Abstract
Background/Objectives: Significant progress in malaria control has been achieved through long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), raising hopes for malaria elimination. However, emerging insecticide resistance threatens these gains. This study assessed the susceptibility of Anopheles gambiae s.l. populations to public health insecticides, examined the frequencies of kdr, Ace-1, and N1575Y mutations, and explored their associations with phenotypic resistance in Dielmo and Ndiop, Senegal. Methods: Anopheles larvae collected between September and December 2022 were reared to adulthood. Adult mosquitoes were exposed to discriminating concentrations of various insecticides following WHO guidelines. Knockdown times (KDT50 and KDT95) for pyrethroids were calculated using the Probit model. RT-qPCR detected target-site mutations (kdr: L1014F and L1014S, Ace-1, N1575Y) and assessed correlations with phenotypic resistance. Species-specific PCR identified species within the An. gambiae complex. Results/Conclusions: The populations of Dielmo and Ndiop showed susceptibility to pirimiphos-methyl and bendiocarb, with no Ace-1 mutation detected. Resistance to DDT and pyrethroids was observed. The knockdown times indicated that alphacypermethrin and lambdacyhalothrin were more effective than permethrin and deltamethrin. The L1014F allele was widespread, while L1014S was absent in Ndiop and low in Dielmo. The N1575Y mutation occurred only in populations with L1014F. The L1014S mutation was significantly associated with resistance to lambdacyhalothrin in both villages and to deltamethrin in Ndiop.
1. Introduction
Substantial progress in malaria control over recent decades has led to a notable decline in both incidence and mortality rates, fostering realistic optimism regarding the potential for disease elimination [1]. This success is largely attributed to the widespread implementation of strategies such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), which rely heavily on the efficacy of insecticides to reduce vector populations and interrupt malaria transmission. Despite these achievements, the emergence and spread of insecticide resistance among malaria vectors present a major challenge to sustaining these control efforts [2,3].
While efforts to explore new classes and combinations of insecticides are ongoing, pyrethroids remain the primary insecticides for treating mosquito nets due to their mosquito-repelling properties and relatively low toxicity to mammals [4]. However, extensive pyrethroid use has led to a marked decrease in their effectiveness, highlighting the urgent need for alternative solutions. Resistance is driven by the selection of resistant alleles, giving certain mosquitoes a survival advantage in environments with high insecticide levels [5], thereby posing a significant threat to future malaria control efforts [6].
Resistance to pyrethroids, DDT, and carbamates has been documented across Africa among Anopheles populations [7,8]. These mosquitoes have developed various survival mechanisms against lethal insecticide doses [9]. These resistance mechanisms include genetic mutations that alter insecticide target sites and enhanced metabolic detoxification pathways.
Knockdown resistance (kdr) mutations, such as L1014F and L1014S, are well-documented contributors to resistance against pyrethroids and DDT and are prevalent in regions across Africa, including West and East Africa [10,11,12]. These mutations disrupt the sodium channel proteins targeted by pyrethroids, leading to cross-resistance. Other target-site mutations, such as G119S in the Ace-1 gene, are associated with resistance to carbamates and organophosphates in An. gambiae s.l. populations [13,14]. Additionally, the N1575Y mutation, located in intracytoplasmic loop linking domains III and IV of sodium channels, has been identified in populations of An. gambiae s.l. carrying the L1014F mutation, further complicating resistance profiles [15,16].
In Senegal, particularly within the Sudanian zone, the dynamics of insecticide resistance and the prevalence of these mutations are not fully understood. Previous studies have highlighted significant resistance to DDT and pyrethroids, but comprehensive data on specific mutations and their roles in resistance remain limited [17,18]. This knowledge gap is critical, as understanding resistance mechanisms in local populations is essential for optimizing vector control strategies. More specifically, in Dielmo village, prior investigations indicated the absence of the kdr L1014S mutation in pyrethroid-resistant An. gambiae populations, with only Anopheles arabiensis and Anopheles coluzzii populations exhibiting the L1014F and L1014S alleles [19]. This suggests that other resistance mechanisms, potentially involving different target sites or metabolic pathways, may be at play in these populations. Additionally, the absence of comprehensive data on the N1575Y and Ace-1 mutations in the Sudanian zone further underscores the need for detailed studies.
Given these evolving resistance dynamics, this study aims to (1) evaluate the current insecticide susceptibility profiles of Anopheles populations from Dielmo and Ndiop to commonly used insecticides, including organochlorines, organophosphates, carbamates, and pyrethroids; (2) assess the frequency and distribution of the key target-site mutations L1014F, L1014S, N1575Y, and G119S; and (3) identify any associations between these genetic markers and the observed resistance phenotypes.
2. Materials and Methods
2.1. Study Area
This study was conducted in two locations in the Sudanian zone of Senegal: Dielmo (13°43′22.2″ N, 16°24′40.1″ W) and Ndiop (13°41′12.8″ N, 16°23′3.2″ W) (Figure 1). Longitudinal studies on malaria determinants have been ongoing in these locations since 1990 and 1993, respectively [20]. Over the years, the epidemiology of malaria has changed significantly, leading to considerations of disease elimination in this area [20,21]. However, a resurgence of malaria cases has been observed in both villages [22,23].
