Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Participants
2.3. Procedures
2.4. Measures
2.4.1. Adverse Childhood Experiences
2.4.2. Control Measures
2.4.3. miRNA Expression
2.5. Data Analysis Plan
3. Results
3.1. ACE Prevalence
3.2. miRNA Associations with Cumulative ACE Exposure
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| KEGG Pathway | Sig. a | Number of Genes | Number of miRNAs |
|---|---|---|---|
| Fatty acid biosynthesis | <0.001 | 6 | 7 |
| Long-term depression | <0.001 | 34 | 12 |
| Hippo signaling pathway | <0.001 | 68 | 14 |
| Signaling pathways regulating pluripotency of stem cells | <0.001 | 71 | 14 |
| Prion diseases | <0.001 | 8 | 8 |
| TGF-β signaling pathway | <0.001 | 38 | 14 |
| Proteoglycans in cancer | <0.001 | 88 | 14 |
| Renal cell carcinoma | <0.001 | 36 | 11 |
| Ubiquitin-mediated proteolysis | <0.001 | 64 | 14 |
| mTOR signaling pathway | <0.001 | 35 | 12 |
| Prostate cancer | 0.001 | 45 | 12 |
| Regulation of actin cytoskeleton | 0.001 | 93 | 14 |
| AMPK signaling pathway | 0.002 | 59 | 13 |
| Axon guidance | 0.002 | 60 | 14 |
| PI3K-Akt signaling pathway | 0.002 | 138 | 14 |
| Adherens junction | 0.002 | 40 | 13 |
| N-Glycan biosynthesis | 0.002 | 21 | 14 |
| WNT signaling pathway | 0.002 | 63 | 14 |
| Focal adhesion | 0.002 | 91 | 14 |
| Gap junction | 0.002 | 38 | 14 |
| Glutamatergic synapse | 0.002 | 50 | 14 |
| Pathways in cancer | 0.002 | 160 | 14 |
| Transcriptional misregulation in cancer | 0.003 | 69 | 14 |
| Adrenergic signaling in cardiomyocytes | 0.003 | 63 | 14 |
| Lysine degradation | 0.003 | 23 | 13 |
| FoxO signaling pathway | 0.003 | 60 | 13 |
| Glioma | 0.003 | 30 | 14 |
| 2-Oxocarboxylic acid metabolism | 0.005 | 9 | 9 |
| cGMP-PKG signaling pathway | 0.011 | 70 | 14 |
| Melanoma | 0.013 | 36 | 12 |
| Fatty acid metabolism | 0.015 | 18 | 12 |
| ErbB signaling pathway | 0.015 | 41 | 14 |
| Rap1 signaling pathway | 0.015 | 87 | 14 |
| Protein processing in endoplasmic reticulum | 0.017 | 67 | 13 |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 0.020 | 35 | 14 |
| Bacterial invasion of epithelial cells | 0.020 | 38 | 14 |
| Choline metabolism in cancer | 0.023 | 47 | 13 |
| Thyroid hormone signaling pathway | 0.026 | 51 | 14 |
| Ras signaling pathway | 0.028 | 89 | 14 |
| Cell cycle | 0.033 | 52 | 13 |
| Oxytocin signaling pathway | 0.039 | 65 | 14 |
| Morphine addiction | 0.039 | 37 | 14 |
| MAPK signaling pathway | 0.047 | 98 | 14 |
| Cholinergic synapse | 0.047 | 49 | 14 |
| Amphetamine addiction | 0.048 | 27 | 13 |
| Endocytosis | 0.049 | 80 | 14 |
| Viral carcinogenesis | 0.049 | 66 | 14 |
| Endocrine and other factor-regulated calcium reabsorption | <0.050 | 17 | 12 |
References
- Anderson, K.N.; Swedo, E.A.; Trinh, E.; Ray, C.M.; Krause, K.H.; Verlenden, J.V.; Clayton, H.B.; Villaveces, A.; Massetti, G.M.; Holditch Niolon, P. Adverse childhood experiences during the COVID-19 pandemic and associations with poor mental health and suicidal behaviors among high school students—Adolescent behaviors and experiences survey, United States, January–June 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, E.; Jarman, I.; McCabe, P.; McCarthy, M.; Provazza, S.; Crosbie, V.; Quigg, Z.; Saini, P. Suicidal crisis among children and young people: Associations with adverse childhood experiences and socio-demographic factors. Int. J. Environ. Res. Public Health 2023, 20, 1251. