Advancements in the Impact of Insect Gut Microbiota on Host Feeding Behaviors
Abstract
1. Introduction
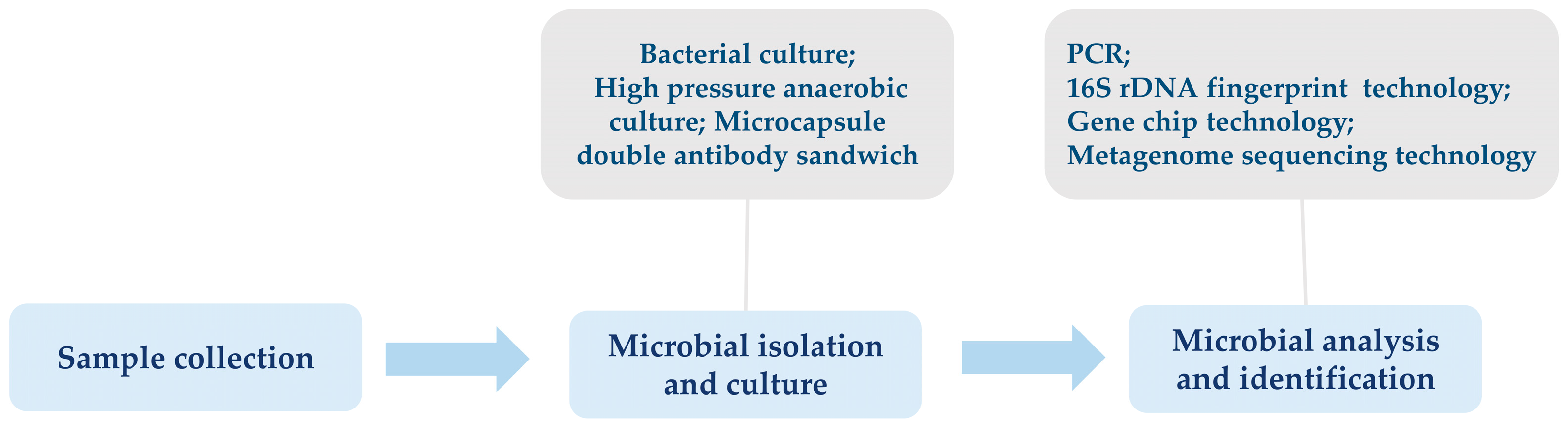
2. Advances in Identification Techniques of Insect Gut Microbiota
3. Differences in Gut Microbiota between Insects Reared in the Laboratory for Multiple Generations and Wild Insects
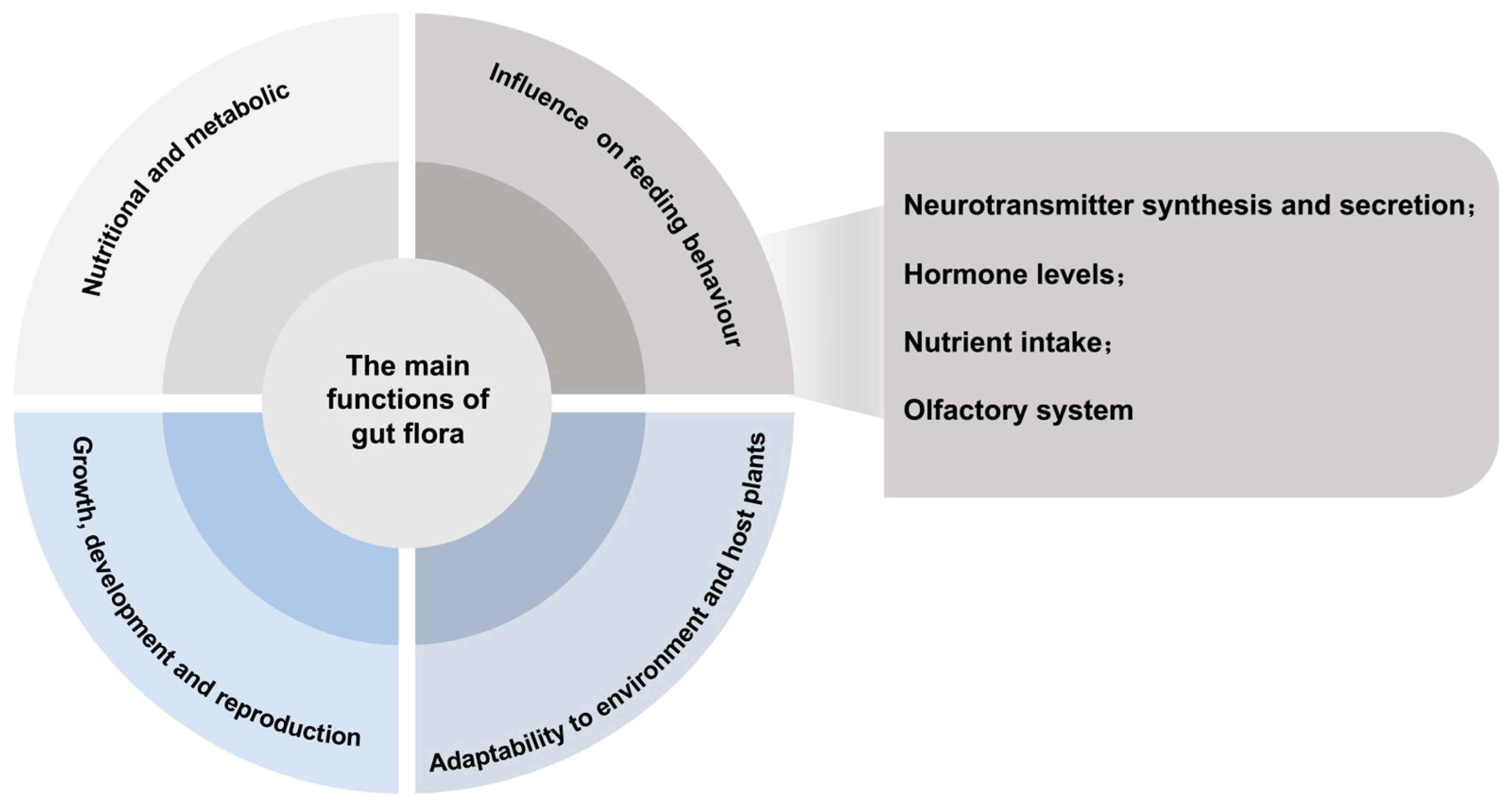
4. Main Functions of Gut Microbiota and Possible Mechanisms Influencing Host Feeding Behaviour
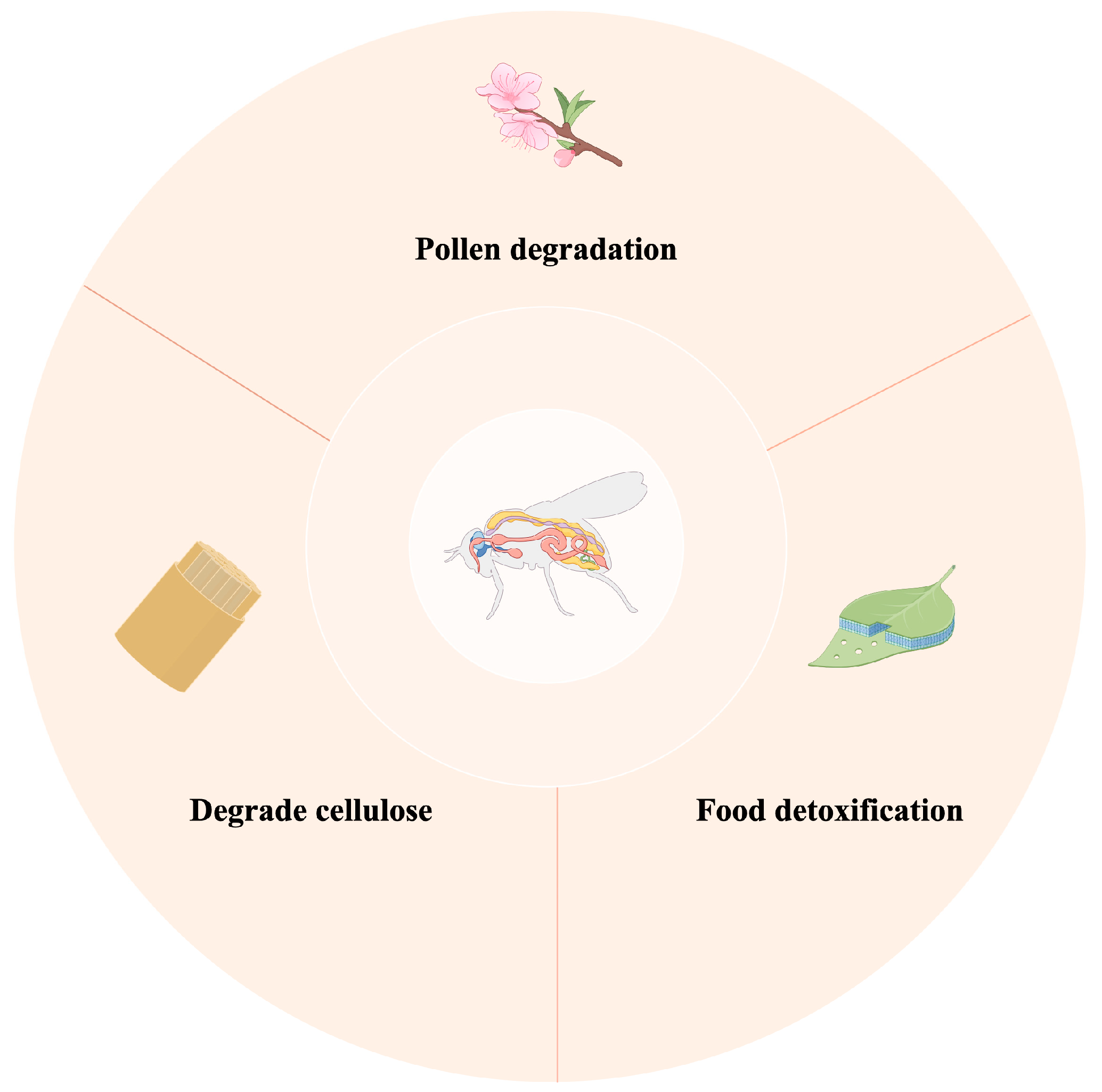
4.1. The Role of Gut Microbiota in Digesting Food