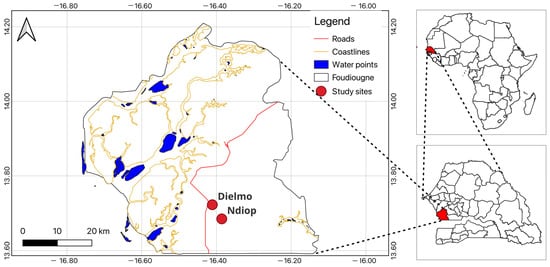
Figure 1.
Map of the study area.
In Dielmo, a small river (Nema) flows year-round, ensuring the constant presence of vectors [24]. In contrast, malaria transmission in Ndiop is seasonal, with vector breeding sites dependent on rainfall [25].
The main economic activity in both villages is agriculture, focused on groundnuts, cashew nuts, mangoes, and vegetables. Consequently, commercial pesticides, including organophosphates, organochlorines, and pyrethroids, are commonly used.
Malaria transmission in Dielmo and Ndiop is primarily maintained by species from the An. gambiae complex, including An. arabiensis, An. gambiae, and An. coluzzii, as well as An. funestus [26].
The two villages were selected based on the persistence of malaria transmission despite the control measures implemented.
2.2. Mosquito Collection and Rearing
Anopheles mosquito larvae were collected over two consecutive days each month from various breeding sites between September and December 2022 from 8:00 am to noon using the methods outlined by Service [27]. Visits were conducted to each site, covering different types of breeding habitats such as river banks, ponds, water tanks, and rain puddles. Upon detecting productive sites, Anopheles larvae and pupae were collected and sorted into bins according to their habitat type and location. These specimens were transported to the laboratory and reared under standard insectary conditions of 70% to 80% relative humidity and 27 °C to 28 °C ambient temperature. Larvae were fed with Tetramin® baby fish food, and emerging adult mosquitoes were provided with a 10% glucose solution before being exposed to insecticides.
2.3. WHO Insecticide Susceptibility Bioassays
Susceptibility assays were conducted following the World Health Organization protocol [28], using three-to-five-day-old unfed female mosquitoes whenever available. Seven insecticides were tested: four pyrethroids (deltamethrin 0.05%, lambdacyhalothrin 0.05%, alphacypermethrin 0.05%, and permethrin 0.75%), one organochlorine (DDT 4%), one organophosphate (pirimiphos-methyl 0.25%), and one carbamate (bendiocarb 0.1%).
Batches of 20 to 26 females were exposed to insecticide-impregnated papers for one hour. For each test, two batches, each consisting of 20 to 25 females, were exposed to untreated papers as controls. The number of knocked-down mosquitoes was recorded after 10, 15, 20, 30, 40, 50, and 60 min of exposure. Following the one-hour exposure period, the mosquitoes were transferred to observation tubes and provided with a 10% glucose solution. The mortality rates of the females were assessed 24 h later.
2.4. Mosquito Species Identification
All An. gambiae s.l. specimens exposed to insecticides underwent species-level identification using a binocular microscope and the morphological identification key of Robert et al. [29]. They were then preserved in Eppendorf tubes containing silica gel and cotton for further analysis.
2.5. Molecular Identification and Genotyping of kdr and Ace-1 Mutations by PCR
Among the tested females, for each month, up to 60 individuals, with 30 per status (dead or alive), were selected for each insecticide for further molecular identification. DNA extraction was performed using the cetyl-trimethyl-ammonium-bromide (CTAB) 2% method [30]. Each specimen was ground using a sterile pestle in 200 μL of CTAB 2%. The tubes were then incubated in a dry bath at 65 °C for 5 min. After cooling, 200 μL of chloroform was added to each tube, mixed thoroughly, and centrifuged at 12,000 rpm for 5 min. The supernatant was transferred to a new tube, and 200 µL of isopropanol was added. The tubes were centrifuged at 12,000 rpm for 15 min, and the supernatant was discarded. The DNA pellet was washed with 200 μL of 70% ethanol, centrifuged at 12,000 rpm for 5 min, air-dried, resuspended in 40 μL of sterile pure water, and stored at −20 °C after overnight incubation at room temperature.
The extracted DNA was used for molecular identification of species within the An. gambiae complex, following the techniques described by Scott et al. [31] and Fanello et al. [32]. The quantitative Polymerase Chain Reaction (qPCR) technique with the Luna Universal Probe assay from New England Biolabs was employed to detect the L1014F, L1014S, N1575Y, and Ace-1 mutations. Genotyping followed protocols by Bass et al. [33] for L1014F (kdr-West) and L1014S (kdr-East), Jones and colleagues [15] for the N1575Y mutation, and Bass and colleagues [34] for the Ace-1 mutation.
2.6. Data Analysis
Mosquito mortality was evaluated at 24 h post-exposure, with validity and susceptibility status determined according to WHO criteria [28]. Tests were considered valid if control mortality was below 5%, required correction using the Abbott formula [35] if between 5% and 20%, and invalid if above 20%.
Population resistance classification was based on mortality rates: confirmed resistance (≤90% mortality), possible resistance (90% and 98%), and susceptibility (≥98%). Mortality rates and allele frequencies were estimated for each population, and data analysis was performed with a 95% confidence interval. Knockdown times (KDT50 and KDT95) were calculated using the Probit model with a 95% confidence interval using the BioRssay package [36]. Comparisons between Anopheles species abundance and mortality rates were performed using the Chi-squared test or Fisher’s exact test, with a significance threshold of 0.05.