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, L.; Leeb, R.T.; Bitsko, R.H.; Carey, K.; Gates, A.; Holland, K.M.; Hartnett, K.P.; Kite-Powell, A.; DeVies, J.; Smith, A.R.; et al. Pediatric emergency department visits associated with mental health conditions before and during the COVID-19 pandemic—United States, January 2019–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.R.; Baumler, E.; Wood, L.; Guillot-Wright, S.; Torres, E.; Thiel, M. The impact of the COVID-19 pandemic on Adolescent Mental Health and Substance Use. J. Adolesc. Health 2022, 71, 277–284. [Google Scholar] [CrossRef]
- Eid, K.; Bjørk, M.H.; Gilhus, N.E.; Torkildsen, Ø. Adverse childhood experiences and the risk of multiple sclerosis development: A review of potential mechanisms. Int. J. Mol. Sci. 2024, 25, 1520. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Huizar, Y.P.; Cundiff JP Schmidt, A.T.; Cribbet, M.R. Risky early family environment and genetic associations with adult metabolic dysregulation. Int. J. Environ. Res. Public Health 2022, 19, 14032. [Google Scholar] [CrossRef]
- Jones, C.M.; Merrick, M.T.; Houry, D.E. Identifying and preventing Adverse Childhood Experiences: Implications for clinical practice. JAMA 2020, 323, 25–26. [Google Scholar] [CrossRef]
- Roberts, A.L.; Zafonte, R.; Chibnik, L.B.; Baggish, A.; Taylor, H.; Baker, J.; Whittington, A.J.; Weisskopf, M.G. Association of adverse childhood experiences with poor neuropsychiatric health and dementia among former professional US football players. JAMA Netw. Open 2022, 5, e223299. [Google Scholar] [CrossRef]
- Vadukapuram, R.; Shah, K.; Ashraf, S.; Srinivas, S.; Elshokiry, A.B.; Trivedi, C.; Mansuri, Z.; Jain, S. Adverse childhood experiences and their impact on sleep in adults: A systematic review. J. Nerv. Ment. Dis. 2022, 210, 397–410. [Google Scholar] [CrossRef]
- Bergquist, B.K.; Schmidt, A.T.; Thomas, A.G. Adverse childhood experiences and negative outcomes among justice-involved youth: Moderating effects of protective factors. Crime Delinq. 2024, 70, 1274–1303. [Google Scholar] [CrossRef]
- De la Rosa, R.; Zablotny, D.; Ye, M.; Bush, N.R.; Hessler, D.; Koita, K.; Bucci, M.; Long, D.; Thakur, N. Biological burden of adverse childhood experiences in children. Psychosom. Med. 2023, 85, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Gette, J.A.; Gissandaner, T.D.; Littlefield, A.K.; Simmons, C.S.; Schmidt, A.T. Modeling the Adverse Childhood Experiences Questionnaire—International Version. Child Maltreatment 2022, 27, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kerker, B.D.; Zhang, J.; Nadeem, E.; Stein RE, K.; Hurlburt, M.S.; Heneghan, A.; Landsverk, J.; McCue Horwitz, S. Adverse childhood experiences and mental health, chronic medical conditions, and development in young children. Acad. Pediatr. 2015, 15, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Avci, G.; Hanten, G.; Schmidt, A.T.; Li, X.; Orsten, K.; Faber, J.; Post, M.; Newsome, M.R. Cognitive contributors to resilience in youth from underserved populations: A brief report. J. Public Ment. Health 2013, 12, 165–170. [Google Scholar] [CrossRef]
- Danese, A.; Baldwin, J.R. Hidden wounds? Inflammatory links between childhood trauma and psychopathology. Annu. Rev. Psychol. 2017, 68, 517–544. [Google Scholar] [CrossRef]
- Miller, E.S.; Sakowicz, A.; Roy, A.; Yang, A.; Sullivan, J.T.; Grobman, W.A.; Wisner, K.L. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. Am. J. Obstet. Gynecol. 2019, 220, 271.e1–271.e10. [Google Scholar] [CrossRef]
