Abstract
With the application and development of high-throughput sequencing technology, the structure and function of insect gut microbiota have been analysed, which lays a foundation for further exploring the intricate relationships between gut microbiota and host feeding behaviour. The microbial community in the insect gut, as an important ecological factor, affects the host’s food selection and nutritional metabolic processes through various mechanisms, which play a key role in population dynamics and ecosystems. The implications of these interactions are profound, affecting agricultural practices, biodiversity, and the broader environment, such as pollination and pest control. In-depth exploration of the molecular mechanism of the interaction between gut microbiota and hosts contributes to the grasp of insect biology and evolution and offers novel avenues for manipulating insect behaviour for practical applications in agriculture and environmental management. This paper focuses on the possible mechanisms of insect gut microbiota regulating host feeding behaviour. It inspires further research on the interaction between gut microbiota and insects affecting host behaviour.
1. Introduction
Insects, as the species with the most successful survival strategies on the earth, are related to their ecological diversity and niche differentiation, as well as their strong fecundity, small size, high energy utilisation efficiency, and rapid evolutionary rate [1]. The ecological diversity and niche differentiation of insects have largely shaped the composition and function of their gut microbiota, which in turn further enhances the adaptability of insects to specific niches and plays an indispensable role in the ecological network [2]. As the main gathering place of insect gut bacteria, the midgut undertakes most of the insect’s nutrient absorption and decomposition and has a rich enzyme system [3]. Studies have shown that in addition to affecting the nutritional metabolism, the midgut microbiota is also involved in the host’s physiological, ecological, and evolutionary processes of the host, forming a close symbiotic relationship with the host [4,5].
In addition, recent studies have revealed that bacteria in the insect gut can also significantly influence host behaviour. Exploring the complex interactions between microbes and their hosts broadens our understanding of animal–microbe symbiosis and provides new perspectives and potential application strategies in agricultural pest management, biological control, and human health [6]. Therefore, this paper provides a systematic review of insect gut microbiota in terms of its identification and main functions. It also focuses on the possible mechanisms by which insect gut microbiota modulate host feeding behaviour, thus providing insights for further exploration of the specific elements of gut microbiota that influence host behaviour.
2. Advances in Identification Techniques of Insect Gut Microbiota
Identifying insect gut microbiota is a multi-step process, including sample collection, microbial isolation and culture, and identification by molecular biology techniques or mass spectrometry (Figure 1). Commonly used culture methods for gut microbiota include bacterial culture, high-pressure anaerobic culture microcapsule double antibody sandwich, and other culture techniques. The morphological characteristics of the colonies are observed and recorded after the culture to carry out preliminary identification [7]. However, due to the high complexity and diversity of most insect gut microbiota, modern research has increasingly favoured non-culture molecular biology techniques for analysing and identifying microbial diversity. For example, polymerase chain reaction (PCR), which amplifies the 16S rRNA gene using universal primers and then sequences the amplified products to identify microbial species [8]; 16S rDNA fingerprinting technique for the analysis of bacterial community structure based on the patterns of bands produced by electrophoresis after PCR amplification [9,10]; gene chip technology, which designs probe targeting a specific microbial species or taxon to identify the microbial composition of a sample by hybridisation; and gene microarray technology, which designs probe targeting a specific microbial species or taxon to identify the microbial composition of a sample by hybridisation. Gene microarray technology to determine the microbial composition of a sample by hybridisation, and macro-genome sequencing technology to sequence the total DNA of all microorganisms in a sample in a high throughput manner to gain a comprehensive understanding of the structure and functional genes of microbial communities without the need for pre-cultivation, etc. [11,12]. In addition, other novel techniques, such as single-cell Raman imaging, in situ hybridisation, etc. [13,14].
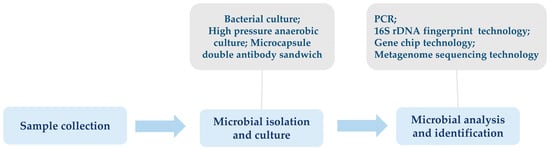
Figure 1.
The basic process of insect gut microbiota identification.
According to the influence of gut microbiota on the host, it is divided into probiotics, neutral bacteria, and pathogenic bacteria. In addition, gut microbiota can be further categorized according to population size and source. For example, according to the number of culturable bacteria, they are divided into major (dominant) flora and secondary flora. The major (dominant) refers to bacteria with large numbers or high population densities in the gut microbiota, typically above 107~108 CFU/g, and includes specialised anaerobic bacteria such as Bacteroides, Eubacterium, Bifidobacterium, Ruminococcus, and Clostridium, which are usually derived from the original flora [15]. Secondary flora with counts below 107~108 CFU/g, mainly aerobic bacteria or facultative anaerobic bacteria, such as Escherichia coli and Streptococcus, are highly mobile and potentially pathogenic, and most belong to the flora of origin or passing flora [13].