Comparisons of kdr mutation frequencies were conducted based on survival or mortality status following the tests using the fmsb package available in the R software (https://cran.r-project.org/web/packages/fmsb/index.html, accessed on 13 September 2023). The relationship between kdr mutations (L1014F and L1014S) and pyrethroid resistance was analyzed using allelic association analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to determine the strength of association between kdr mutations and resistance, with statistical significance determined using a p-value of <0.05.
All statistical analyses and graphs were created using R software version 4.3.2. [37].
3. Results
3.1. Insecticide Resistance Profile
Insecticide susceptibility tests on Anopheles populations from Dielmo and Ndiop are summarized in Table 1. In all bioassays, the control tests showed mortality rates below 5%, so no corrections were required in the tested sample data. A total of 2139 specimens were exposed to the WHO-recommended discriminating concentrations. Anopheles mosquitoes showed complete susceptibility to pirimiphos-methyl and bendiocarb at both sites. However, resistance to DDT and pyrethroids was observed, with mortality rates ranging from 31% to 88% (Table 1).

Table 1.
Percentage mortality rates of An. gambiae s.l. populations from Dielmo and Ndiop, Senegal, exposed to different insecticides from September to December 2022.
Mortality rates with lambdacyhalothrin decreased over time, from September to December in Dielmo and from September to October in Ndiop (Table 1). This decrease was statistically significant (p < 0.05) at both sites. Conversely, a significant increase in mortality rates with deltamethrin was observed in Dielmo between November and December (Table 1).
3.2. Knockdown Effects and Dynamics of Pyrethroids
Table 2 presents the mean knockdown times (KDT50 and KDT95) for 50% and 95% of mosquitoes, respectively. In both sites, deltamethrin had the shortest KDT50 times, at 25 min, followed by permethrin at 28 min for Ndiop and 31 min for Dielmo. Similar profiles were observed for KDT95 (Table 2).

Table 2.
Knockdown times in minutes for An. gambiae s.l. populations from Dielmo and Ndiop to different insecticides from September to December 2022.
The dynamics of pyrethroid action showed that resistance was associated with a low insecticide action dynamic (Figure 2). None of the four tested pyrethroids achieved 100% knockdown after 60 min of exposure. The highest mean knockdown (KD) values were observed with deltamethrin in Dielmo at 50 and 60 min (90.4%) and in Ndiop at 60 min (94%), as well as with permethrin at 88% in Dielmo and 91% in Ndiop after 60 min. Conversely, alphacypermethrin showed the lowest knockdown rates.
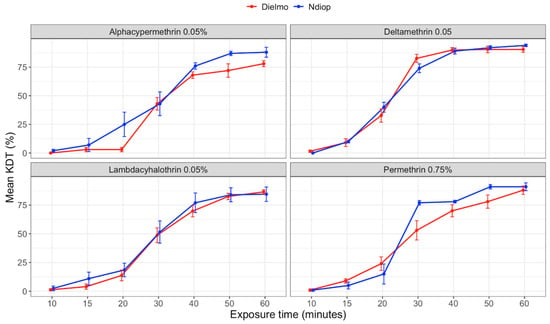
Figure 2.
Variations in mean pyrethroid KDT rates according to exposure times for An. gambiae s.l. populations from Dielmo and Ndiop to different insecticides from September to December 2022.
3.3. Species Composition
Species-specific PCR analysis on 729 mosquitoes from Dielmo and 412 mosquitoes from Ndiop (out of the 2139 specimens exposed to insecticides) identified An. arabiensis as the predominant species, comprising 84.9% in Dielmo and 83.5% in Ndiop. An. gambiae was the second most abundant species, while An. coluzzii was less common, accounting for 3.6% in Dielmo and 3.4% in Ndiop. Hybrids between An. gambiae and An. coluzzii were rare, with only three individuals (0.4%) in Dielmo and five individuals (1.2%) in Ndiop (Table 3). The species proportions were similar in both sites, with no statistically significant difference (p > 0.05).

Table 3.
Species composition within An. gambiae complex in Dielmo and Ndiop, Senegal, from September to December 2022.
3.4. Genotypic and Allelic Frequencies of the L1014F and L1014S Mutations
All six genotypes at the 1014 locus were identified in An. arabiensis populations, while four were found in An. coluzzii and three in An. gambiae (Table 4). The homozygous wild-type LL and heterozygous LS genotypes were most common in An. arabiensis, with frequencies of 37.3% and 29.3% in Dielmo and 32% and 34% in Ndiop, respectively. In these populations, 32.6% (106/325) in Dielmo and 31.4% (60/191) in Ndiop were the homozygous susceptible LL genotype (Table 4). Heterozygotes resistant to the L1014F mutation constituted 31.1% (101/325) in Dielmo and 29.8% (57/191) in Ndiop, while resistance to the L1014S mutation was observed in 24% (78/325) and 26.2% (50/191), respectively.

Table 4.
Genotypic and allelic frequencies of kdr mutations in the populations of An. gambiae complex species in Dielmo and Ndiop, Senegal, from September to December 2022.