- Dennis, E.L.; Caeyenberghs, K.; Hoskinson, K.R.; Merkley, T.L.; Suskauer, S.J.; Asarnow, R.F.; Babikian, T.; Bartnik-Olson, B.; Bickart, K.; Bigler, E.D.; et al. White matter disruption in pediatric traumatic brain injury: Results from ENIGMA pediatric moderate to severe traumatic brain injury. Neurology 2021, 97, 298–309. [Google Scholar] [CrossRef]
- Keleher, F.; Lindsey HKerestes, R.; Amiri, H.; Asarnow, R.; Bartnik-Olson, B.; Bigler, E.; Caeyenberghs, K.; Esopenko, C.; Ewing-Cobbs, L.; Giza, C.; et al. Multimodal analysis of secondary cerebellar alterations after pediatric traumatic brain injury. JAMA Netw. Open 2023, 6, e2343410. [Google Scholar] [CrossRef]
- Schmidt, A.T.; Hanten, G.R.; Li, X.; Vasquez, A.C.; Wilde, E.A.; Chapman, S.B.; Levin, H.S. Decision making performance after pediatric traumatic brain injury: Trajectory of recovery and relationship to age and gender. Int. J. Dev. Neurosci. 2012, 30, 225–230. [Google Scholar] [CrossRef]
- Schmidt, A.T.; Lindsey, H.M.; Dennis, E.; Wilde, E.A.; Biekman, B.D.; Chu, Z.D.; Hanten, G.R.; Formon, D.L.; Spruiell, M.S.; Hunter, J.V.; et al. Diffusion tensor imaging correlates of resilience following adolescent traumatic brain injury. Cogn. Behav. Neurol. 2021, 34, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.A.; Schmidt, A.T.; Hanten, G.R.; Levin, H.S. Executive dysfunction in children and adolescents with behavior disorders and traumatic brain injury. Child Neuropsychol. 2020, 26, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Max, J.E.; Wilde, E.A.; Bigler, E.D.; MacLeod, M.; Vasquez, A.C.; Schmidt, A.T.; Chapman, S.B.; Hotz, G.; Yang, T.T.; Levin, H.S. Psychiatric disorders after traumatic brain injury: A prospective longitudinal controlled study. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.T.; Martin, R.B.; Ozturk, A.; Kates, W.R.; Wharam, M.; Mahone, E.M.; Horska, A. Neuroimaging and neuropsychological follow-up study in a pediatric brain tumor patient treated with surgery and radiation. Neurocase 2010, 16, 74–90. [Google Scholar] [CrossRef]
- Schmidt, A.T.; Hanten, G.; Li, X.; Wilde, E.A.; Ibarra, A.; Chu, Z.D.; Helbling, A.R.; Shah, S.; Levin, H.S. Emotional prosody and diffusion tensor imaging in children after traumatic brain injury. Brain Inj. 2013, 27, 1528–1535. [Google Scholar] [CrossRef]
- Schmidt, A.T.; Li, X.; Zhang-Rutledge, K.; Hanten, G.R.; Levin, H.S. A history of low birth weight alters recovery following a future head injury: A case series. Child Neuropsychol. 2014, 20, 495–508. [Google Scholar] [CrossRef]
- Wilde, E.A.; Merkley, T.L.; Bigler, E.D.; Max, J.E.; Schmidt, A.T.; Ayoub, K.W.; McCauley, S.R.; Hunter, J.V.; Hanten, G.; Li, X.; et al. Longitudinal changes in cortical thickness in children after traumatic brain injury: Relationship with behavioral regulation and emotional control. Int. J. Dev. Neurosci. 2012, 30, 267–276. [Google Scholar] [CrossRef]
- Rasmussen LJ, H.; Moffitt, T.E.; Arseneault, L.; Danese, A.; Eugen-Olsen, J.; Fisher, H.L.; Harrington, H.; Houts, R.; Matthews, T.; Sugden, K.; et al. Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. 2020, 174, 38–47. [Google Scholar] [CrossRef]
- Dieckmann, L.; Cole, S.; Kumsta, R. Stress genomics revisited: Gene co-expression analysis identifies molecular signatures associated with childhood adversity. Transl. Psychiatry 2020, 10, 34. [Google Scholar] [CrossRef]
