Insecticide Resistance and Target-Site Mutations kdr, N1575Y, and Ace-1 in Anopheles gambiae s.l. Populations in a Low-Malaria-Transmission Zone in the Sudanian Region of Senegal
Abstract
1. Introduction
2. Materials and Methods
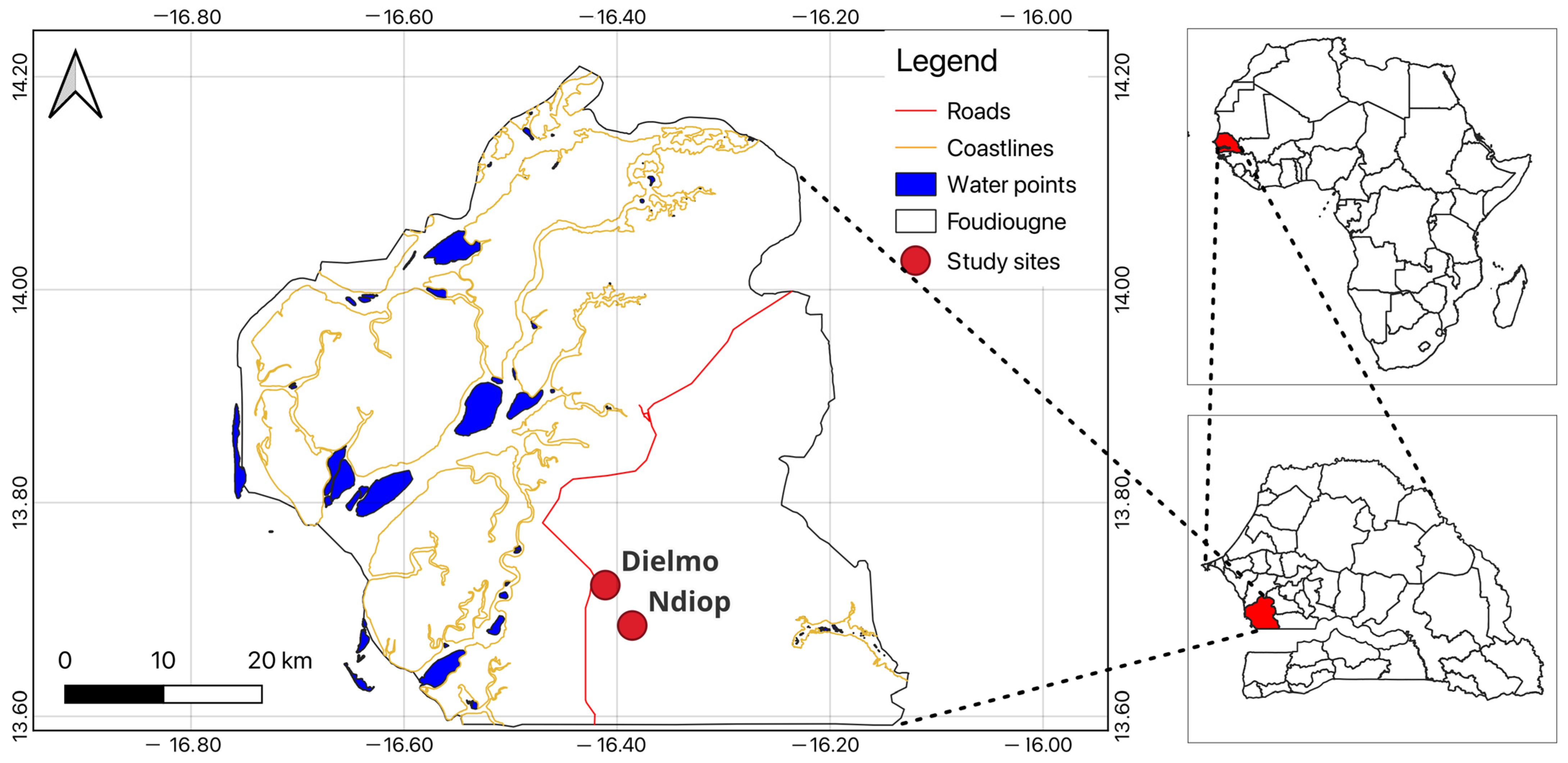
2.1. Study Area
2.2. Mosquito Collection and Rearing
2.3. WHO Insecticide Susceptibility Bioassays
2.4. Mosquito Species Identification
2.5. Molecular Identification and Genotyping of kdr and Ace-1 Mutations by PCR
2.6. Data Analysis
3. Results