The L1014F allele was present in all populations from both sites, whereas the L1014S allele was absent in An. gambiae females from Ndiop and found at a low frequency (1%) in Dielmo (Table 4). Among An. coluzzii and An. gambiae, the L1014F allele was more frequent than the L1014S allele, while in An. arabiensis, the 1014S allele was predominant. No significant difference in L1014F allele frequencies was observed between the two sites for each species.
3.5. Genotypic and Allelic Frequencies of the N1575Y and Ace-1 Mutations
Overall, the N1575Y mutation was absent in An. arabiensis and An. coluzzii at both sites and present at a low frequency in An. gambiae from Dielmo (2% in two specimens). The homozygous resistant genotype YY was not observed in any species at either site.
In An. arabiensis, the wild-type N allele was present at a frequency of 100% in both Dielmo and Ndiop. Similarly, An. coluzzii populations also showed a 100% frequency at both sites. For An. gambiae, the N allele frequency was 98% in Dielmo and 100% in Ndiop, with the Y allele detected only in Dielmo (Table 5).

Table 5.
Genotypic and allelic frequencies of the N1575Y mutation in the populations of An. gambiae complex species in Dielmo and Ndiop, Senegal, from September to December 2022.
The Ace-1 mutation was investigated in 241 specimens of An. gambiae s.l. from Dielmo and Ndiop. All these specimens were susceptible to bendiocarb and pirimiphos-methyl. The Ace-1 mutation was not identified in any of the tested specimens.
3.6. Association between the L1014F and L1014S Mutations and Pyrethroid Resistance
The analysis of the L1014F and L1014S mutations in relation to pyrethroid resistance in An. gambiae s.l. populations from Dielmo and Ndiop revealed significant associations for certain combinations of mutations and insecticides.
For the L1014F mutation, no significant association with resistance to the tested pyrethroids—alphacypermethrin, deltamethrin, lambdacyhalothrin, and permethrin—was observed at either sites. The odds ratios (ORs) for L1014F varied but did not indicate a strong relationship with insecticide resistance (Table 6), suggesting that this mutation is not a major determinant of pyrethroid resistance in these populations.

Table 6.
Association between the L1014F mutation and pyrethroid resistance in the populations of An. gambiae complex species in Dielmo and Ndiop, Senegal, from September to December 2022.
In contrast, the L1014S mutation was significantly associated with resistance to some pyrethroids. In Ndiop, significant correlations were found between the L1014S mutation and resistance to lambdacyhalothrin (p = 0.01) and deltamethrin (p = 0.03). In Dielmo, this mutation was notably linked to resistance to lambdacyhalothrin (p = 0.0002). However, no significant correlation was observed between the L1014S mutation and resistance to alphacypermethrin and permethrin at either site or to deltamethrin in Dielmo (p > 0.05) (Table 7). The odds ratios indicated that individuals with the L1014S mutation were more likely to exhibit resistance to these pyrethroids.

Table 7.
Correlation between the L1014S mutation and pyrethroid resistance in the populations of An. gambiae complex species in Dielmo and Ndiop, Senegal, from September to December 2022.
4. Discussion
This study aimed to evaluate the insecticide resistance profiles of species within the An. gambiae complex and to determine the frequencies of the kdr (L1014F and L1014S), Ace-1, and N1575Y mutations in the Sudanian zone of Senegal. Species-specific PCR analysis identified An. arabiensis as the predominant species at both study sites, followed by An. gambiae and An. coluzzii. This composition is consistent with other studies in West Africa, where An. arabiensis is frequently reported as the dominant vector species in areas with significant agricultural activity [17,38,39]. The similarity in species proportions between the sites suggests a stable vector community structure, crucial for developing targeted vector control interventions. This stability aligns with the adaptability of An. arabiensis to arid environments and its preference for habitats such as riverbanks and ponds, which are primary breeding sites in our study area [19].
Insecticide susceptibility tests revealed resistance to DDT and pyrethroids, with mortality rates ranging from 31% to 88%. These findings are consistent with numerous studies across Africa documenting widespread resistance to these insecticides in Anopheles populations [28]. Similar results have been observed across different regions of Senegal [11,17,18]. This cross-resistance to pyrethroids and DDT may be attributed to historical insecticide use in vector control and agricultural practices [18,40,41,42]. Resistance to pyrethroids is particularly concerning given their widespread use in long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), cornerstone strategies for malaria vector control [28]. The observed decrease in mortality rates with lambdacyhalothrin over time in both Dielmo and Ndiop highlights the dynamic nature of insecticide resistance and suggests that continuous monitoring and timely adaptation of vector control strategies are essential. This trend mirrors findings from other regions, where prolonged use of pyrethroids has led to significant increases in resistant mosquito populations [11,17,18]. The concurrent use of pyrethroids in insecticide-treated bed nets [21] and the intense agricultural use of pyrethroids likely contribute to the observed resistance in Dielmo and Ndiop.
The complete susceptibility of the mosquito populations to pirimiphos-methyl and bendiocarb underscores the potential utility of these insecticides in managing resistance and controlling malaria transmission. In contrast, resistance to bendiocarb and pirimiphos-methyl has been observed in other regions of Senegal, such as urban Dakar and in the Sudanian zone [19,42].