- Levine, M.E.; Cole, S.W.; Weir, D.R.; Crimmins, E.M. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc. Sci. Med. 2015, 130, 16–22. [Google Scholar] [CrossRef]
- Marie-Mitchell, A.; Cole, S.W. Adverse childhood experiences and transcriptional response in school-age children. Dev. Psychopathol. 2021, 34, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Dwivedi, Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol. Psychiatry 2023, 28, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.-M.; Huang, C.-M.; Zhu, X.-Y.; Bian, F.; Pan, S.-M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef] [PubMed]
- Hollins, S.L.; Cairns, M.J. MicroRNA: Small RNA mediators of the brains genomic response to environmental stress. Prog. Neurobiol. 2016, 143, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Improta Caria, A.C.; Nonaka CK, V.; Pereira, C.S.; Soares MB, P.; Macambira, S.G.; Souza, B.S.d.F. Exercise training-induced changes in microRNAs: Beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Kaushik, D.K.; Kundu, K.; Basu, A. MicroRNA-29b modulates Japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor α-induced protein 3. J. Neurochem. 2014, 129, 143–154. [Google Scholar] [CrossRef]
- Bergman, P.; James, T.; Kular, L.; Ruhrmann, S.; Kramarova, T.; Kvist, A.; Supic, G.; Gillett, A.; Pivarcsi, A.; Jagodic, M. Next-generation sequencing identifies microRNAs that associate with pathogenic autoimmune neuroinflammation in rats. J. Immunol. 2013, 190, 4066–4075. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Liu, Y.; Liu, D.; Jiang, H.; Pan, F. FKBP5 and specific microRNAs via glucocorticoid receptor in the basolateral amygdala involved in the susceptibility to depressive disorder in early adolescent stressed rats. J. Psychiatr. Res. 2017, 95, 102–113. [Google Scholar] [CrossRef]
- Sillivan, S.E.; Jamieson, S.; de Nijs, L.; Jones, M.; Snijders, C.; Klengel, T.; Joseph, N.F.; Krauskopf, J.; Kleinjans, J.; Vinkers, C.H.; et al. MicroRNA regulation of persistent stress-enhanced memory. Mol. Psychiatry 2020, 25, 965–976. [Google Scholar] [CrossRef]
- Chen, R.J.; Kelly, G.; Sengupta, A.; Heydendael, W.; Nicholas, B.; Beltrami, S.; Luz, S.; Peixoto, L.; Able, T.; Bhatnagar, S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience 2015, 305, 36–48. [Google Scholar] [CrossRef]
- Volk, N.; Pape, J.C.; Engel, M.; Zannas, A.S.; Cattane, N.; Cattaneo, A.; Binder, E.B.; Chen, A. Amygdalar microRNA-15a is essential for coping with chronic stress. Cell Rep. 2016, 17, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Le, T.; Wei, R.; Jiao, Y. Knockdown of JMJD1C, a target of has-miR-590-3p, inhibits mitochondrial dysfunction and oxidative stress in MPP+-treated MES23.5 and SH-SY5Y cells. Cell. Mol. Biol. 2016, 62, 39–45. [Google Scholar]
- Honda, M.; Kuwano, Y.; Katsuura-Kamano, S.; Kamezaki, Y.; Fujita, K.; Akaike, Y.; Kano, S.; Nishida, K.; Masuda, K.; Rokutan, K. Chronic academic stress increases a group of microRNAs in peripheral blood. PLoS ONE 2013, 8, e75960. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Dwivedi, Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol. Psychiatry 2020, 25, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg MM, J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Jiang, H.; Du, J.; Na, Z.; Hao, W.; Yu, S.; Zhao, M. Decreased expression of plasma microRNA in patients with methamphetamine (MA) use disorder. J. Neuroimmune Pharmacol. 2016, 11, 542–548. [Google Scholar] [CrossRef]