3.1. Insecticide Resistance Profile
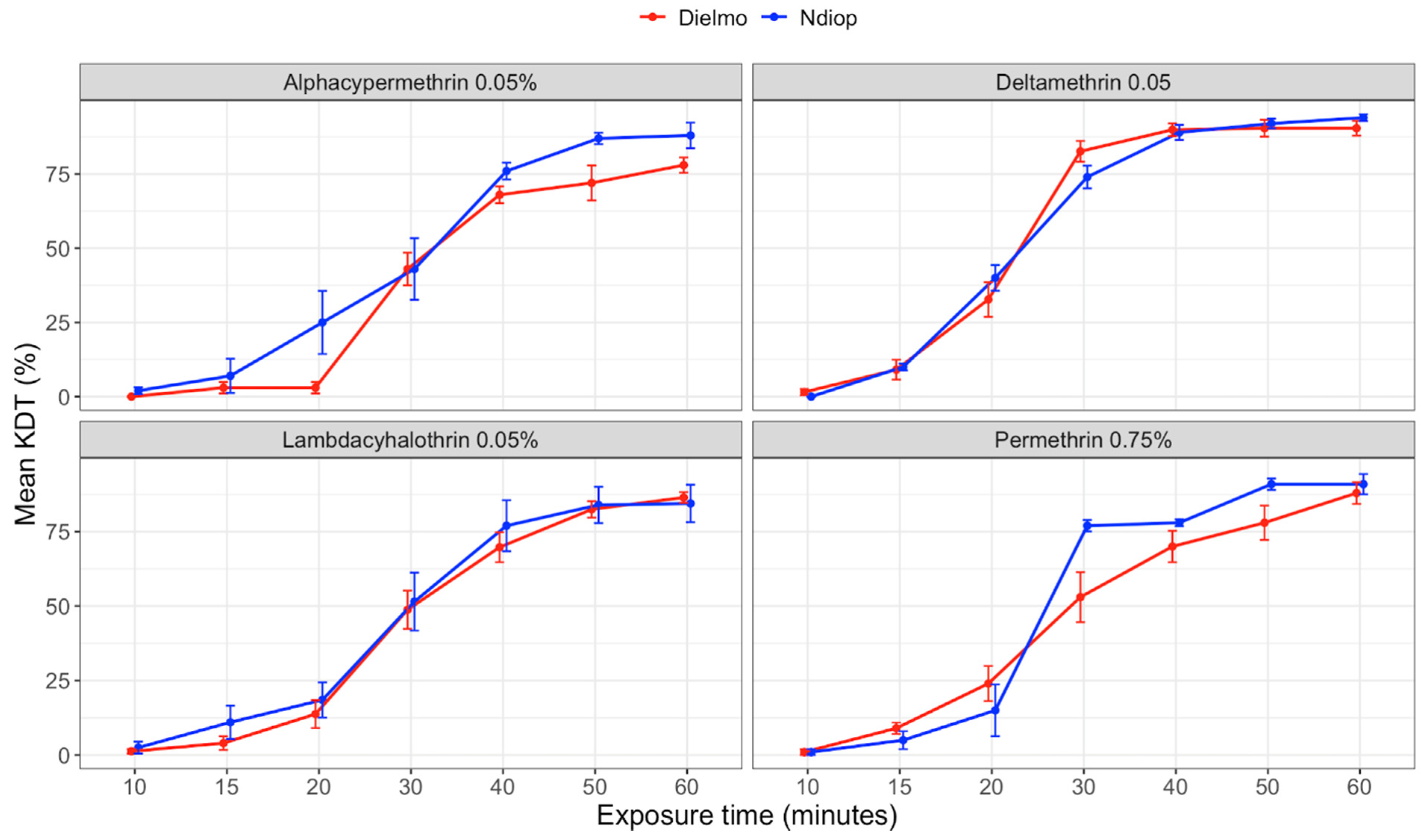
3.2. Knockdown Effects and Dynamics of Pyrethroids
3.3. Species Composition
3.4. Genotypic and Allelic Frequencies of the L1014F and L1014S Mutations
3.5. Genotypic and Allelic Frequencies of the N1575Y and Ace-1 Mutations
3.6. Association between the L1014F and L1014S Mutations and Pyrethroid Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; Available online: https://iris.who.int/handle/10665/252038 (accessed on 11 September 2023).