3. Differences in Gut Microbiota between Insects Reared in the Laboratory for Multiple Generations and Wild Insects
Insects reared in captivity in the laboratory are not exposed to bacteria in their natural habitat, including microorganisms that benefit the insect’s life activities. The antimicrobial agents in the diet of captive insect larvae and the pH reduce the likelihood of larval contamination with harmful microorganisms. Still, this situation largely reduces the chance of horizontal transmission of beneficial organisms. In other studies, it has also been found that wild Triatoma infestans have a greater diversity of hindgut bacteria compared to laboratory-cultured insects, the microbial composition of T. infestans in the laboratory does not reflect the complete collection of gut microbes in wild T. infestans, and extrapolating the gut microbiota profiles of wild insects by studying laboratory insects alone may not be possible [16]. The general assumption is that microbial diversity contributes to the vital activities of insects; however, insects raised artificially in laboratory environments are, perhaps, less functionally diverse due to a reduction in bacterial diversity and increased susceptibility [17].
4. Main Functions of Gut Microbiota and Possible Mechanisms Influencing Host Feeding Behaviour
The gut microbiota of insects plays multiple important functions in host insects [18,19,20]. There are complex and close interactions between insect gut microbiota and their hosts, involving regulation of nutrient metabolism, growth and reproduction, and feeding behaviour regulation [21,22] (Figure 2), and in-depth studies of these functions and the possible mechanisms of their feeding behaviours are of great significance to the understanding of insect ecology and microbial interrelationships.
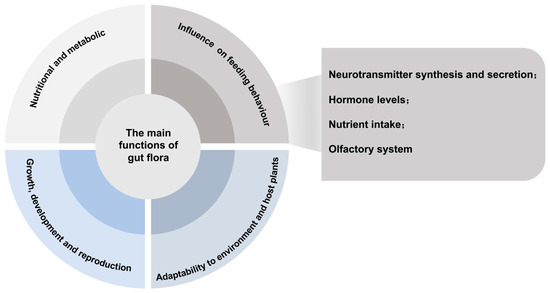
Figure 2.
The main functions of gut microbiota and the possible mechanisms affecting host feeding behaviour.
4.1. The Role of Gut Microbiota in Digesting Food
Insect gut microbiota play multiple roles in their digestion of food (Figure 3). In phytophagous insects, the microbial community in the gut can degrade cellulose, e.g., microbes in the gut of the termite help the host break down lignocelllose [23] efficiently. Some insects specialise in pollen as their main source of nutrition. The pollen grains are protected by a shell that the insect must overcome to gain access to abundant nutrients. Bees work in close collaboration with their intestinal commensal bacteria, which encode a series of enzyme genes, such as pectin-degrading enzymes, glycoside hydrolases, and polysaccharide hydrolases, which help bees efficiently utilise the carbohydrate resources of pollen [24,25,26].
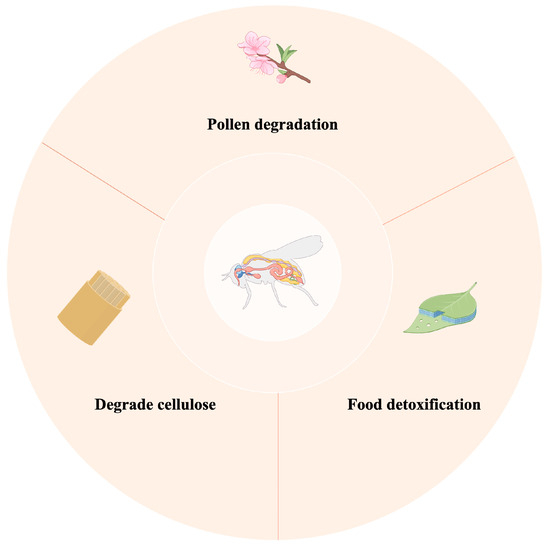
Figure 3.
The role of gut microbiota in digesting food.