- Van der Auwera, S.; Ameling, S.; Nauck, M.; Volzke, H.; Volker, U.; Grabe, H.J. Association between different dimensions of childhood traumatization and plasma micro-RNA levels in a clinical psychiatric sample. J. Psychiatr. Res. 2021, 139, 113–119. [Google Scholar] [CrossRef]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Morel, L.; Regan, M.; Higashimori, H.; Ng, S.K.; Esau, C.; Vidensky, S.; Rothstein, J.; Yang, Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J. Biol. Chem. 2013, 288, 7105–7116. [Google Scholar] [CrossRef]
- Rani, K.; Mukherjee, R.; Singh, E.; Kumar, S.; Sharma, V.; Vishwakarma, P.; Bharti, P.S.; Nikolajeff, F.; Dinda, A.K.; Goyal, V.; et al. Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Park. Relat. Disord. 2019, 67, 21–23. [Google Scholar] [CrossRef]
- Sullivan, R.; Montgomery, A.; Scipioni, A.; Jhaveri, P.; Schmidt, A.T.; Hicks, S. Confounding factors impacting microRNA biomarkers in human saliva: Methodological and biological considerations. Genes 2022, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Ignacio, C.; Gentile, K.; Middleton, F.A. Salivary miRNA profiles identify children with autism spectrum disorder, correlate with adaptive behavior, and implicate ASD candidate genes involved in neurodevelopment. BMC Pediatr. 2016, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Johnson, J.; Carney, M.C.; Bramley, H.; Olympia, R.P.; Loeffert, A.C.; Thomas, N.J. Overlapping MicroRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J. Neurotrauma 2018, 35, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.J.; Erkanli, A.; Fairbank, J.A.; Angold, A. The prevalence of potentially traumatic events in childhood and adolescence. J. Trauma. Stress 2005, 15, 99–112. [Google Scholar] [CrossRef]
- Copeland, W.E.; Keeler, G.; Angold, A.; Costello, E.J. Traumatic events and postraumatic stress in childhood. Arch. Gen. Psychiatry 2007, 64, 577–584. [Google Scholar] [CrossRef]
- Kempker, S.M.; Schmidt, A.T.; Espinosa, E.M. Understanding the influence of mental health diagnosis and gender on placement decisions for justice-involved youth. J. Youth Adolesc. 2017, 46, 1562–1581. [Google Scholar] [CrossRef]
- Maloney, K.A.; Schmidt, A.T. Juvenile delinquency and antisocial behavior: A brief overview of theories, definitions, and statistics. In The SAGE Encyclopedia of Criminal Psychology; Morgan, R.D., Olver, M.E., Stockdale, K.C., Eds.; SAGE Publications: New York, NY, USA, 2019. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Center for Youth Wellness. Adverse Childhood Experiences Questionnaire (ACE-Q) Teen Self-Report; Center for Youth Wellness: San Francisco, CA, USA, 2015. [Google Scholar]
- Hall, A.; Perez, A.; West, X.; Brown, M.; Kim, E.; Salih, Z.; Aronoff, S. The association of adverse childhood experiences and resilience with health outcomes in adolescents: An observational study. Glob. Pediatr. Health 2021, 8, 2333794X20982433. [Google Scholar] [CrossRef]
- Kellum, C.E.; Kemp, K.M.; Mrug, S.; Pollock, J.S.; Seifert, M.E.; Feig, D.I. Adverse childhood experiences are associated with vascular changes in adolescents that are risk factors for future cardiovascular disease. Pediatr. Nephrol. 2023, 38, 2155–2163. [Google Scholar] [CrossRef]
