Gene Expression Modulation of Markers Involved in Bone Formation and Resorption by Bisphenol A, Bisphenol F, Bisphenol S, and Bisphenol AF
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isolation and Primary Culture of Human Osteoblasts
2.3. Treatments
2.4. Effect of BPA, BPF, BPS, and BPAF on RANKL, OPG, TGF-β1, TGFR1, TGFR2, TGFR3 and VEGF Gene Expression of Human Osteoblasts
2.5. Statistical Analysis
3. Results
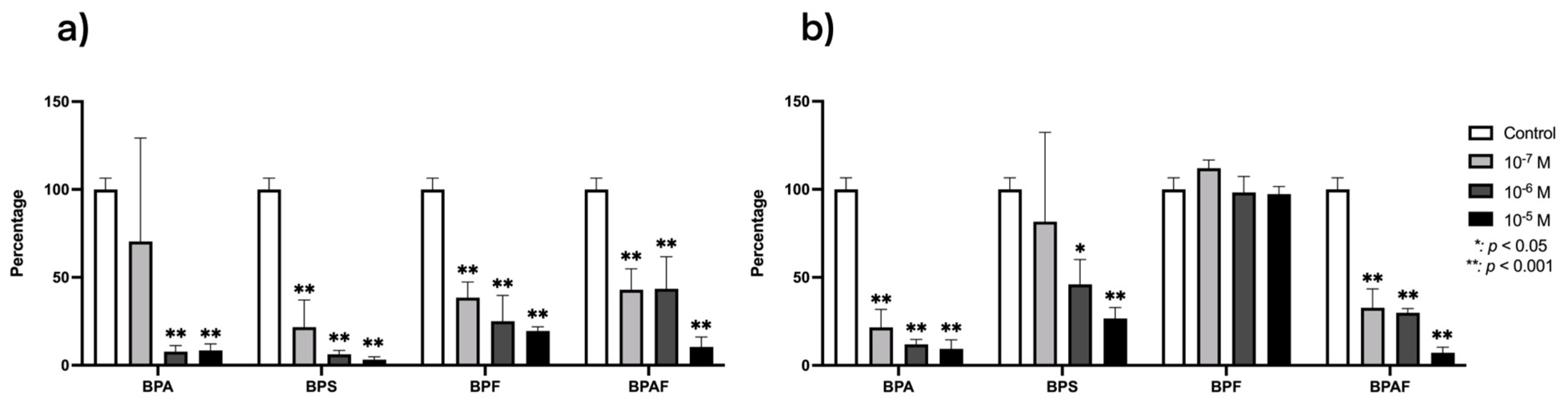
3.1. Effect of BPs on the Modulation of RANKL and OPG Gene Expression
3.2. Effect of BPs on the Modulation of TGF-β1 and Its Receptors Gene Expression
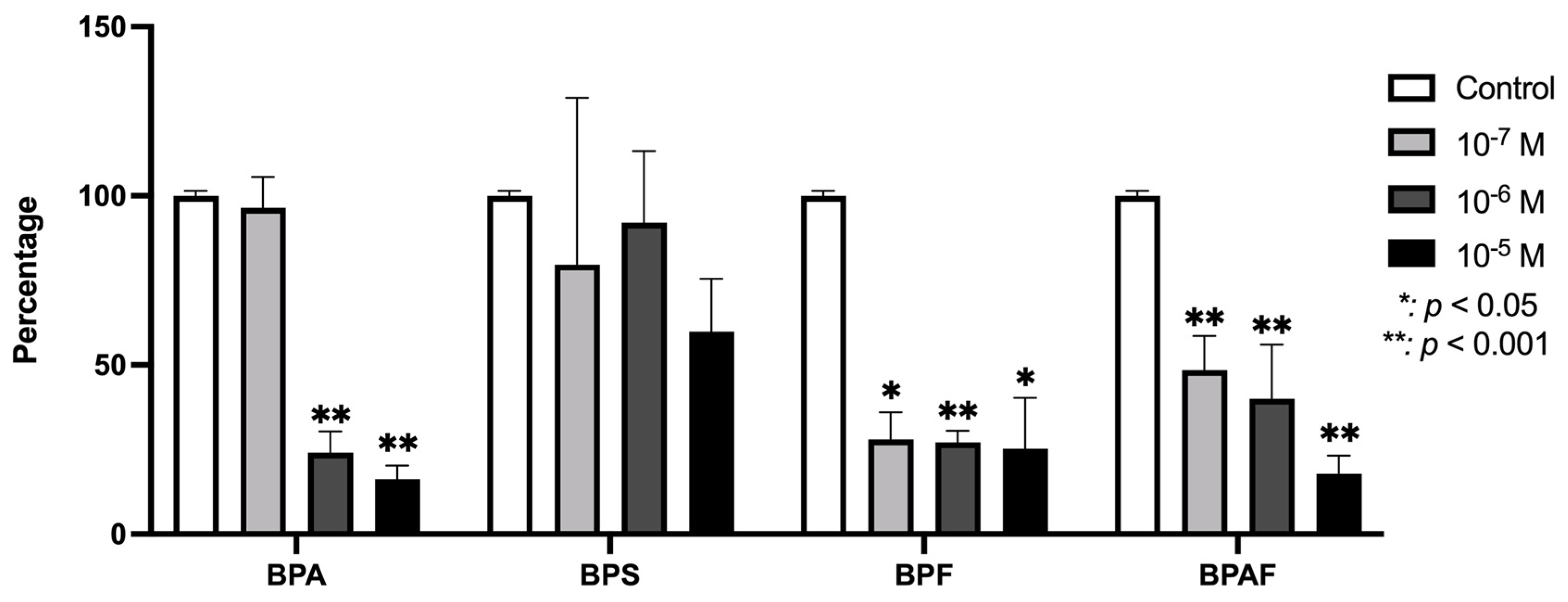
3.3. Effect of BPs on the Modulation of VEGF Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Huang, Y.; Bajis, R.; Sahih, M.; Li, Y.-P.; Dai, K.; Zhang, X. Osteoblastogenesis Regulation Signals in Bone Remodeling. Osteoporos. Int. 2012, 23, 1653–1663. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kondo, F.; Katayama, Y. Human Exposure to Bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Geens, T.; Goeyens, L.; Kannan, K.; Neels, H.; Covaci, A. Levels of Bisphenol-A in Thermal Paper Receipts from Belgium and Estimation of Human Exposure. Sci. Total Environ. 2012, 435–436, 30–33. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.-J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol A Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; Alice van Vugt-Lussenburg, B.M.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are Structural Analogues to Bisphenol a Safe Alternatives? Toxicol. Sci. 2014, 139, 35–47. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative Study of the Endocrine-Disrupting Activity of Bisphenol A and 19 Related Compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yasuhara, A. Quantities of Bisphenol a Leached from Plastic Waste Samples. Chemosphere 1999, 38, 2569–2576. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Yaglov, V.V. Endocrine Disruptors as a New Etiologic Factor of Bone Tissue Diseases (Review). Sovrem. Tekhnologii Med. 2021, 13, 84–94. [Google Scholar] [CrossRef]
- Turan, S. Endocrine Disrupting Chemicals and Bone. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, R.; Lisco, G.; Giagulli, V.A.; Settembrini, S.; De Pergola, G.; Guastamacchia, E.; Lombardi, G.; Triggiani, V. Bone Disruption and Environmental Pollutants. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 704–715. [Google Scholar] [CrossRef]
- Hiroi, H.; Tsutsumi, O.; Momoeda, M.; Takai, Y.; Osuga, Y.; Taketani, Y. Differential Interactions of Bisphenol A And17β-Estradiol with Estrogen Receptor α (ERα) and ERβ. Endocr. J. 1999, 46, 773–778. [Google Scholar] [CrossRef]