- Tene-Fossog, B.; Fotso-Toguem, Y.G.; Amvongo-Adjia, N.; Ranson, H.; Wondji, C.S. Temporal variation of high-level pyrethroid resistance in the major malaria vector Anopheles gambiae s.l. in Yaoundé, Cameroon, is mediated by target-site and metabolic resistance. Med. Vet. Entomol. 2022, 36, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; McKenzie, F.E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 2016, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Hodoșan, C.; Gîrd, C.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Nistor, L.; Bărbuică, I.S.; Marin, Ș.C.; Mihalache, A.; Popa, L. Pyrethrins and Pyrethroids: A Comprehensive Review of Natural Occurring Compounds and Their Synthetic Derivatives. Plants 2023, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- Namountougou, M.; Simard, F.; Baldet, T.; Diabaté, A.; Ouédraogo, J.B.; Martin, T.; Dabiré, R.K. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS ONE 2012, 7, e48412. [Google Scholar] [CrossRef]
- Yadouleton, A.W.; Padonou, G.; Asidi, A.; Moiroux, N.; Bio-Banganna, S.; Corbel, V.; N’guessan, R.; Gbenou, D.; Yacoubou, I.; Gazard, K.; et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar. J. 2010, 9, 83. [Google Scholar] [CrossRef]
- Oyewole, I.O.; Mustapha, K.; Adedeji, O.C.; Adeogun, D.; Awolola, S. Susceptibility pattern of Anopheles mosquito to different classes of insecticides in selected communities in Ila-Orangun, Southwest Nigeria. Int. J. Mosq. Res. 2018, 5, 106–111. [Google Scholar]
- Olatunbosun-Oduola, A.; Abba, E.; Adelaja, O.; Taiwo-Ande, A.; Poloma-Yoriyo, K.; Samson-Awolola, T. Widespread Report of Multiple Insecticide Resistance in Anopheles gambiae s.l. Mosquitoes in Eight Communities in Southern Gombe, North-Eastern Nigeria. J. Arthropod-Borne Dis. 2019, 13, 50–61. [Google Scholar]
- Djogbénou, L. Lutte antivectorielle contre le paludisme et résistance des vecteurs aux insecticides en Afrique. Méd. Trop. 2009, 69, 160–164. [Google Scholar]
- Ranson, H.; Jensen, B.; Vulule, J.M.; Wang, X.; Hemingway, J.; Collins, F.H. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Niang, E.H.A.; Konaté, L.; Diallo, M.; Faye, O.; Dia, I. Patterns of insecticide resistance and knock down resistance (kdr) in malaria vectors An. arabiensis, An. coluzzii and An. gambiae from sympatric areas in Senegal. Parasites Vectors 2016, 9, 71. [Google Scholar] [CrossRef]
- Keita, K.; Camara, D.; Barry, Y.; Ossè, R.; Wang, L.; Sylla, M.; Miller, D.; Leite, L.; Schopp, P.; Lawrence, G.G.; et al. Species identification and resistance status of Anopheles gambiae s.l. (Diptera: Culicidae) mosquitoes in Guinea. J. Med. Entomol. 2017, 54, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, J.; Yawson, A.E.; Weetman, D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae s.s. and Anopheles coluzzii across southern Ghana. Malar. J. 2013, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Keïta, M.; Kané, F.; Thiero, O.; Traoré, B.; Zeukeng, F.; Sodio, A.B.; Traoré, S.F.; Djouaka, R.; Doumbia, S.; Sogoba, N. Acetylcholinesterase (ace-1R) target site mutation G119S and resistance to carbamates in Anopheles gambiae (sensu lato) populations from Mali. Parasites Vectors 2020, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Liyanapathirana, M.; Agossa, F.R.; Weetman, D.; Ranson, H.; Donnelly, M.J.; Wilding, C.S. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2012, 109, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Oyeniyi, A.T.; Adeogun, A.O.; Idowu, E.T.; Oboh, B.; Olakiigbe, A.; Adesalu, O.; Jimoh, T.; Awolola, T.S. First report of N1575Y mutation in pyrethroid resistant Anopheles gambiae s.l. in Nigeria. Sci. Afr. 2020, 10, e00645. [Google Scholar] [CrossRef]
- Sabaly, P.; Ngom, E.H.M.; Gueye, N.A.; Gueye, A.; Diallo, M.; Dia, I. Differential insecticide resistance in Anopheles arabiensis populations in the seaside area of Mbour and its suburbs in Senegal. Heliyon 2023, 9, e21968. [Google Scholar] [CrossRef]