- Bethell, C.D.; Carle, A.; Hudziak, J.; Gombojav, N.; Powers, K.; Wade, R.; Braveman, P. Methods to Assess Adverse Childhood Experiences of Children and Families: Toward Approaches to Promote Child Well-being in Policy and Practice. Acad. Pediatr. 2017, 17 (Suppl. 7), S51–S69. [Google Scholar] [CrossRef]
- Meinck, F.; Neelakantan, L.; Steele, B.; Jochim, J.; Davies, L.M.; Boyes, M.; Barlow, J.; Dunne, M. Measuring Violence Against Children: A COSMIN Systematic Review of the Psychometric Properties of Child and Adolescent Self-Report Measures. Trauma Violence Abus. 2023, 24, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.L.; Jerman, P.; Boparai SK, P.; Koita, K.; Briner, S.; Bucci, M.; Harris, N.B. Review of tools for measuring exposure to adversity in children and adolescents. J. Pediatr. Health Care 2018, 32, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Elmore, A.L.; Crouch, E. The associations of adverse childhood experiences with anxiety and depression in children and youth, 8 to 17 years of age. Acad. Pediatr. 2020, 20, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Murakami, Y. Principal component analysis based feature extraction approach to identify circulating microRNA biomarkers. PLoS ONE 2013, 8, e66714. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkuoni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acid Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gissandaner, T.D.; Gette, J.A.; Schmidt, A.T.; Littlefield, A.K. Mitigating the relation between adverse childhood experiences and perceived stress: The role of resilience. Advers. Resil. Sci. 2022, 3, 53–63. [Google Scholar] [CrossRef]
- Olson, C.L. Practical considerations in choosing a MANOVA test statistic: A rejoinder to Stevens. Psychol. Bull. 1979, 86, 1350–1352. [Google Scholar] [CrossRef]
- Eve, M.; Gandawijaya, J.; Yang, L.; Oguro-Ando, A. Neuronal cell adhesion molecules may mediate neuroinflammation in autism spectrum disorder. Front. Psychiatry 2022, 13, 842755. [Google Scholar] [CrossRef]
- Harrison, E.B.; Emanual, K.; Lamberty, B.G.; Morsey, B.M.; Li, M.; Kelso, M.L.; Yelamanchili, S.V.; Fox, H.S. Induction of miR-155 after brain injury promotes type 1 interferon and has a neuroprotective effect. Front. Mol. Neurosci. 2017, 28, 228. [Google Scholar] [CrossRef]
- Sha, R.; Zhang, B.; Han, X.; Peng, J.; Zheng, C.; Zhang, F.; Huang, X. Electroacupuncture alleviates ischemic brain injury by inhibiting the miR-223/NLRP3 pathway. Med. Sci. Monit. 2019, 25, 4723–4733. [Google Scholar] [CrossRef] [PubMed]
- Cukovic, D.; Bagla, S.; Ukasik, D.; Stemmer, P.M.; Jena, B.P.; Naik, A.R.; Sood, S.; Asano, E.; Luat, A.; Chugani, D.C.; et al. Exosomes in epilepsy of tuberous sclerosis complex: Carriers of pro-inflammatory microRNAs. Noncoding RNA 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G. RNA and oxidative stress in Alzheimer’s disease: Focus on microRNAs. Oxidative Med. Cell. Longev. 2020, 2020, 2638130. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, J.; He, F.P.; Bao, W.X.; Zheng, T.T.; Zhou, D.M.; Pan, H.Y.; Zhang, H.; Zhang, X.Q.; He, X.; et al. Down-regulated expression of microRNA-338-5p contributes to neuropathology in Alzheimer’s disease. FASEB J. 2019, 33, 4404–4417. [Google Scholar] [CrossRef]
- Song, Y.; Hu, M.; Zhang, J.; Teng, Z.Q.; Chen, C. A novel mechanism of synaptic and cognitive impairment mediated via microRNA-30b in Alzheimer’s disease. eBioMedicine 2019, 39, 409–421. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; He, J.H. Transcriptomic analysis reveals essential microRNAs after peripheral nerve injury. Neural Regen. Res. 2021, 16, 1865–1870. [Google Scholar] [CrossRef]