- Kurosawa, T.; Hiroi, H.; Tsutsumi, O.; Ishikawa, T.; Osuga, Y.; Fujiwara, T.; Inoue, S.; Muramatsu, M.; Momoeda, M.; Taketani, Y. The Activity of Bisphenol A Depends on Both the Estrogen Receptor Subtype and the Cell Type. Endocr. J. 2002, 49, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Maduranga Karunarathne, W.A.H.; Choi, Y.H.; Park, S.R.; Lee, C.-M.; Kim, G.-Y. Bisphenol A Inhibits Osteogenic Activity and Causes Bone Resorption via the Activation of Retinoic Acid-Related Orphan Receptor α. J. Hazard. Mater. 2022, 438, 129458. [Google Scholar] [CrossRef]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine Disruptor Bisphenol A Strongly Binds to Human Estrogen-Related Receptor γ (ERRγ) with High Constitutive Activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Tohmé, M.; Prud’homme, S.M.; Boulahtouf, A.; Samarut, E.; Brunet, F.; Bernard, L.; Bourguet, W.; Gibert, Y.; Balaguer, P.; Laudet, V. Estrogen-Related Receptor γ Is an in Vivo Receptor of Bisphenol A. FASEB J. 2014, 28, 3124–3133. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, Á. Bisphenol-A Acts as a Potent Estrogen via Non-Classical Estrogen Triggered Pathways. Mol. Cell. Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Jiang, L.; Liu, X.; Geng, C.; Wang, W.; Zhong, L.; Yang, G.; Chen, M. Bisphenol A Induces Oxidative Stress-Associated DNA Damage in INS-1 Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 769, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Pang, K.-L.; Mark-Lee, W.F. A Review on the Effects of Bisphenol A and Its Derivatives on Skeletal Health. Int. J. Med. Sci. 2018, 15, 1043–1050. [Google Scholar] [CrossRef]
- Thent, Z.C.; Froemming, G.R.A.; Muid, S. Bisphenol A Exposure Disturbs the Bone Metabolism: An Evolving Interest towards an Old Culprit. Life Sci. 2018, 198, 1–7. [Google Scholar] [CrossRef] [PubMed]
- García-Recio, E.; Costela-Ruiz, V.J.; Melguizo-Rodriguez, L.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C.; de Luna-Bertos, E. Repercussions of Bisphenol A on the Physiology of Human Osteoblasts. Int. J. Mol. Sci. 2022, 23, 5349. [Google Scholar] [CrossRef]
- García-Recio, E.; Costela-Ruiz, V.J.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; García-Martínez, O.; Ruiz, C.; De Luna-Bertos, E. Modulation of Osteogenic Gene Expression by Human Osteoblasts Cultured in the Presence of Bisphenols BPF, BPS, or BPAF. Int. J. Mol. Sci. 2023, 24, 4256. [Google Scholar] [CrossRef]
- García-Recio, E.; Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Ramos-Torrecillas, J.; Illescas-Montes, R.; De Luna-Bertos, E.; Ruiz, C. Effects of Bisphenol F, Bisphenol S, and Bisphenol AF on Cultured Human Osteoblasts. Arch. Toxicol. 2023, 97, 1899–1905. [Google Scholar] [CrossRef]
- García-Martínez, O.; Díaz-Rodríguez, L.; Rodríguez-Pérez, L.; De Luna-Bertos, E.; Reyes Botella, C.; Ruiz, C.C. Effect of Acetaminophen, Ibuprofen and Methylprednisolone on Different Parameters of Human Osteoblast-like Cells. Arch. Oral. Biol. 2011, 56, 317–323. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Rodríguez-Martínez, J.B.; Ramos-Torrecillas, J.; Vallecillo-Capilla, M.F.; Ruiz, C.; García-Martínez, O.; Reyes-Botella, C. Proliferation and Osteogenic Differentiation of Osteoblast-like Cells Obtained from Two Techniques for Harvesting Intraoral Bone Grafts. Clin. Oral. Investig. 2013, 17, 1349–1356. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Ruiz, C.; García-Martínez, O. Bisphosphonate Modulation of the Gene Expression of Different Markers Involved in Osteoblast Physiology: Possible Implications in Bisphosphonate-Related Osteonecrosis of the Jaw. Int. J. Med. Sci. 2018, 15, 359–367. [Google Scholar] [CrossRef]