- Gueye, O.K.; Tchouakui, M.; Dia, A.K.; Faye, M.B.; Ahmed, A.A.; Wondji, M.J.; Nguiffo, D.N.; Mugenzi, L.M.J.; Tripet, F.; Konaté, L.; et al. Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403. [Google Scholar] [CrossRef]
- Thiaw, O.; Doucouré, S.; Sougoufara, S.; Bouganali, C.; Konaté, L.; Diagne, N.; Faye, O.; Sokhna, C. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar. J. 2018, 17, 123. [Google Scholar] [CrossRef]
- Trape, J.F.; Tall, A.; Sokhna, C.; Ly, A.B.; Diagne, N.; Ndiath, O.; Mazenot, C.; Richard, V.; Badiane, A.; Dieye-Ba, F.; et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: A 22 year longitudinal study. Lancet Infect. Dis. 2014, 14, 476–488. [Google Scholar] [CrossRef]
- Wotodjo, A.N.; Doucoure, S.; Diagne, N.; Sarr, F.D.; Parola, P.; Gaudart, J.; Sokhna, C. Another challenge in malaria elimination efforts: The increase of malaria among adults after the implementation of long-lasting insecticide-treated nets (LLINs) in Dielmo, Senegal. Malar. J. 2018, 17, 384. [Google Scholar] [CrossRef]
- Wotodjo, A.N.; Doucoure, S.; Gaudart, J.; Diagne, N.; Diene Sarr, F.; Faye, N.; Tall, A.; Raoult, D.; Sokhna, C. Malaria in Dielmo, a Senegal village: Is its elimination possible after seven years of implementation of long-lasting insecticide-treated nets? PLoS ONE 2017, 12, e0179528. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Sandfort, M.; Mbodj, A.F.; Diouf, B.; Talla, C.; Faye, J.; Sane, R.; Thiam, L.G.; Thiam, A.; Badiane, A.; et al. Fine-scale Spatiotemporal Mapping of Asymptomatic and Clinical Plasmodium falciparum Infections: Epidemiological Evidence for Targeted Malaria Elimination Interventions. Clin. Infect. Dis. 2021, 73, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Fontenille, D.; Lochouarn, L.; Diagne, N.; Sokhna, C.; Lemasson, J.J.; Diatta, M.; Konate, L.; Faye, F.; Rogier, C.; Trape, J.F. High annual and seasonal variations in malaria transmission by anophelines and vector species composition in Dielmo, a holoendemic area in Senegal. Am. J. Trop. Med. Hyg. 1997, 56, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Fontenille, D.; Lochouarn, L.; Diatta, M.; Sokhna, C.; Dia, I.; Diagne, N.; Lemasson, J.J.; Ba, K.; Tall, A.; Rogier, C.; et al. Four years’ entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 647–652. [Google Scholar] [CrossRef]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef]
- Service, M.W. Mosquito Ecology, Field Sampling Methods Vector Biology and Control, 2nd ed.; Liverpool School of Tropical Medicine: Liverpool, UK, 1993. [Google Scholar]
- WHO. Procédures Pour Tester la Résistance aux Insecticides Chez les Moustiques Vecteurs du Paludisme; WHO: Geneva, Switzerland, 2017; Available online: https://iris.who.int/bitstream/handle/10665/259741/9789242511574-fre.pdf (accessed on 6 September 2023).
- Robert, V.; Ndiaye, E.H.; Rahola, N.; Le Goff, G.; Bousses, P.; Diallo, D.; Le Goff, V.; Mariamé, L.; Diallo, M. Clés dichotomiques illustrées d’identification des femelles et des larves de moustiques (Diptera: Culicidae) du Burkina Faso, Cap-Vert, Gambie, Mali, Mauritanie, Niger, Sénégal et Tchad. 2022. Available online: https://mosquito-taxonomic-inventory.myspecies.info/node/1364 (accessed on 8 September 2023).
- Morlais, I.; Ponçon, N.; Simard, F.; Cohuet, A.; Fontenille, D. Intraspecific nucleotide variation in Anopheles gambiae: New insights into the biology of malaria vectors. Am. J. Trop. Med. Hyg. 2004, 71, 795–802. [Google Scholar] [CrossRef]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef]
- Fanello, C.; Santolamazza, F.; della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 2002, 16, 461–464. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Donnelly, M.J.; Williamson, M.S.; Ranson, H.; Ball, A.; Vontas, J.; Field, L.M. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 2007, 6, 111. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, P.; Pocquet, N.; Labbé, P.; Milesi, P. BioRssay: An R package for analyses of bioassays and probit graphs. Parasites Vectors 2022, 15, 35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; p. 145. Available online: https://www.r-project.org/ (accessed on 3 August 2023).