- Pearson-Leary, J.; Eacret, D.; Chen, R.; Takano, H.; Nicholas, B.; Bhatnagar, S. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry 2017, 7, e1160. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Singh, N.; Mehrpouya-Bahrami, P.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. AhR activation by TCDD (2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin) attenuates pertussis toxin-induced inflammatory responses by differential regulation of tregs and Th17 cells through specific targeting by microRNA. Front. Microbiol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Kamath, S.P.; Choolani, M.; Lu, J.; Dheen, S.T. microRNA-200b modulates microglia-mediated neuroinflammation via the cJun/MAPK pathway. J. Neurochem. 2014, 130, 388–401. [Google Scholar] [CrossRef]
- Ureña-Peralta, J.R.; Alfonso-Loeches, S.; Cuesta-Diaz, C.M.; García-García, F.; Guerri, C. Deep sequencing and miRNA profiles in alcohol-induced neuroinflammation and the TLR4 response in mice cerebral cortex. Sci. Rep. 2018, 8, 15913. [Google Scholar] [CrossRef]
- Yakovleva, K.D.; Dmitrenko, D.V.; Panina, I.S.; Usoltseva, A.A.; Gazenkampf, K.A.; Konovalenko, O.V.; Kantimirova, E.A.; Novitsky, M.A.; Nasyrova, R.F.; Shnayder, N.A. Expression Profile of miRs in mesial temporal lobe epilepsy: Systematic review. Int. J. Mol. Sci. 2022, 23, 951. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Li, Y.; Cui, L.; Wu, K.; Luo, H. Astaxanthin inhibits microglia M1 activation against inflammatory injury triggered by lipopolysaccharide through down-regulating miR-31-5p. Life Sci. 2021, 267, 118943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ye, J.; Nie, Y.; Ashraf, U.; Zohaib, A.; Duan, X.; Fu, Z.F.; Song, Y.; Chen, H.; Cao, S. MicroRNA-15b modulates Japanese encephalitis virus-mediated inflammation via targeting RNF125. J. Immunol. 2015, 195, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Geng, L.; Streck, D.L.; Dermody, J.J.; Toruner, G.A. MicroRNA expression changes in association with changes in interleukin-1ß/interleukin10 ratios produced by monocytes in autism spectrum disorders: Their association with neuropsychiatric symptoms and comorbid conditions (observational study). J. Neuroinflamm. 2017, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Liguori, M.; Nuzziello, N.; Introna, A.; Consiglio, A.; Licciulli, F.; D’Errico, E.; Scarafino, A.; Distaso, E.; Simone, I.L. Dysregulation of MicroRNAs and target genes networks in peripheral blood of patients with sporadic amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2018, 11, 288. [Google Scholar] [CrossRef]
- Fry, R.C.; Rager, J.E.; Bauer, R.; Sebastian, E.; Peden, D.B.; Jaspers, I.; Alexis, N.E. Air toxics and epigenetic effects: Ozone altered microRNAs in the sputum of human subjects. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L1129–L1137. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Ma, J.; Luo, S.; Chen, S.; Gu, Q. MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum. Cell 2018, 31, 106–115. [Google Scholar] [CrossRef]
- Gilgoff, R.; Singh, L.; Koita, K.; Gentile, B.; Marques, S.S. Adverse Childhood Experiences, Outcomes, and Interventions. Pediatr. Clin. N. Am. 2020, 67, 259–273. [Google Scholar] [CrossRef]