Interestingly, while the populations were resistant, we observed a significant increase in the mortality rates with deltamethrin in Dielmo between November and December. This difference could be attributed to seasonal variations in mosquito exposure to insecticides or the implementation of effective resistance management strategies, shown to delay resistance onset and restore susceptibility in vector populations [43].
The knockdown times (KDT50 and KDT95) for the tested pyrethroids revealed a low insecticide action dynamic, with none of the four pyrethroids achieving 100% knockdown after 60 min of exposure. Deltamethrin and permethrin had the shortest KDT50 times, indicating a quicker knockdown effect compared to other pyrethroids. However, the overall low knockdown rates reflect the reduced efficacy of these insecticides in the studied populations, indicative of the resistance mechanisms that impair their immediate action [2].
Genotypic analysis of the L1014F and L1014S mutations in An. gambiae s.l. populations revealed interesting patterns. All six genotypes described at the 1014 locus were found in An. arabiensis populations, while only four were present in An. coluzzii and three in An. gambiae. The predominance of the L1014F allele across An. gambiae and An. coluzzii populations and the relatively low frequency of the L1014S allele are consistent with other findings documenting the widespread presence of L1014F in malaria vectors across Africa [42,44]. This predominance of the L1014F mutation within these populations in our study is consistent with prior findings in Senegal [18,45] and elsewhere [46,47]. The geographic variability in the distribution of the L1014S mutation, being more prevalent in Ndiop than in Dielmo, underscores the need for localized studies to understand specific resistance profiles in different areas [11,48,49].
The association between kdr mutations and pyrethroid resistance showed that the L1014F mutation was not significantly correlated with resistance to the tested pyrethroids. This is consistent with previous studies indicating that while L1014F contributes to resistance, it may not always be the primary determinant of resistance levels [19,50]. Conversely, the L1014S mutation showed significant associations with resistance to lambdacyhalothrin and deltamethrin, particularly in Ndiop. This suggests that the L1014S mutation plays a more critical role in conferring resistance to specific pyrethroids in these populations [51,52,53]. The lack of significant correlation between the L1014S mutation and resistance to alphacypermethrin and permethrin in Dielmo further highlights the complexity of resistance mechanisms and the possible involvement of other genetic or metabolic factors [54].
Regarding the N1575Y mutation, initially reported by Gueye et al. [18] in Senegal, this mutation was exclusively identified in the heterozygous state in Dielmo and was present in only two An. gambiae specimens. This mutation has also been reported in other studies in West Africa [15,55]. However, its absence in An. arabiensis populations at both sites is consistent with findings from Ethiopia [15,56]. The low frequency of this mutation suggests that it may not yet play a significant role in resistance in the studied populations. Similarly, the absence of the Ace-1 mutation in the tested specimens indicates that this mutation is not currently contributing to resistance to carbamates and organophosphates in these populations.
Overall, our findings underscore the importance of continuous surveillance and localized strategies to manage insecticide resistance. The complex interplay of different resistance mechanisms, including target-site mutations and metabolic detoxification, necessitates an integrated approach to vector control. Future studies should focus on elucidating the roles of additional genetic mutations and metabolic pathways in resistance and evaluating the effectiveness of alternative insecticides and integrated vector management strategies.
5. Conclusions
The high levels of resistance to pyrethroids and DDT in Anopheles populations from Dielmo and Ndiop, coupled with the presence of kdr mutations, pose significant challenges to malaria control efforts. This study highlights the critical need to assess the expression level of detoxification genes involved in metabolic resistance and to investigate behavioral resistance in vectors for adaptive management strategies. Continuous resistance monitoring is essential to sustain gains in malaria control and work towards malaria elimination. This is particularly important as some species exhibiting resistance to pyrethroids were not carriers of kdr or N1575Y mutations. A comprehensive understanding of this mechanism is crucial for the design of targeted and sustainable vector control interventions.
Author Contributions
I.D. and A.G. conceived and designed the study. A.G., E.H.M.N., B.B.N., M.L.D., F.A.S. and M.G. led the entomology field activities and participated in data collection. I.D., A.G., B.B.N., E.H.N. and B.D. carried out the molecular analysis. I.D. and A.G. analyzed the data and wrote the manuscript with contributions from D.D., B.D., M.N., M.D., I.D. and A.G. drafted the original manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European and Developing Countries Clinical Trials Partnership (Grant no. TMA2018SF-2468) and the Ministère de la Recherche Scientifique et de l’Innovation du Senegal through the project SGCI2-CRDI-MESRI.
Institutional Review Board Statement
The study presented here was conducted in mosquito breeding sites around villages. It did not involve endangered or protected species or humans. No specific permission was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this study have been presented within this article, and any further information regarding this study can be reasonably requested from the corresponding author.
Acknowledgments
The authors would like to thank the populations of Dielmo and Ndiop for facilitating our study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; Available online: https://iris.who.int/handle/10665/252038 (accessed on 11 September 2023).