4.2. Gut Microbiota Influences Nutritional and Metabolic Functions of Insects
4.3. Influence on the Growth, Development, and Reproduction of Host Insects
4.4. Regulating the Adaptation of Insects to Their Environment and Host Plants
4.5. Influence of Gut Bacteria on Host Insect Feeding Behaviour and Possible Mechanisms
4.5.1. Influence on Host Insect Neurotransmitter Synthesis and Secretion
4.5.2. Hormone Levels Affecting Host Insects
4.5.3. Influencing Nutrient Intake of Host Insects
4.5.4. Effects on the Host’s Olfactory System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Wu, J.; Deng, X.Y.; Lin, L.B.; Liu, S.; Li, J.L. Advances in research on insect gut microbiota and their functions. Chin. J. Appl. Entomol. 2015, 52, 1345–1352. [Google Scholar]
- Gould, A.L.; Zhang, V.; Lamberti, L.; Jones, E.W.; Obadia, B.; Korasidis, N.; Gavryushkin, A.; Carlson, J.M.; Beerenwinkel, N.; Ludington, W.B. Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. USA 2018, 115, E11951–E16190. [Google Scholar] [CrossRef]
- Basile, E.J.; Launico, M.V.; Sheer, A.J. Physiology, Nutrient Absorption. 2023. Available online: https://europepmc.org/article/NBK/nbk597379 (accessed on 20 December 2023).