- Urio, N.H.; Pinda, P.G.; Ngonzi, A.J.; Muyaga, L.L.; Msugupakulya, B.J.; Finda, M.; Matanila, G.S.; Mponzi, W.; Ngowo, H.S.; Kahamba, N.F.; et al. Effects of agricultural pesticides on the susceptibility and fitness of malaria vectors in rural south-eastern Tanzania. Parasites Vectors 2020, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Sy, O.; Sarr, P.C.; Assogba, B.S.; Nourdine, M.A.; Ndiaye, A.; Konaté, L.; Faye, O.; Donnelly, M.J.; Gaye, O.; Weetman, D.; et al. Residual malaria transmission and the role of Anopheles arabiensis and Anopheles melas in central Senegal. J. Med. Entomol. 2023, 60, 546–553. [Google Scholar] [CrossRef]
- Bigoga, J.D.; Ndangoh, D.N.; Awono-Ambene, P.H.; Patchoke, S.; Fondjo, E.; Leke, R.G. Pyrethroid resistance in Anopheles gambiae from the rubber cultivated area of Niete, South Region of Cameroon. Acta Trop. 2012, 124, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kabula, B.; Tungu, P.; Malima, R.; Rowland, M.; Minja, J.; Wililo, R.; Ramsan, M.; McElroy, P.D.; Kafuko, J.; Kulkarni, M.; et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med. Vet. Entomol. 2014, 28, 244–252. [Google Scholar] [CrossRef]
- Dia, A.K.; Guèye, O.K.; Niang, E.A.; Diédhiou, S.M.; Sy, M.D.; Konaté, A.; Samb, B.; Diop, A.; Konaté, L.; Faye, O. Insecticide resistance in Anopheles arabiensis populations from Dakar and its suburbs: Role of target site and metabolic resistance mechanisms. Malar. J. 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, N.P.; Weetman, D.; Hastings, I.M. Insecticide resistance management strategies for public health control of mosquitoes exhibiting polygenic resistance: A comparison of sequences, rotations, and mixtures. Evol. Appl. 2023, 16, 936–959. [Google Scholar] [CrossRef] [PubMed]
- Agossa, F.R.; Gnanguenon, V.; Anagonou, R.; Azondekon, R.; Aïzoun, N.; Sovi, A.; Oké-Agbo, F.; Sèzonlin, M.; Akogbéto, M.C. Impact of Insecticide Resistance on the Effectiveness of Pyrethroid-Based Malaria Vectors Control Tools in Benin: Decreased Toxicity and Repellent Effect. PLoS ONE 2015, 10, e0145207. [Google Scholar] [CrossRef]
- Diallo, M.; Hamid-Adiamoh, M.; Sy, O.; Sarr, P.C.; Manneh, J.; Ndiath, M.O.; Gaye, O.; Faye, O.; Konaté, L.; Sesay, A.K.; et al. Evolution of the Pyrethroids Target-Site Resistance Mechanisms in Senegal: Early Stage of the Vgsc-1014F and Vgsc-1014S Allelic Frequencies Shift. Genes 2021, 12, 1948. [Google Scholar] [CrossRef]
- Kouamé, R.M.A.; Lynd, A.; Kouamé, J.K.I.; Vavassori, L.; Abo, K.; Donnelly, M.J.; Edi, C.; Lucas, E. Widespread occurrence of copy number variants and fixation of pyrethroid target site resistance in Anopheles gambiae (s.l.) from southern Côte d’Ivoire. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 3, 100117. [Google Scholar] [CrossRef] [PubMed]
- Stica, C.; Jeffries, C.L.; Irish, S.R.; Barry, Y.; Camara, D.; Yansane, I.; Kristan, M.; Walker, T.; Messenger, L.A. Characterizing the molecular and metabolic mechanisms of insecticide resistance in Anopheles gambiae in Faranah, Guinea. Malar. J. 2019, 18, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, M.; Tan, R.; Deng, C.; Huang, B.; Wu, Z.; Zheng, S.; Guo, W.; Tuo, F.; Yuan, Y.; et al. Presence of L1014F Knockdown-Resistance Mutation in Anopheles gambiae s.s. From São Tomé and Príncipe. Front. Cell. Infect. Microbiol. 2021, 11, 633905. [Google Scholar] [CrossRef] [PubMed]