- Völkel, W.; Kiranoglu, M.; Fromme, H. Determination of Free and Total Bisphenol A in Human Urine to Assess Daily Uptake as a Basis for a Valid Risk Assessment. Toxicol. Lett. 2008, 179, 155–162. [Google Scholar] [CrossRef]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of Bisphenol A in Humans Following a Single Oral Administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tayama, S. Metabolism and Cytotoxicity of Bisphenol A and Other Bisphenols in Isolated Rat Hepatocytes. Arch. Toxicol. 2000, 74, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-Tertiary-Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Relationship between Urinary Bisphenol A Levels and Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and Reproductive Health: Update of Experimental and Human Evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-Osteoclast Interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK-RANKL-OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local Communication on and within Bone Controls Bone Remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Heufelder, A.E. Role of Receptor Activator of Nuclear Factor-kappaB Ligand and Osteoprotegerin in Bone Cell Biology. J. Mol. Med. 2001, 79, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral. Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG System. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef]
- Huang, F.-M.; Chang, Y.-C.; Lee, S.-S.; Yang, M.-L.; Kuan, Y.-H. Expression of Pro-Inflammatory Cytokines and Mediators Induced by Bisphenol A via ERK-NFκB and JAK1/2-STAT3 Pathways in Macrophages. Environ. Toxicol. 2019, 34, 486–494. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; ElAmin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A Induces Hepatotoxicity through Oxidative Stress in Rat Model. Oxid. Med. Cell Longev. 2012, 2012, 194829. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of Oxidative Stress by Bisphenol A and Its Pleiotropic Effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Huang, M.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol A and Its Analogues Bisphenol S, Bisphenol F and Bisphenol AF Induce Oxidative Stress and Biomacromolecular Damage in Human Granulosa KGN Cells. Chemosphere 2020, 253, 126707. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Marcucci, G.; Favilli, F.; Zonefrati, R.; Mavilia, C.; Galli, G.; Tanini, A.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Role of GSH/GSSG Redox Couple in Osteogenic Activity and Osteoclastogenic Markers of Human Osteoblast-like SaOS-2 Cells. FEBS J. 2013, 280, 867–879. [Google Scholar] [CrossRef]