In addition, gut microbiota exhibit potential food detoxification functions, as the effective nutritional value of many food sources relies on their non-toxic state, and certain plant cell wall components undergo microbial-mediated hydrolysis to both detoxify and convert them into nutrients that can be fully utilised by the host [27,28]. In particular, the gut microbiota of insects that feed on toxic plants play a crucial role in processing and detoxifying such food toxicity, ensuring that insects obtain the energy and nutrients they need to survive from a unique and complex food chain [29]. Many insects avoid plant tannins during feeding, which reduces the protein content of the food. Many microorganisms produce tannase, an enzyme that allows insects to be more adapted to local food sources [30].
4.2. Gut Microbiota Influences Nutritional and Metabolic Functions of Insects
Gut microbiota plays an indispensable role in insect digestion and nutrient absorption processes [31,32,33]. Intestinal microorganisms also play an important role in insect hosts, such as participating in insect growth and development, adapting to the environment, and drug resistance [34]. They not only help the host insect to decompose and transform food components efficiently but also provide a variety of essential nutrients such as vitamins, amino acids, and nitrogen sources and are deeply involved in the anabolic process of these substances [35,36,37].
Moreover, the gut microbiota of insects can enhance the recycling of urea and uric acid, aid insects in fending off infections from foreign pathogenic microorganisms to sustain internal balance and support the host’s normal physiological functions. Insects are also rich in microorganisms on their surfaces, manure, and growth environments, which can affect their physiological functions [38]. The relationship between the growth environment of insects and the host gut microbiota needs more research and exploration.
4.3. Influence on the Growth, Development, and Reproduction of Host Insects
Gut microbiota largely influence the growth and development process of the host through direct interaction with host cells and regulation of host endocrine hormone signalling pathways. Experimental results have shown that Drosophila larvae cultured in a sterile environment exhibit significant differences, such as slowed growth rates and developmental delays, compared to Drosophila larvae with a normal microbiome [39,40]. However, when these sterile Drosophila larvae were re-implanted with appropriate amounts of Acetobacter pomorum, their developmental rates and growth levels could return to control standards. In addition to Drosophila, the gut microbiota of other non-model organisms has also been found to function in ways that affect the biological characteristics of host insects. In the case of Drosophila Suzuki, the complete replacement of brewer’s yeast adversely affected female fecundity, pupal weight, adult survival, and recovery rates, thus suggesting that Enterobacter sp. AA26 cannot fulfil the protein requirements of D. suzukii when yeast is absent from the diet. Interestingly, the partial yeast replacement did not present severe effects (apart from the adult recovery rate), suggesting that halving the yeast quantity is still sufficient to produce fit adults. Results in Ceratitis capitata indicated that the addition of Enterobacter sp. AA26 increased pupae and adult production and decreased rearing duration for several developmental stages. Enterobacter sp. AA26 proved to be an adequate nutritional source for C. capitata larvae [41]. The Enterobacter sp. AA26 gut symbiont has been shown to shorten immature developmental stages, increase fecundity, extend survival, and improve male mate competitiveness and female mate acceptance in Mediterranean fruit fly [42]. Alterations in gut microbiota dynamics and their impact on the feeding and growth of Spodoptera frugiperda following antibiotic treatment are being investigated. Antibiotic treatment suppresses the gut bacteria of Spodoptera frugiperda, leading to decreased food intake and body weight of the larvae and an extension in the developmental period [43]. Insect gut microbiota exerts diverse influences on insect reproduction, including enhancing reproductive rates, boosting reproductive efficiency, altering reproductive modes, and modifying the adaptability of offspring [44]. Additional studies have similarly revealed the importance of gut microbiota for aphid growth and reproduction: aphids showed a decrease in growth, developmental rate, and reproductive capacity when antibiotics were used to eliminate microbes from the aphid gut [45]. Similar effects have been demonstrated in stink bugs (Megacopta punctatissima), where the elimination of gut microbiota by antibiotics resulted in stagnant development, sterility, and a significant increase in mortality of stink bug individuals [46]. Additionally, alterations in the gut microbiota can stimulate the host’s immune mechanisms to resist pathogen invasion and influence insect development [47,48]. This further confirms that gut microbiota is critical for healthy growth and survival at all insect life cycle stages [49].
4.4. Regulating the Adaptation of Insects to Their Environment and Host Plants
The conditions of their surroundings largely govern the life activities of insects, and among the many environmental factors, the temperature has a particularly significant effect [50]. The range of temperatures that insects can tolerate and adapt to, and their resilience to change, is a central element in determining their geographic distribution patterns. A large body of evidence reveals the important role of symbiotic microorganisms on the environmental adaptability of insects [51], among which the effect of endosymbiotic bacteria on the heat tolerance of insects is a central focus of research in this field, especially the interrelationships between endosymbiotic bacteria and their insect hosts, which has become a central research element in understanding how insects respond to global climate change, especially the increase in temperature [52]. These endosymbiotic bacteria can be passed from one generation to the next within the insect and directly influence host physiology, such as metabolism, immune responses, and tolerance thresholds to temperature extremes [53]. It has been shown that the diversity of secondary bacteria in aphids increases when aphids feed on a wider variety of host plants. It is inferred that symbiotic flora may expand the range of host plant lineages that host insects can adapt to and utilise by influencing their ability to utilise different plant resources.