- Koukpo, C.Z.; Fassinou, A.J.Y.H.; Ossè, R.A.; Agossa, F.R.; Sovi, A.; Sewadé, W.T.; Aboubakar, S.; Assogba, B.S.; Akogbeto, M.C.; Sezonlin, M. The current distribution and characterization of the L1014F resistance allele of the kdr gene in three malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis) in Benin (West Africa). Malar. J. 2019, 18, 175. [Google Scholar] [CrossRef]
- Kayode, F.I.; Taiwo, I.E.; Adeogun, A.O.; Olalekan, O.; Chimdalu, I.P.; Olayilola, O.I.; Amos, O.T.; Nkemeh, C.L.; Otubanjo, O.A.; Oladosu, Y.; et al. Low frequency of knockdown resistance mutation (L1014F) and the efficacy of PBO synergist in multiple insecticide-resistant populations of Anopheles gambiae in Ikorodu, Lagos State, Nigeria. Afr. Health Sci. 2023, 23, 255–261. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Manu, Y.A.; Tukur, Z.; Irving, H.; Wondji, C.S. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect. Dis. 2014, 14, 441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kabula, B.; Kisinza, W.; Tungu, P.; Ndege, C.; Batengana, B.; Kollo, D.; Malima, R.; Kafuko, J.; Mohamed, M.; Magesa, S. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop. Med. Int. Health 2014, 19, 331–341. [Google Scholar] [CrossRef]
- Kabula, B.; Tungu, P.; Rippon, E.J.; Steen, K.; Kisinza, W.; Magesa, S.; Mosha, F.; Donnelly, M.J. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 2016, 15, 289. [Google Scholar] [CrossRef]
- Meiwald, A.; Clark, E.; Kristan, M.; Edi, C.; Jeffries, C.L.; Pelloquin, B.; Irish, S.R.; Walker, T.; Messenger, L.A. Association of Reduced Long-Lasting Insecticidal Net Efficacy and Pyrethroid Insecticide Resistance With Overexpression of CYP6P4, CYP6P3, and CYP6Z1 in Populations of Anopheles coluzzii From Southeast Côte d’Ivoire. J. Infect. Dis. 2022, 225, 1424–1434. [Google Scholar] [CrossRef]
- Toé, K.H.; N’Falé, S.; Dabiré, R.K.; Ranson, H.; Jones, C.M. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015, 16, 146. [Google Scholar] [CrossRef]
- Alemayehu, E.; Asale, A.; Eba, K.; Getahun, K.; Tushune, K.; Bryon, A.; Morou, E.; Vontas, J.; Van Leeuwen, T.; Duchateau, L.; et al. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasites Vectors 2017, 10, 407. [Google Scholar] [CrossRef] [PubMed]
| Sites | Insecticides | September | October | November | December | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | MR (95% CI) | N | MR (95% CI) | N | MR (95% CI) | N | MR (95% CI) | ||
| Dielmo | Lamdacyhalothrin (0.05%) | 100 | 85 (76.5–91.4) | 100 | 62 (51.7–75.5) | 100 | 71 (61.1–79.6) | 100 | 31 (22.1–41) |
| Alphacypermethrin (0.05%) | - | - | 100 | 62 (51.7–75.5) | - | - | - | - | |
| Deltamethrin (0.05%) | - | - | - | - | 102 | 52.9 (42.8–62.9) | 99 | 75.7 (66.1–83.8) | |
| Permethrin (0.75%) | - | - | 100 | 82 (73.1–89.0) | - | - | - | - | |
| Bendiocarb (0.1%) | 100 | 100 (96.4–100) | - | - | 82 | 100 (95.6–100) | - | - | |