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Lu, D.H.; Huang, Y.M.; Kong, Y.; Tao, T.; Zhu, X. Gut microecology: Why our microbes could be key to our health. Biomed. Pharmacother. 2020, 131, 110784. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in agricultural pest management and their environmental risks: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10507–10532. [Google Scholar] [CrossRef]
- Wornell, K.; Pardesi, B.; Lee, K.; Boycheva, S.; Roberton, A.M.; White, W.L. High-throughput Method for novel medium development for culture of anaerobic gut bacteria. Curr. Protoc. 2022, 2, e463. [Google Scholar] [CrossRef]
- Geng, J.; Sui, Z.X.; Dou, W.H.; Miao, Y.H.; Wang, T.; Wei, X.F.; Chen, S.C.; Zhang, Z.Q.; Xiao, J.H.; Huang, D.W. 16S rRNA gene sequencing reveals specific gut microbes common to medicinal insects. Front. Microbiol. 2022, 13, 892767. [Google Scholar] [CrossRef]
- Cao, L.; Ning, K. Metagenomics of insect gut: New borders of microbial big data. Acta Microbiol. Sin. 2018, 58, 964–984. [Google Scholar]
- Choubey, J.; Choudhari, J.K.; Sahariah, B.P.; Verma, M.K.; Banerjee, A. Chapter 25—Molecular Tools: Advance Approaches to Analyze Diversity of Microbial Community; Elsevier: Amsterdam, The Netherlands, 2021; pp. 507–520. [Google Scholar]
- Deutscher, A.T.; Burke, C.M.; Darling, A.E.; Riegler, M.; Reynolds, O.L.; Chapman, T.A. Near full-length 16S rRNA gene next-generation sequencing revealed Asaia as a common midgut bacterium of wild and domesticated Queensland fruit fly larvae. Microbiome 2018, 6, 85. [Google Scholar] [CrossRef]
- Hosokawa, M.; Endoh, T.; Kamata, K.; Arikawa, K.; Nishikawa, Y.; Kogawa, M.; Saeki, T.; Yoda, T.; Takeyama, H. Strain-level profiling of viable microbial community by selective single-cell genome sequencing. Sci. Rep. 2022, 12, 4443. [Google Scholar] [CrossRef]
- Diao, Z.L.; Li, J.M. Metagenomic Next-generation Sequencing: Current Status, Challenges and Prospects of Clinical Application. Med. J. Peking Union Med. Coll. Hosp. 2023, 14, 905–910. [Google Scholar]
- Wang, D.Q.; He, P.S.; Wang, Z.J.; Li, G.Y.; Majed, N.; Gu, A.Z. Advances in single cell Raman spectroscopy technologies for biological and environmental applications. Curr. Opin. Biotech. 2020, 64, 218–229. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Waltmann, A.; Willcox, A.C.; Balasubramanian, S.; Borrini Mayori, K.; Mendoza Guerrero, S.; Salazar Sanchez, R.S.; Roach, J.; Condori Pino, C.; Gilman, R.H.; Bern, C. Hindgut microbiota in laboratory-reared and wild Triatoma infestans. PLoS Negl. Trop. Dis. 2019, 13, e0007383. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Chapman, T.A.; Shuttleworth, L.A.; Riegler, M.; Reynolds, O.L. Tephritid-microbial interactions to enhance fruit fly performance in sterile insect technique programs. BMC Microbiol. 2019, 19, 287. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.G.; Xu, H.X.; Liu, Y.H.; Lu, Z.X. Effects of host plant and insect generation on shaping of the gut microbiota in the rice leaffolder, Cnaphalocrocis medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef]
- Wu, X.L.; Xia, X.F.; Chen, J.H.; Gurr, G.M.; You, M.S. Effects of different diets on the diversity of larval gut bacteria of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2019, 62, 1172–1185. [Google Scholar]
- Huerta-García, A.; Álvarez-Cervantes, J. The gut microbiota of insects: A potential source of bacteria and metabolites. Int. J. Trop. Insect Sci. 2024, 44, 13–30. [Google Scholar] [CrossRef]
- Miyazawa, H.; Aulehla, A. Revisiting the role of metabolism during development. Development 2018, 145, dev131110. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Watanabe, H.; Tokuda, G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef]
- Shahimi, S.; Lamri, M.F.; Mutalib, S.A.; Khalid, R.M.; Tab, M.M.; Khairuddin, F. Gene expression of microbial gelatinase activity for porcine gelatine identification. Food Chem. 2021, 355, 129586. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Raut, A.M.; Pavan, S.Z.; Banu, A.N. Diversity of micro-flora in insect gut and their potential role in insect metabolism. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 33–40. [Google Scholar]