- Tene-Fossog, B.; Fotso-Toguem, Y.G.; Amvongo-Adjia, N.; Ranson, H.; Wondji, C.S. Temporal variation of high-level pyrethroid resistance in the major malaria vector Anopheles gambiae s.l. in Yaoundé, Cameroon, is mediated by target-site and metabolic resistance. Med. Vet. Entomol. 2022, 36, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; McKenzie, F.E. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar. J. 2016, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Hodoșan, C.; Gîrd, C.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Nistor, L.; Bărbuică, I.S.; Marin, Ș.C.; Mihalache, A.; Popa, L. Pyrethrins and Pyrethroids: A Comprehensive Review of Natural Occurring Compounds and Their Synthetic Derivatives. Plants 2023, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- Namountougou, M.; Simard, F.; Baldet, T.; Diabaté, A.; Ouédraogo, J.B.; Martin, T.; Dabiré, R.K. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS ONE 2012, 7, e48412. [Google Scholar] [CrossRef]
- Yadouleton, A.W.; Padonou, G.; Asidi, A.; Moiroux, N.; Bio-Banganna, S.; Corbel, V.; N’guessan, R.; Gbenou, D.; Yacoubou, I.; Gazard, K.; et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar. J. 2010, 9, 83. [Google Scholar] [CrossRef]
- Oyewole, I.O.; Mustapha, K.; Adedeji, O.C.; Adeogun, D.; Awolola, S. Susceptibility pattern of Anopheles mosquito to different classes of insecticides in selected communities in Ila-Orangun, Southwest Nigeria. Int. J. Mosq. Res. 2018, 5, 106–111. [Google Scholar]
- Olatunbosun-Oduola, A.; Abba, E.; Adelaja, O.; Taiwo-Ande, A.; Poloma-Yoriyo, K.; Samson-Awolola, T. Widespread Report of Multiple Insecticide Resistance in Anopheles gambiae s.l. Mosquitoes in Eight Communities in Southern Gombe, North-Eastern Nigeria. J. Arthropod-Borne Dis. 2019, 13, 50–61. [Google Scholar]
- Djogbénou, L. Lutte antivectorielle contre le paludisme et résistance des vecteurs aux insecticides en Afrique. Méd. Trop. 2009, 69, 160–164. [Google Scholar]
- Ranson, H.; Jensen, B.; Vulule, J.M.; Wang, X.; Hemingway, J.; Collins, F.H. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Niang, E.H.A.; Konaté, L.; Diallo, M.; Faye, O.; Dia, I. Patterns of insecticide resistance and knock down resistance (kdr) in malaria vectors An. arabiensis, An. coluzzii and An. gambiae from sympatric areas in Senegal. Parasites Vectors 2016, 9, 71. [Google Scholar] [CrossRef]
- Keita, K.; Camara, D.; Barry, Y.; Ossè, R.; Wang, L.; Sylla, M.; Miller, D.; Leite, L.; Schopp, P.; Lawrence, G.G.; et al. Species identification and resistance status of Anopheles gambiae s.l. (Diptera: Culicidae) mosquitoes in Guinea. J. Med. Entomol. 2017, 54, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, J.; Yawson, A.E.; Weetman, D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae s.s. and Anopheles coluzzii across southern Ghana. Malar. J. 2013, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Keïta, M.; Kané, F.; Thiero, O.; Traoré, B.; Zeukeng, F.; Sodio, A.B.; Traoré, S.F.; Djouaka, R.; Doumbia, S.; Sogoba, N. Acetylcholinesterase (ace-1R) target site mutation G119S and resistance to carbamates in Anopheles gambiae (sensu lato) populations from Mali. Parasites Vectors 2020, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Liyanapathirana, M.; Agossa, F.R.; Weetman, D.; Ranson, H.; Donnelly, M.J.; Wilding, C.S. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2012, 109, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Oyeniyi, A.T.; Adeogun, A.O.; Idowu, E.T.; Oboh, B.; Olakiigbe, A.; Adesalu, O.; Jimoh, T.; Awolola, T.S. First report of N1575Y mutation in pyrethroid resistant Anopheles gambiae s.l. in Nigeria. Sci. Afr. 2020, 10, e00645. [Google Scholar] [CrossRef]
- Sabaly, P.; Ngom, E.H.M.; Gueye, N.A.; Gueye, A.; Diallo, M.; Dia, I. Differential insecticide resistance in Anopheles arabiensis populations in the seaside area of Mbour and its suburbs in Senegal. Heliyon 2023, 9, e21968. [Google Scholar] [CrossRef]
- Gueye, O.K.; Tchouakui, M.; Dia, A.K.; Faye, M.B.; Ahmed, A.A.; Wondji, M.J.; Nguiffo, D.N.; Mugenzi, L.M.J.; Tripet, F.; Konaté, L.; et al. Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403. [Google Scholar] [CrossRef]
- Thiaw, O.; Doucouré, S.; Sougoufara, S.; Bouganali, C.; Konaté, L.; Diagne, N.; Faye, O.; Sokhna, C. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar. J. 2018, 17, 123. [Google Scholar] [CrossRef]
- Trape, J.F.; Tall, A.; Sokhna, C.; Ly, A.B.; Diagne, N.; Ndiath, O.; Mazenot, C.; Richard, V.; Badiane, A.; Dieye-Ba, F.; et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: A 22 year longitudinal study. Lancet Infect. Dis. 2014, 14, 476–488. [Google Scholar] [CrossRef]