| Pirimiphos-methyl (0.25%) | 100 | 100 (96.4–100) | - | - | 100 | 100 (96.4–100) | 98 | 100 (96.3–100) | |
| DDT (4%) | - | - | 75 | 82.7 (72.2–90.4) | - | - | - | - | |
| Ndiop | Lamdacyhalothrin (0.05%) | 100 | 78 (68.6–85.7) | 100 | 57 (46.7–66.9) | - | - | - | - |
| Alphacypermethrin (0.05%) | - | - | 100 | 87 (78.8–92.9) | - | - | - | - | |
| Deltamethrin (0.05%) | - | - | - | - | 100 | 77 (67.5–84.8) | - | - | |
| Permethrin (0.75%) | - | - | 100 | 88 (80.0–93.6) | - | - | - | - | |
| Bendiocarb (0.1%) | 100 | 100 (96.4–100) | - | - | - | - | - | - | |
| Pirimiphos-methyl (0.25%) | 100 | 100 (96.4–100) | - | - | - | - | - | - | |
| DDT (4%) | - | - | 100 | 85 (76.5–91.4) | - | - | - | - |
| Sites | KDT | Insecticides | |||
|---|---|---|---|---|---|
| Alphacypermethrin (0.05%) | Deltamethrin (0.05%) | Lambdacyhalothrin (0.05%) | Permethrin (0.75%) | ||
| Dielmo | KDT50 (95% CI) | 39 (12–211) | 25 (8.86–117) | 33 (15–96) | 31 (14–88) |
| KDT95 (95% CI) | 98 (27–667) | 64 (19–366) | 80 (32–263) | 78 (31–258) | |
| Ndiop | KDT50 (95% CI) | 31 (11–140) | 25 (11–75) | 31 (8.9–216) | 28 (9.77–132) |
| KDT95 (95% CI) | 76 (23–428) | 58 (23–202) | 87 (21–805) | 61 (19–340) | |
| Sites | Mosquito Species | N | |||
|---|---|---|---|---|---|
| An. arabiensis | An. gambiae | An. coluzzii | Hybrids | ||
| Dielmo | 619 (84.9) | 81 (11.1) | 26 (3.6) | 3 (0.4) | 729 |
| Ndiop | 344 (83.5) | 49 (11.9) | 14 (3.4) | 5 (1.2) | 412 |
| Sites | Species | Genotypes | Allelic Frequencies (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FF | FS | LF | LL | LS | SS | F | L | S | ||
| Dielmo | An. arabiensis | 2 | 21 | 49 | 98 | 77 | 16 | 0.14 | 0.61 | 0.25 |
| An. coluzzii | 0 | 0 | 12 | 4 | 1 | 0 | 0.35 | 0.62 | 0.03 | |
| An. gambiae | 0 | 1 | 40 | 4 | 0 | 0 | 0.46 | 0.53 | 0.01 | |
| Ndiop | An. arabiensis | 1 | 16 | 28 | 48 | 50 | 6 | 0.15 | 0.58 | 0.26 |
| An. coluzzii | 0 | 1 | 4 | 4 | 0 | 0 | 0.28 | 0.67 | 0.06 | |
| An. gambiae | 0 | 0 | 25 | 8 | 0 | 0 | 0.38 | 0.62 | 0 | |
| Sites | Species | Genotypes | Allelic Frequencies | ||||
|---|---|---|---|---|---|---|---|
| Nb | NN | NY | YY | N | Y | ||
| Dielmo | An. arabiensis | 259 | 259 | 0 | 0 | 100 | 0 |
| An. gambiae | 45 | 43 | 2 | 0 | 98 | 2 | |
| An. coluzzii | 16 | 16 | 0 | 0 | 100 | 0 | |
| Ndiop | An. arabiensis | 148 | 148 | 0 | 0 | 100 | 0 |
| An. gambiae | 33 | 33 | 0 | 0 | 100 | 0 | |
| An. coluzzii | 9 | 9 | 0 | 0 | 100 | 0 | |
| Sites | Insecticides | Status | L1014F | L1014L | Total | Freq | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Dielmo | Alphacypermethrin (0.05%) | Survivors | 14 | 40 | 54 | 26 | 1 | ||
| Dead | 17 | 41 | 58 | 29 | 1.18 | 0.52–2.72 | 0.69 | ||
| Deltamethrin (0.05%) | Survivors | 15 | 83 | 98 | 15 | 1 | |||
| Dead | 10 | 90 | 100 | 10 | 0.61 | 0.26–1.44 | 0.26 | ||
| Lambdacyhalothrin (0.05%) | Survivors | 23 | 77 | 100 | 23 | 1 | |||
| Dead | 29 | 107 | 136 | 21 | 0.9 | 0.49–1.69 | 0.76 | ||
| Permethrin (0.75%) | Survivors | 10 | 26 | 36 | 28 | 1 | |||
| Dead | 9 | 51 | 60 | 15 | 0.46 | 0.17–1.27 | 0.13 | ||
| Ndiop | Alphacypermethrin (0.05%) | Survivors | 5 | 19 | 24 | 21 | 1 | ||