- Cai, S.B.; Wu, G.; Dong, Z.X.; Lin, L.B.; Guo, J.; Zhang, Q.L. Colonization dynamics of the gut flora in western honey bee workers within 7-day post-emergence. Apidologie 2022, 53, 44. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect microbial symbionts: Ecology, interactions, and biological significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y.J. Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Garcia, F.C.; Moya, A.; Latorre, A. Insects potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef]
- Tobias, N.J.; Eberhard, F.E.; Guarneri, A.A. Enzymatic biosynthesis of B-complex vitamins is supplied by diverse microbiota in the Rhodnius prolixus anterior midgut following Trypanosoma cruzi infection. Comput. Struct. Biotechnol. J. 2020, 18, 3395–3401. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhang, F.; Lu, X.M. Diversity and functional roles of the gut microbiota in Lepidopteran insects. Microorganisms 2022, 10, 1234. [Google Scholar] [CrossRef]
- Li, Y.Z.; Chang, L.Y.; Xu, K.; Zhang, S.H.; Gao, F.J.; Fan, Y.S. Research progresses on the function and detection methods of insect gut microbes. Microorganisms 2023, 11, 1208. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef]
- Guilhot, R.; Xuéreb, A.; Lagmairi, A.; Olazcuaga, L.; Fellous, S. Microbiota acquisition and transmission in Drosophila flies. iScience 2023, 26, 107656. [Google Scholar] [CrossRef]
- Nikolouli, K.; Sassù, F.; Ntougias, S.; Stauffer, C.; Cáceres, C.; Bourtzis, K. Enterobacter sp. AA26 as a Protein Source in the Larval Diet of Drosophila suzukii. Insects 2021, 12, 923. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Ntougias, S.; Papadopoulos, N.T.; Bourtzis, K.; Cáceres, C. Enterobacter sp. AA26 gut symbiont as a protein source for Mediterranean fruit fly mass-rearing and sterile insect technique applications. BMC Microbiol. 2019, 19, 288. [Google Scholar] [CrossRef]
- Lü, D.; Dong, Y.; Yan, Z.; Liu, X.Y.; Zhang, Y.J.; Yang, D.B.; He, K.L.; Wang, Z.Y.; Wang, P.; Yuan, X.Q. Dynamics of gut microflora across the life cycle of Spodoptera frugiperda and its effects on the feeding and growth of larvae. Pest Manag. Sci. 2023, 79, 173–182. [Google Scholar] [CrossRef]
- Shan, H.W.; Xia, X.J.; Feng, Y.L.; Wu, W.; Li, H.J.; Sun, Z.T.; Li, J.M.; Chen, J.P. The plant-sucking insect selects assembly of the gut microbiota from environment to enhance host reproduction. npj Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef]
- Selcho, M.; Pauls, D. Linking physiological processes and feeding behaviors by octopamine. Curr. Opin. Insect Sci. 2019, 36, 125–130. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kutsuna, N.; Daubnerová, I.; Roller, L.; Žitňan, D.; Nagasawa, H.; Nagata, S. Enteroendocrine peptides regulate feeding behavior via controlling intestinal contraction of the silkworm Bombyx mori. PLoS ONE 2019, 14, e0219050. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.M.; Zhang, X.Y.; Zhang, K.X.; Li, Y.; Yin, Y.S.; Zhang, R.L.; Zhang, Z. Beneficial bacteria in the intestines of housefly larvae promote larval development and humoral phenoloxidase activity, while harmful bacteria do the opposite. Front. Immunol. 2022, 13, 938972. [Google Scholar] [CrossRef]
- Zeng, T.; Jaffar, S.; Xu, Y.J.; Qi, Y.X. The intestinal immune defense system in insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.M.; Zhang, K.X.; Yin, Y.S.; Zhang, X.Y.; Zhang, Q.; Kong, X.X.; Tang, L.Y.; Zhang, R.; Zhang, Z. Serratia marcescens in the intestine of housefly larvae inhibits host growth by interfering with gut microbiota. Parasites Vectors 2023, 16, 196. [Google Scholar] [CrossRef]
- Mason, C.J.; Shikano, I. Hotter days, stronger immunity? Exploring the impact of rising temperatures on insect gut health and microbial relationships. Curr. Opin. Insect Sci. 2023, 59, 101096. [Google Scholar] [CrossRef]