- Wotodjo, A.N.; Doucoure, S.; Diagne, N.; Sarr, F.D.; Parola, P.; Gaudart, J.; Sokhna, C. Another challenge in malaria elimination efforts: The increase of malaria among adults after the implementation of long-lasting insecticide-treated nets (LLINs) in Dielmo, Senegal. Malar. J. 2018, 17, 384. [Google Scholar] [CrossRef]
- Wotodjo, A.N.; Doucoure, S.; Gaudart, J.; Diagne, N.; Diene Sarr, F.; Faye, N.; Tall, A.; Raoult, D.; Sokhna, C. Malaria in Dielmo, a Senegal village: Is its elimination possible after seven years of implementation of long-lasting insecticide-treated nets? PLoS ONE 2017, 12, e0179528. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Sandfort, M.; Mbodj, A.F.; Diouf, B.; Talla, C.; Faye, J.; Sane, R.; Thiam, L.G.; Thiam, A.; Badiane, A.; et al. Fine-scale Spatiotemporal Mapping of Asymptomatic and Clinical Plasmodium falciparum Infections: Epidemiological Evidence for Targeted Malaria Elimination Interventions. Clin. Infect. Dis. 2021, 73, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Fontenille, D.; Lochouarn, L.; Diagne, N.; Sokhna, C.; Lemasson, J.J.; Diatta, M.; Konate, L.; Faye, F.; Rogier, C.; Trape, J.F. High annual and seasonal variations in malaria transmission by anophelines and vector species composition in Dielmo, a holoendemic area in Senegal. Am. J. Trop. Med. Hyg. 1997, 56, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Fontenille, D.; Lochouarn, L.; Diatta, M.; Sokhna, C.; Dia, I.; Diagne, N.; Lemasson, J.J.; Ba, K.; Tall, A.; Rogier, C.; et al. Four years’ entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 647–652. [Google Scholar] [CrossRef]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef]
- Service, M.W. Mosquito Ecology, Field Sampling Methods Vector Biology and Control, 2nd ed.; Liverpool School of Tropical Medicine: Liverpool, UK, 1993. [Google Scholar]
- WHO. Procédures Pour Tester la Résistance aux Insecticides Chez les Moustiques Vecteurs du Paludisme; WHO: Geneva, Switzerland, 2017; Available online: https://iris.who.int/bitstream/handle/10665/259741/9789242511574-fre.pdf (accessed on 6 September 2023).
- Robert, V.; Ndiaye, E.H.; Rahola, N.; Le Goff, G.; Bousses, P.; Diallo, D.; Le Goff, V.; Mariamé, L.; Diallo, M. Clés dichotomiques illustrées d’identification des femelles et des larves de moustiques (Diptera: Culicidae) du Burkina Faso, Cap-Vert, Gambie, Mali, Mauritanie, Niger, Sénégal et Tchad. 2022. Available online: https://mosquito-taxonomic-inventory.myspecies.info/node/1364 (accessed on 8 September 2023).
- Morlais, I.; Ponçon, N.; Simard, F.; Cohuet, A.; Fontenille, D. Intraspecific nucleotide variation in Anopheles gambiae: New insights into the biology of malaria vectors. Am. J. Trop. Med. Hyg. 2004, 71, 795–802. [Google Scholar] [CrossRef]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef]
- Fanello, C.; Santolamazza, F.; della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 2002, 16, 461–464. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Donnelly, M.J.; Williamson, M.S.; Ranson, H.; Ball, A.; Vontas, J.; Field, L.M. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 2007, 6, 111. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, P.; Pocquet, N.; Labbé, P.; Milesi, P. BioRssay: An R package for analyses of bioassays and probit graphs. Parasites Vectors 2022, 15, 35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; p. 145. Available online: https://www.r-project.org/ (accessed on 3 August 2023).
- Urio, N.H.; Pinda, P.G.; Ngonzi, A.J.; Muyaga, L.L.; Msugupakulya, B.J.; Finda, M.; Matanila, G.S.; Mponzi, W.; Ngowo, H.S.; Kahamba, N.F.; et al. Effects of agricultural pesticides on the susceptibility and fitness of malaria vectors in rural south-eastern Tanzania. Parasites Vectors 2020, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Sy, O.; Sarr, P.C.; Assogba, B.S.; Nourdine, M.A.; Ndiaye, A.; Konaté, L.; Faye, O.; Donnelly, M.J.; Gaye, O.; Weetman, D.; et al. Residual malaria transmission and the role of Anopheles arabiensis and Anopheles melas in central Senegal. J. Med. Entomol. 2023, 60, 546–553. [Google Scholar] [CrossRef]
- Bigoga, J.D.; Ndangoh, D.N.; Awono-Ambene, P.H.; Patchoke, S.; Fondjo, E.; Leke, R.G. Pyrethroid resistance in Anopheles gambiae from the rubber cultivated area of Niete, South Region of Cameroon. Acta Trop. 2012, 124, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Kabula, B.; Tungu, P.; Malima, R.; Rowland, M.; Minja, J.; Wililo, R.; Ramsan, M.; McElroy, P.D.; Kafuko, J.; Kulkarni, M.; et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med. Vet. Entomol. 2014, 28, 244–252. [Google Scholar] [CrossRef]