| Dead | 9 | 51 | 60 | 15 | 0.67 | 0.20–2.26 | 0.51 | ||
| Deltamethrin (0.05%) | Survivors | 12 | 34 | 46 | 26 | 1 | |||
| Dead | 5 | 37 | 42 | 12 | 0.38 | 0.12–1.20 | 0.09 | ||
| Lambdacyhalothrin (0.05%) | Survivors | 13 | 45 | 58 | 22 | 1 | |||
| Dead | 21 | 47 | 68 | 31 | 1.55 | 0.69–3.45 | 0.29 | ||
| Permethrin (0.75%) | Survivors | 5 | 19 | 24 | 21 | 1 | |||
| Dead | 8 | 54 | 62 | 13 | 0.56 | 0.16–1.93 | 0.36 |
| Sites | Insecticides | Statuts | L1014S | L1014L | Total | Freq | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Dielmo | Alphacypermethrin (0.05%) | Survivors | 15 | 39 | 54 | 28 | 1 | ||
| Dead | 8 | 52 | 60 | 13 | 0.4 | 0.15–1.04 | 0.06 | ||
| Deltamethrin (0.05%) | Survivors | 29 | 69 | 98 | 30 | 1 | |||
| Dead | 18 | 82 | 100 | 18 | 0.52 | 0.27–1.02 | 0.06 | ||
| Lambdacyhalothrin (0.05%) | Survivors | 29 | 73 | 102 | 28 | 1 | |||
| Dead | 14 | 126 | 140 | 10 | 0.28 | 0.14–0.56 | 0.0002 | ||
| Permethrin (0.75%) | Survivors | 11 | 25 | 36 | 31 | 1 | |||
| Dead | 9 | 51 | 60 | 15 | 0.4 | 0.15–1.09 | 0.07 | ||
| Ndiop | Alphacypermethrin (0.05%) | Survivors | 7 | 17 | 24 | 29 | 1 | ||
| Dead | 7 | 53 | 60 | 12 | 0.32 | 0.09–1.04 | 0.05 | ||
| Deltamethrin (0.05%) | Survivors | 18 | 28 | 46 | 39 | 1 | |||
| Dead | 7 | 33 | 40 | 18 | 0.33 | 0.12–0.90 | 0.03 | ||
| Lambdacyhalothrin (0.05%) | Survivors | 17 | 41 | 58 | 29 | 1 | |||
| Dead | 8 | 62 | 70 | 11 | 0.31 | 0.12–0.79 | 0.01 | ||
| Permethrin (0.75%) | Survivors | 7 | 17 | 24 | 29 | 1 | |||
| Dead | 11 | 51 | 62 | 18 | 0.52 | 0.17–1.56 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gueye, A.; Ngom, E.H.M.; Ndoye, B.B.; Dione, M.L.; Diouf, B.; Ndiaye, E.H.; Sy, F.A.; Guèye, M.; Niang, M.; Diallo, D.; et al. Insecticide Resistance and Target-Site Mutations kdr, N1575Y, and Ace-1 in Anopheles gambiae s.l. Populations in a Low-Malaria-Transmission Zone in the Sudanian Region of Senegal. Genes 2024, 15, 1331. https://doi.org/10.3390/genes15101331
Gueye A, Ngom EHM, Ndoye BB, Dione ML, Diouf B, Ndiaye EH, Sy FA, Guèye M, Niang M, Diallo D, et al. Insecticide Resistance and Target-Site Mutations kdr, N1575Y, and Ace-1 in Anopheles gambiae s.l. Populations in a Low-Malaria-Transmission Zone in the Sudanian Region of Senegal. Genes. 2024; 15(10):1331. https://doi.org/10.3390/genes15101331
Chicago/Turabian StyleGueye, Assiyatou, El Hadji Malick Ngom, Baye Bado Ndoye, Mamadou Lamine Dione, Babacar Diouf, El Hadji Ndiaye, Faty Amadou Sy, Marième Guèye, Makhtar Niang, Diawo Diallo, and et al. 2024. "Insecticide Resistance and Target-Site Mutations kdr, N1575Y, and Ace-1 in Anopheles gambiae s.l. Populations in a Low-Malaria-Transmission Zone in the Sudanian Region of Senegal" Genes 15, no. 10: 1331. https://doi.org/10.3390/genes15101331
APA StyleGueye, A., Ngom, E. H. M., Ndoye, B. B., Dione, M. L., Diouf, B., Ndiaye, E. H., Sy, F. A., Guèye, M., Niang, M., Diallo, D., Diallo, M., & Dia, I. (2024). Insecticide Resistance and Target-Site Mutations kdr, N1575Y, and Ace-1 in Anopheles gambiae s.l. Populations in a Low-Malaria-Transmission Zone in the Sudanian Region of Senegal. Genes, 15(10), 1331. https://doi.org/10.3390/genes15101331