- Hill, L.; Barnett, J.E.; Ward, J.A.; Morton, A.R.; Schmidt, A.T. Trauma-informed care for justice-involved youth: A narrative review and synthesis. Juv. Fam. Court J. 2023, 74, 21–33. [Google Scholar] [CrossRef]
- Lorenc, T.; Lester, S.; Sutcliffe, K.; Stansfield, C.; Thomas, J. Interventions to support people exposed to adverse childhood experiences: Systematic review of systematic reviews. BMC Public Health 2020, 20, 657. [Google Scholar] [CrossRef]
- Roitbak, T. MicroRNAs and regeneration in animal models of CNS disorders. Neurochem. Res. 2019, 45, 188–203. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.-J. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
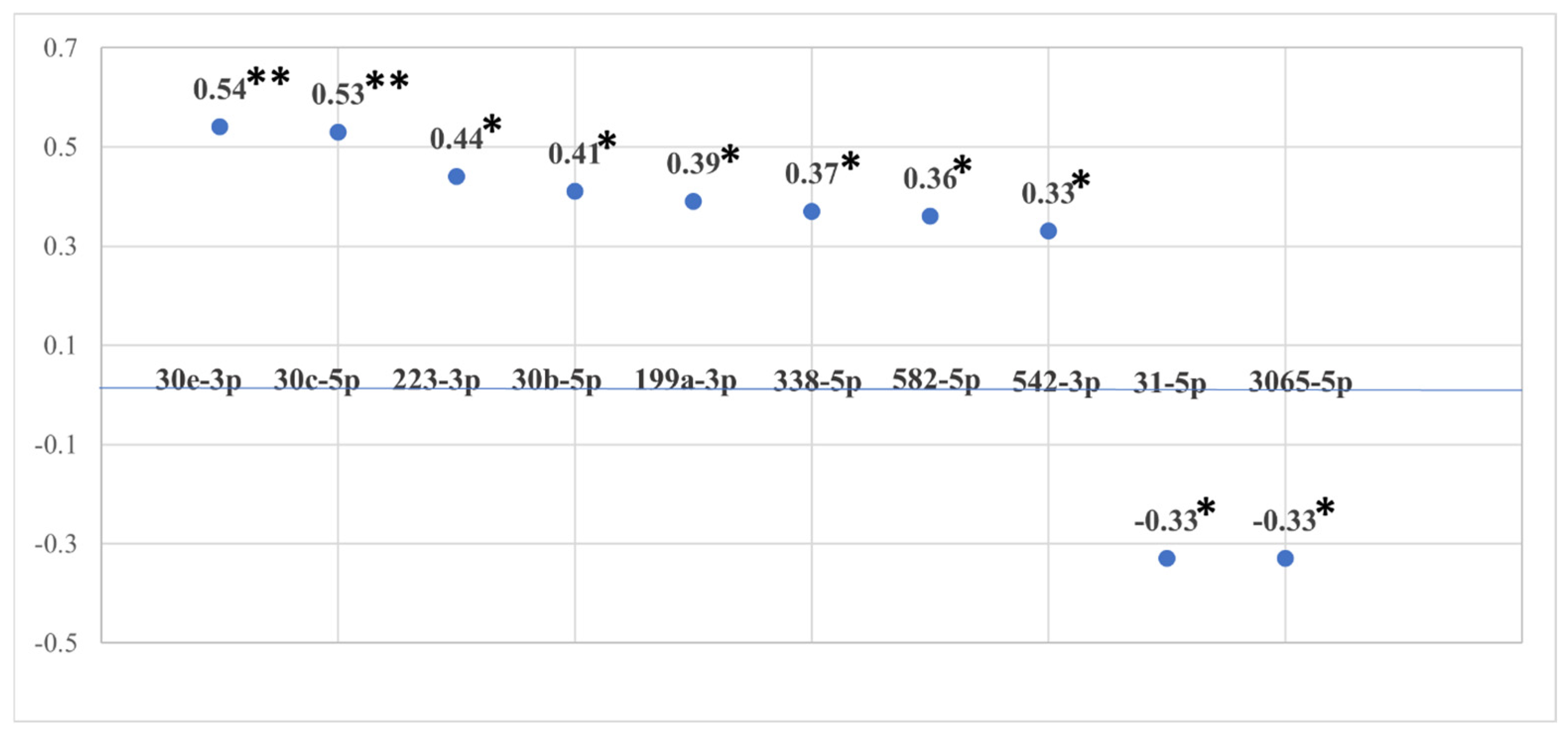
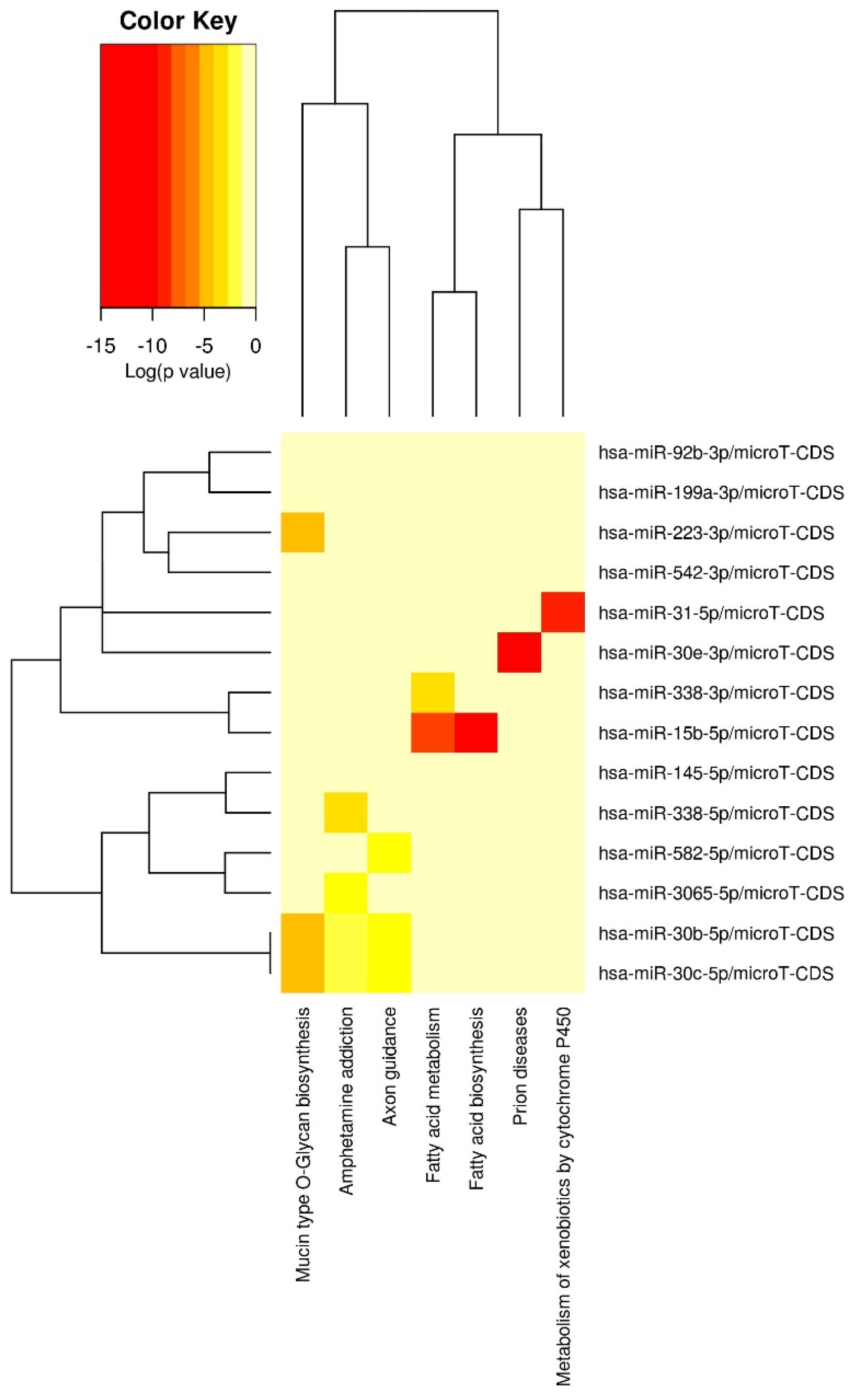
| Source | Dependent Variable | Mean Square | F | Sig. a | Partial Eta Squared |
|---|---|---|---|---|---|
| ACEs | miR-223-3p | 1.10 | 19.32 | <0.001 | 0.32 |
| miR-30e-3p | 0.45 | 17.53 | <0.001 | 0.30 | |
| miR-30b-5p | 0.32 | 8.55 | 0.01 | 0.17 | |
| miR-30c-5p | 0.31 | 12.50 | 0.002 | 0.23 | |
| miR-365a-3p | 0.14 | 4.94 | 0.04 | 0.10 | |
| miR-582-5p | 0.14 | 2.58 | 0.11 | 0.05 | |
| miR-199a-3p | 0.28 | 6.67 | 0.02 | 0.14 | |
| miR-338-5p | 0.17 | 4.66 | 0.04 | 0.10 |
| MicroRNA | Mean | Standard Deviation |
|---|---|---|
| miR-223-3p | 0.24 | 0.29 |
| miR-30e-3p | 0.12 | 0.19 |
| miR-30b-5p | 0.02 | 0.24 |
| miR-30c-5p | 0.074 | 0.21 |
| miR-365a-3p | −0.09 | 0.21 |
| miR-582-5p | 0.10 | 0.23 |
| miR-199a-3p | 0.18 | 0.22 |
| miR-338-5p | 0.12 | 0.20 |
| Source | Dependent Variable | Mean Square | F | Sig. a | Partial Eta Squared |
|---|---|---|---|---|---|
| ACEs | miR-223-3p | 1.27 | 23.17 | <0.001 | 0.05 |
| miR-30e-3p | 0.42 | 16.39 | <0.001 | 0.01 | |
| miR-30b-5p | 0.21 | 5.74 | 0.03 | 0.04 | |
| miR-30c-5p | 0.30 | 11.61 | 0.005 | 0.00 | |
| miR-365a-3p | 0.08 | 2.90 | 0.10 | 0.07 | |
| miR-582-5p | 0.06 | 1.20 | 0.27 | 0.05 | |
| miR-199a-3p | 0.30 | 7.47 | 0.01 | 0.00 | |
| miR-338-5p | 0.14 | 3.83 | 0.07 | 0.00 | |
| Internalizing Scores | miR-223-3p | 0.12 | 2.19 | 0.37 | 0.37 |
| miR-30e-3p | 0.01 | 0.59 | 0.70 | 0.29 | |
| miR-30b-5p | 0.06 | 1.73 | 0.38 | 0.12 | |
| miR-30c-5p | 0.01 | 0.33 | 0.74 | 0.22 | |
| miR-365a-3p | 0.09 | 3.28 | 0.56 | 0.06 | |
| miR-582-5p | 0.12 | 2.38 | 0.52 | 0.03 | |
| miR-199a-3p | 0.00 | 0.07 | 0.90 | 0.16 | |
| miR-338-5p | 0.00 | 0.03 | 0.85 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, A.T.; Hicks, S.D.; Bergquist, B.K.; Maloney, K.A.; Dennis, V.E.; Bammel, A.C. Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample. Genes 2024, 15, 1433. https://doi.org/10.3390/genes15111433
Schmidt AT, Hicks SD, Bergquist BK, Maloney KA, Dennis VE, Bammel AC. Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample. Genes. 2024; 15(11):1433. https://doi.org/10.3390/genes15111433
Chicago/Turabian StyleSchmidt, Adam T., Steven D. Hicks, Becca K. Bergquist, Kelsey A. Maloney, Victoria E. Dennis, and Alexandra C. Bammel. 2024. "Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample" Genes 15, no. 11: 1433. https://doi.org/10.3390/genes15111433
APA StyleSchmidt, A. T., Hicks, S. D., Bergquist, B. K., Maloney, K. A., Dennis, V. E., & Bammel, A. C. (2024). Preliminary Evidence for Neuronal Dysfunction Following Adverse Childhood Experiences: An Investigation of Salivary MicroRNA Within a High-Risk Youth Sample. Genes, 15(11), 1433. https://doi.org/10.3390/genes15111433





