mechanisms related to radiotherapy sensitivity in rectal cancer based on multiple datasets. J. Transl. Med. 2023, 21, 176. [Google Scholar] [CrossRef]
- Zhong, H.; Shi, Q.; Wen, Q.; Chen, J.; Li, X.; Ruan, R.; Zeng, S.; Dai, X.; Xiong, J.; Li, L.; et al. Pan-cancer analysis reveals potential of FAM110A as a prognostic and immunological biomarker in human cancer. Front. Immunol. 2023, 14, 1058627. [Google Scholar] [CrossRef]
- Hochmann, S.; Ou, K.; Poupardin, R.; Mittermeir, M.; Textor, M.; Ali, S.; Wolf, M.; Ellinghaus, A.; Jacobi, D.; Elmiger, J.A.J.; et al. The enhancer landscape predetermines the skeletal regeneration capacity of stromal cells. Sci. Transl. Med. 2023, 15, eabm7477. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Mo, C.; Guo, J.; Qin, J.; Zhang, X.; Sun, Y.; Wei, H.; Cao, D.; Zhang, Y.; Zhao, C.; Xiong, Y.; et al. Single-cell transcriptomics of LepR-positive skeletal cells reveals heterogeneous stress-dependent stem and progenitor pools. EMBO J. 2022, 41, e108415. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, X.; Cui, Y.; Song, Y.; Wang, S.; Geng, Y.; Li, R.; Gao, W.; Fu, D. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 2021, 41, 101915. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zheng, A.; Liu, Y.; Zhang, X.; Wang, X.; Wu, J.; She, W.; Lv, K.; Cao, L.; Jiang, X. Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact. Mater. 2021, 6, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dong, J.; Fan, X.; Yong, J.; Yang, M.; Liu, Y.; Zhang, X.; Lv, L.; Wen, L.; Qiao, J.; et al. Characterization of mesenchymal stem cells in human fetal bone marrow by single-cell transcriptomic and functional analysis. Signal Transduct. Target. Ther. 2023, 8, 126. [Google Scholar] [CrossRef]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 2022, 102, 379–410. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Alves, L.N.R.; Meira, D.D.; Merigueti, L.P.; Casotti, M.C.; Ventorim, D.D.P.; Almeida, J.F.F.; de Sousa, V.P.; Sant’ana, M.C.; da Cruz, R.G.C.; Louro, L.S.; et al. Biomarkers in Breast Cancer: An Old Story with a New End. Genes 2023, 14, 1364. [Google Scholar] [CrossRef]
- Marie, P.J.; Fromigué, O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen. Med. 2006, 1, 539–548. [Google Scholar] [CrossRef]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Nantavisai, S.; Pisitkun, T.; Osathanon, T.; Pavasant, P.; Kalpravidh, C.; Dhitavat, S.; Makjaroen, J.; Sawangmake, C. Systems biology analysis of osteogenic differentiation behavior by canine mesenchymal stem cells derived from bone marrow and dental pul. Sci. Rep. 2020, 10, 20703. [Google Scholar] [CrossRef]
- Zha, K.; Tian, Y.; Panayi, A.C.; Mi, B.; Liu, G. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Front. Cell Dev. Biol. 2022, 10, 824812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, S.; Peng, Y.; Zhang, J. Lamc1 promotes osteogenic differentiation and inhibits adipogenic differentiation of bone marrow-derived mesenchymal stem cells. Sci. Rep. 2024, 14, 19592. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Barilani, M.; Rossi, R.L.; Jalal, M.M.K.; Rusconi, F.; Buono, G.; Ragni, E.; Cantarella, G.; Simpson, H.A.R.W.; Péault, B.; et al. FOXP1 circular RNA sustains mesenchymal stem cell identity via microRNA inhibition. Nucleic Acids Res. 2019, 47, 5325–5340. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, S.; Li, F.; Xu, C.; Tu, W.; Chen, Y. A novel link between melatonin and circ_0005753/PTBP1/TXNIP regulatory network in the modulation of osteogenic potential in mesenchymal stem cells. Chem. Biol. Drug Des. 2024, 103, e14380. [Google Scholar] [CrossRef]
- Fujie, A.; Funayama, A.; Miyauchi, Y.; Sato, Y.; Kobayashi, T.; Kanagawa, H.; Katsuyama, E.; Hao, W.; Tando, T.; Watanabe, R.; et al. Bcl6 promotes osteoblastogenesis through Stat1 inhibition. Biochem. Biophys. Res. Commun. 2015, 457, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Diesel, L.F.D.C.; Dos Santos, B.P.; Bellagamba, B.C.; Neto, A.S.P.; Ely, P.B.; Meirelles, L.D.S.; Nardi, N.B.; Camassola, M. Stability of Reference Genes during Tri-Lineage Differentiation of Human Adipose-Derived Stromal Cells. J. Stem Cells 2015, 10, 225–242. [Google Scholar]
- Pfeffer, G.; Lee, G.; Pontifex, C.S.; Fanganiello, R.D.; Peck, A.; Weihl, C.C.; Kimonis, V. Multisystem Proteinopathy Due to VCP Mutations: A Review of Clinical Heterogeneity and Genetic Diagnosis. Genes 2022, 13, 963. [Google Scholar] [CrossRef]