- Dia, A.K.; Guèye, O.K.; Niang, E.A.; Diédhiou, S.M.; Sy, M.D.; Konaté, A.; Samb, B.; Diop, A.; Konaté, L.; Faye, O. Insecticide resistance in Anopheles arabiensis populations from Dakar and its suburbs: Role of target site and metabolic resistance mechanisms. Malar. J. 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, N.P.; Weetman, D.; Hastings, I.M. Insecticide resistance management strategies for public health control of mosquitoes exhibiting polygenic resistance: A comparison of sequences, rotations, and mixtures. Evol. Appl. 2023, 16, 936–959. [Google Scholar] [CrossRef] [PubMed]
- Agossa, F.R.; Gnanguenon, V.; Anagonou, R.; Azondekon, R.; Aïzoun, N.; Sovi, A.; Oké-Agbo, F.; Sèzonlin, M.; Akogbéto, M.C. Impact of Insecticide Resistance on the Effectiveness of Pyrethroid-Based Malaria Vectors Control Tools in Benin: Decreased Toxicity and Repellent Effect. PLoS ONE 2015, 10, e0145207. [Google Scholar] [CrossRef]
- Diallo, M.; Hamid-Adiamoh, M.; Sy, O.; Sarr, P.C.; Manneh, J.; Ndiath, M.O.; Gaye, O.; Faye, O.; Konaté, L.; Sesay, A.K.; et al. Evolution of the Pyrethroids Target-Site Resistance Mechanisms in Senegal: Early Stage of the Vgsc-1014F and Vgsc-1014S Allelic Frequencies Shift. Genes 2021, 12, 1948. [Google Scholar] [CrossRef]
- Kouamé, R.M.A.; Lynd, A.; Kouamé, J.K.I.; Vavassori, L.; Abo, K.; Donnelly, M.J.; Edi, C.; Lucas, E. Widespread occurrence of copy number variants and fixation of pyrethroid target site resistance in Anopheles gambiae (s.l.) from southern Côte d’Ivoire. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 3, 100117. [Google Scholar] [CrossRef] [PubMed]
- Stica, C.; Jeffries, C.L.; Irish, S.R.; Barry, Y.; Camara, D.; Yansane, I.; Kristan, M.; Walker, T.; Messenger, L.A. Characterizing the molecular and metabolic mechanisms of insecticide resistance in Anopheles gambiae in Faranah, Guinea. Malar. J. 2019, 18, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, M.; Tan, R.; Deng, C.; Huang, B.; Wu, Z.; Zheng, S.; Guo, W.; Tuo, F.; Yuan, Y.; et al. Presence of L1014F Knockdown-Resistance Mutation in Anopheles gambiae s.s. From São Tomé and Príncipe. Front. Cell. Infect. Microbiol. 2021, 11, 633905. [Google Scholar] [CrossRef] [PubMed]
- Koukpo, C.Z.; Fassinou, A.J.Y.H.; Ossè, R.A.; Agossa, F.R.; Sovi, A.; Sewadé, W.T.; Aboubakar, S.; Assogba, B.S.; Akogbeto, M.C.; Sezonlin, M. The current distribution and characterization of the L1014F resistance allele of the kdr gene in three malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis) in Benin (West Africa). Malar. J. 2019, 18, 175. [Google Scholar] [CrossRef]
- Kayode, F.I.; Taiwo, I.E.; Adeogun, A.O.; Olalekan, O.; Chimdalu, I.P.; Olayilola, O.I.; Amos, O.T.; Nkemeh, C.L.; Otubanjo, O.A.; Oladosu, Y.; et al. Low frequency of knockdown resistance mutation (L1014F) and the efficacy of PBO synergist in multiple insecticide-resistant populations of Anopheles gambiae in Ikorodu, Lagos State, Nigeria. Afr. Health Sci. 2023, 23, 255–261. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Manu, Y.A.; Tukur, Z.; Irving, H.; Wondji, C.S. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect. Dis. 2014, 14, 441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kabula, B.; Kisinza, W.; Tungu, P.; Ndege, C.; Batengana, B.; Kollo, D.; Malima, R.; Kafuko, J.; Mohamed, M.; Magesa, S. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop. Med. Int. Health 2014, 19, 331–341. [Google Scholar] [CrossRef]
- Kabula, B.; Tungu, P.; Rippon, E.J.; Steen, K.; Kisinza, W.; Magesa, S.; Mosha, F.; Donnelly, M.J. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 2016, 15, 289. [Google Scholar] [CrossRef]
- Meiwald, A.; Clark, E.; Kristan, M.; Edi, C.; Jeffries, C.L.; Pelloquin, B.; Irish, S.R.; Walker, T.; Messenger, L.A. Association of Reduced Long-Lasting Insecticidal Net Efficacy and Pyrethroid Insecticide Resistance With Overexpression of CYP6P4, CYP6P3, and CYP6Z1 in Populations of Anopheles coluzzii From Southeast Côte d’Ivoire. J. Infect. Dis. 2022, 225, 1424–1434. [Google Scholar] [CrossRef]
- Toé, K.H.; N’Falé, S.; Dabiré, R.K.; Ranson, H.; Jones, C.M. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015, 16, 146. [Google Scholar] [CrossRef]
- Alemayehu, E.; Asale, A.; Eba, K.; Getahun, K.; Tushune, K.; Bryon, A.; Morou, E.; Vontas, J.; Van Leeuwen, T.; Duchateau, L.; et al. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasites Vectors 2017, 10, 407. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).