- Kassem, M.; Kveiborg, M.; Eriksen, E.F. Production and Action of Transforming Growth Factor-Beta in Human Osteoblast Cultures: Dependence on Cell Differentiation and Modulation by Calcitriol. Eur. J. Clin. Investig. 2000, 30, 429–437. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.F.; Diwan, A.D. The Role of BMP-7 in Chondrogenic and Osteogenic Differentiation of Human Bone Marrow Multipotent Mesenchymal Stromal Cells In Vitro. J. Cell Biochem. 2010, 109, 406–416. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Kang, J.S.; Liu, C.; Derynck, R. New Regulatory Mechanisms of TGF-Beta Receptor Function. Trends Cell Biol. 2009, 19, 385–394. [Google Scholar] [CrossRef]
- Hinck, A.P. Structural Studies of the TGF-Βs and Their Receptors - Insights into Evolution of the TGF-β Superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef]
- Jann, J.; Gascon, S.; Roux, S.; Faucheux, N. Influence of the TGF-β Superfamily on Osteoclasts/Osteoblasts Balance in Physiological and Pathological Bone Conditions. Int. J. Mol. Sci. 2020, 21, 7597. [Google Scholar] [CrossRef]
- Hwang, J.K.; Min, K.H.; Choi, K.H.; Hwang, Y.C.; Jeong, I.-K.; Ahn, K.J.; Chung, H.-Y.; Chang, J.S. Bisphenol A Reduces Differentiation and Stimulates Apoptosis of Osteoclasts and Osteoblasts. Life Sci. 2013, 93, 367–372. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-Derived VEGF Regulates Osteoblast Differentiation and Bone Formation during Bone Repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ma, L.; Kyrkanides, S. Effects of Vascular Endothelial Growth Factor on Osteoblasts and Osteoclasts. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 366–373. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| RANKL | ATACCCTGATGAAAGGAGGA | GGGGCTCAATCTATATCTCG |
| OPG | ATGCAACACAGCACAACATA | GTTGCCGTTTTATCCTCTCT |
| TGF-β1 | TGAACCGGCCTTTCCTGCTTCTCATG | GCGGAAGTCAATGTACAGCTGCCGC |
| TGFB-R1 | ACTGGCAGCTGTCATTGCTGGACCAG | CTGAGCCAGAACCTGACGTTGTCATATCA |
| TGFB-R2 | GGCTCAACCACCAGGGCATCCAGAT | CTCCCCGAGAGCCTGTCCAGATGCT |
| TGFB-R3 | ACCGTGATGGGCATTGCGTTTGCA | GTGCTCTGCGTGCTGCCGATGCTGT |
| VEGF | CCTTGCTGCTCTACCTCCAC | CACACAGGATGGCTTGAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Recio, E.; González-Acedo, A.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Ruiz, C. Gene Expression Modulation of Markers Involved in Bone Formation and Resorption by Bisphenol A, Bisphenol F, Bisphenol S, and Bisphenol AF. Genes 2024, 15, 1453. https://doi.org/10.3390/genes15111453
García-Recio E, González-Acedo A, Manzano-Moreno FJ, De Luna-Bertos E, Ruiz C. Gene Expression Modulation of Markers Involved in Bone Formation and Resorption by Bisphenol A, Bisphenol F, Bisphenol S, and Bisphenol AF. Genes. 2024; 15(11):1453. https://doi.org/10.3390/genes15111453
Chicago/Turabian StyleGarcía-Recio, Enrique, Anabel González-Acedo, Francisco Javier Manzano-Moreno, Elvira De Luna-Bertos, and Concepción Ruiz. 2024. "Gene Expression Modulation of Markers Involved in Bone Formation and Resorption by Bisphenol A, Bisphenol F, Bisphenol S, and Bisphenol AF" Genes 15, no. 11: 1453. https://doi.org/10.3390/genes15111453
APA StyleGarcía-Recio, E., González-Acedo, A., Manzano-Moreno, F. J., De Luna-Bertos, E., & Ruiz, C. (2024). Gene Expression Modulation of Markers Involved in Bone Formation and Resorption by Bisphenol A, Bisphenol F, Bisphenol S, and Bisphenol AF. Genes, 15(11), 1453. https://doi.org/10.3390/genes15111453