- Yang, M.; Miao, Y.-R.; Xie, G.-Y.; Luo, M.; Hu, H.; Kwok, H.F.; Feng, J.; Guo, A.-Y. ICBatlas: A Comprehensive Resource for Depicting Immune Checkpoint Blockade Therapy Characteristics from Transcriptome Profiles. Cancer Immunol. Res. 2022, 10, 1398–1406. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, D. Deep learning shapes single-cell data analysis. Nat. Rev. Mol. Cell Biol. 2022, 23, 303–304. [Google Scholar] [CrossRef]
- Castiglioni, S.; Di Fede, E.; Bernardelli, C.; Lettieri, A.; Parodi, C.; Grazioli, P.; Colombo, E.A.; Ancona, S.; Milani, D.; Ottaviano, E.; et al. KMT2A: Umbrella Gene for Multiple Diseases. Genes 2022, 13, 514. [Google Scholar] [CrossRef]
- Gao, Y.; Chi, Y.; Chen, Y.; Wang, W.; Li, H.; Zheng, W.; Zhu, P.; An, J.; Duan, Y.; Sun, T.; et al. Multi-omics analysis of human mesenchymal stem cells shows cell aging that alters immunomodulatory activity through the downregulation of PD-L1. Nat. Commun. 2023, 14, 4373. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, S.; Song, Z.; Chen, S.; Lin, X.; Sun, H.; Zhou, P.; Peng, Q.; Du, S.; Zheng, S.; et al. Macrophage polarization states in atherosclerosis. Front. Immunol. 2023, 14, 1185587. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dror, J.; Shalamov, M.; Sonnenblick, A. The History of Early Breast Cancer Treatment. Genes 2022, 13, 960. [Google Scholar] [CrossRef]
- Deng, C.; Dong, K.; Liu, Y.; Chen, K.; Min, C.; Cao, Z.; Wu, P.; Luo, G.; Cheng, G.; Qing, L.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote the survival of skin flaps after ischaemia-reperfusion injury via mTOR/ULK1/FUNDC1 pathways. J. Nanobiotechnol. 2023, 21, 340. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Blood and Immune System | Protein Expressed in the Database of the HUMAN PROTEIN ALTAS | |||
|---|---|---|---|---|---|
| Bone Marrow | Lymph Node | Tonsil | Spleen | ||
| CXCL12 | High | Low | Low | Low | Bone marrow poietic cells showed strong nuclear positivity. |
| PTBP1 | High | High | High | Medium | Most normal tissues displayed strong nuclear positivity. |
| PKM2 | Low | High | High | High | Cytoplasmic expression in most tissues, hepatocytes, neurons, and most soft tissues was negative. |
| H2AFZ | High | High | Medium | High | Ubiquitous nuclear expression. |
| NUDT1 | Medium | High | High | Medium | Most normal tissues showed moderate to strong cytoplasmic staining. |
| ANGPT1 | High | Medium | Medium | Medium | Ubiquitous cytoplasmic expression. |
| PPAGR | Low | Not detected | High | Low | Squamous epithelia, glandular cells in the gastrointestinal tract, gall bladder, urinary bladder, placenta, and epididymis showed moderate to strong nuclear positivity. |
| MME | Medium | Low | Medium | High | B-lymphocytes, myoepithelium, stromal cells, and some glandular epithelia displayed strong cytoplasmic positivity. |
| RPS6KA2 | Medium | Low | High | Medium | Most of the normal tissues displayed moderate nuclear and cytoplasmic positivity. |
| TTPAL | Medium | High | High | High | Most normal tissues displayed moderate to strong cytoplasmic staining with a granular pattern. |
| BCL6 | High | High | High | Medium | Nuclear expression, mainly in lymphoid tissues. |
| CTNNB1 | Low | Not detected | High | Not detected | Membranous expression was observed in most tissues. |
| STAT5A | Medium | High | High | Low | Cytoplasmic and nuclear expression in a few tissues, most abundant in subsets of lymphoid cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Liu, T.; Li, L.; He, L.; Pan, S.; Liu, Y. Exploration of Key Regulatory Factors in Mesenchymal Stem Cell Continuous Osteogenic Differentiation via Transcriptomic Analysis. Genes 2024, 15, 1568. https://doi.org/10.3390/genes15121568
Pan Y, Liu T, Li L, He L, Pan S, Liu Y. Exploration of Key Regulatory Factors in Mesenchymal Stem Cell Continuous Osteogenic Differentiation via Transcriptomic Analysis. Genes. 2024; 15(12):1568. https://doi.org/10.3390/genes15121568
Chicago/Turabian StylePan, Yu, Tao Liu, Linfeng Li, Liang He, Shu Pan, and Yuwei Liu. 2024. "Exploration of Key Regulatory Factors in Mesenchymal Stem Cell Continuous Osteogenic Differentiation via Transcriptomic Analysis" Genes 15, no. 12: 1568. https://doi.org/10.3390/genes15121568
APA StylePan, Y., Liu, T., Li, L., He, L., Pan, S., & Liu, Y. (2024). Exploration of Key Regulatory Factors in Mesenchymal Stem Cell Continuous Osteogenic Differentiation via Transcriptomic Analysis. Genes, 15(12), 1568. https://doi.org/10.3390/genes15121568