- Lemoine, M.M.; Engl, T.; Kaltenpoth, M. Microbial symbionts expanding or constraining abiotic niche space in insects. Curr. Opin. Insect Sci. 2020, 39, 14–20. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, L.Y.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef]
- Jia, Y.C.; Jin, S.; Hu, K.K.; Geng, L.; Han, C.H.; Kang, R.X.; Pang, Y.X.; Ling, E.J.; Tan, E.K.; Pan, Y.F. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef]
- Shi, Z.H.; Hou, Y.M. Current understanding on the mechanism of the interactions between insects and gut microbiota and its implications in the pest control. J. Enviromrntal Entomol. 2020, 42, 798–805. [Google Scholar]
- Kang, X.L.; Zhang, J.Y.; Wang, D.; Zhao, Y.M.; Han, X.L.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. 2019, 15, e1008331. [Google Scholar] [CrossRef]
- Petruccelli, E.; Lark, A.; Mrkvicka, J.A.; Kitamoto, T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J. Neurogenet. 2020, 34, 55–68. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.A.; Yao, Z.C.; Awan, U.A.; Zhang, Z.Y.; Zheng, W.W.; Zhang, H.Y. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Wu, G. Potential effects of gut microbiota on host cancers: Focus on immunity, DNA damage, cellular pathways, and anticancer therapy. ISME J. 2023, 17, 1535–1551. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Q.; Dai, M.; Shu, Q.L.; Li, X.; Guo, X.Q.; Wang, Y.F.; Wei, J.; Liu, W.; Dai, Y. Tachinid parasitoid Exorista japonica affects the utilization of diet by changing gut microbial composition in the silkworm, Bombyx mori. Arch. Insect Biochem. Physiol. 2023, 113, e22011. [Google Scholar] [CrossRef]
- Lesperance, D.N.A.; Broderick, N.A. Gut bacteria mediate nutrient availability in drosophila diets. Appl. Environ. Microbiol. 2020, 87, e01401-20. [Google Scholar] [CrossRef]
- Bai, S.A.; Yao, Z.C.; Raza, M.F.; Cai, Z.H.; Zhang, H.Y. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef]
- Liberti, J.; Engel, P. The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13. [Google Scholar] [CrossRef]
- Trevelline, B.K.; Kohl, K.D. The gut microbiome influences host diet selection behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2117537119. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Harrold, J.A.; Dovey, T.M.; Blundell, J.E.; Halford, J.C.G. CNS regulation of appetite. Neuropharmacology 2012, 63, 3–17. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile organic compounds (VOCs) as environmental pollutants: Occurrence and mitigation using nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, S.K.; Xu, L.T. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. npj Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, H.; Yang, X.; Gao, Y.; Lu, Y.Y.; Cheng, D.F. Gut bacteria induce oviposition preference through ovipositor recognition in fruit fly. Commun. Biol. 2022, 5, 973. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Du, Y.W.; Shi, X.B.; Zhao, L.C.; Yuan, G.G.; Zhao, W.W.; Huang, G.H.; Chen, G. Chinese cabbage changes its release of volatiles to defend against spodoptera litura. Insects 2022, 13, 73. [Google Scholar] [CrossRef]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.J.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, L.; Li, D.; Chen, Z.; Luo, Y.; Zhou, J.; Luo, B.; Yan, R.; Liu, H.; Wang, L. Advancements in the Impact of Insect Gut Microbiota on Host Feeding Behaviors. Genes 2024, 15, 1320. https://doi.org/10.3390/genes15101320
Wang Y, Wang L, Li D, Chen Z, Luo Y, Zhou J, Luo B, Yan R, Liu H, Wang L. Advancements in the Impact of Insect Gut Microbiota on Host Feeding Behaviors. Genes. 2024; 15(10):1320. https://doi.org/10.3390/genes15101320
Chicago/Turabian StyleWang, Yikang, Liang Wang, Di Li, Zhenfu Chen, Yang Luo, Juan Zhou, Bo Luo, Rong Yan, Hui Liu, and Lingjun Wang. 2024. "Advancements in the Impact of Insect Gut Microbiota on Host Feeding Behaviors" Genes 15, no. 10: 1320. https://doi.org/10.3390/genes15101320
APA StyleWang, Y., Wang, L., Li, D., Chen, Z., Luo, Y., Zhou, J., Luo, B., Yan, R., Liu, H., & Wang, L. (2024). Advancements in the Impact of Insect Gut Microbiota on Host Feeding Behaviors. Genes, 15(10), 1320. https://doi.org/10.3390/genes15101320