4.5. Influence of Gut Bacteria on Host Insect Feeding Behaviour and Possible Mechanisms
The influence of gut bacteria on the feeding behaviour of host insects has become a hotspot at the intersection of ecology, microbiology, and neurobiology in recent years [54]. The mechanisms of their influence may involve the production and regulation of host neurotransmitters, hormone levels, nutrient intake, and the insects’ olfactory systems.
4.5.1. Influence on Host Insect Neurotransmitter Synthesis and Secretion
Gut microbiota plays a key role in the regulation of neurotransmitters in insects. Neurotransmitters, as key chemicals for inter-neuronal signalling [55], play an important role in the regulation of appetite in insects. Studies have shown that gut microbiota can modulate insect feeding behaviour by influencing neurotransmitter synthesis and host release mechanisms [56]. For example, certain gut microbiota may promote the production and release of specific neurotransmitters, thereby enhancing the insect’s appetite; conversely, other microorganisms may inhibit these processes, decreasing the insect’s desire to feed. Jia Y et al. [57] showed that food intake could be controlled by regulating the expression level of octopamine in Drosophila. When the activity of this neurotransmitter was inhibited, Drosophila feeding was significantly reduced. In addition, octopamine, as a neurotransmitter widely found in the arthropod nervous system, is involved in and regulates various physiological functions and is particularly important in connecting the physiological state of insects with their feeding behaviours. A series of experiments by Selcho M et al. [45] further confirmed the effect of octopamine on the feeding behaviours of insects. They found that octopamine positively stimulated feeding activity and that this stimulatory effect was closely related to the concentration of octopamine in the insect brain. Octopamine regulates foraging behaviour and food intake by activating specific neuronal pathways related to feeding behaviour.
4.5.2. Hormone Levels Affecting Host Insects
The gut microbiota community interacts with the endocrine system of insects through diverse mechanisms, which in turn affects their feeding behaviour [46]. Specifically, gut bacteria can modulate energy use and the desire to feed by regulating insect hormone levels, such as glucagon, insulin and other hormones related to energy metabolism and appetite [58]. 20-Hydroxyecdysone is a moulting hormone commonly found in insects, and it has a variety of bioactive functions. This hormone interacts with dopamine receptors and significantly affects feeding behaviour and pupation. Kang et al. [59] revealed that 20-Hydroxyecdysone binds to dopamine receptors in Lepidoptera, inhibiting feeding and facilitating entry into the pupal stage. They further explored the mechanism behind this interaction, i.e., the binding of 20-Hydroxyecdysone to the dopamine receptor activates specific signalling pathways, modulating neuronal activity, ultimately leading to reduced feeding and enhanced pupation behaviour. DopEcR (a G-protein-coupled dopamine/ecdysterone receptor), as a dual G-protein-coupled receptor, recognises the catecholamine hormone dopamine and also responds to the steroid hormone ecdysteroid. In Drosophila, ecdysone and dopamine are involved in the stress response [60]. In the agricultural pest Helicoverpa armigera, DopEcR is a key switch integrating dopamine and 20-Hydroxyecdysone (20E) signalling, which determines whether larvae will continue to remain actively feeding and growing or switch to sedentary behaviour and begin to enter the pupal stage [38]. 20-Hydroxyecdysone concentrations are low in the feeding stage of larvae and high in the preparation for the pupal stage. In contrast, dopamine concentrations were higher during the active feeding period of larvae and decreased when they were about to pupate.
4.5.3. Influencing Nutrient Intake of Host Insects
Gut microbiota has a significant impact on the selectivity of host nutrient absorption, and the insect gut microbiota synergistically regulates food digestion in the host through various mechanisms [61,62]. These microorganisms secrete key digestive enzymes that break down complex macromolecules in food into smaller molecular fragments that are more readily absorbed, thus significantly affecting the ability of insects to acquire and utilise desired nutrients from food, which in turn may indirectly influence their food selection preferences [30]. Li et al. [63] found a significant effect of Exorista japonica on the intestinal microbial composition of Bombyx mori. After the parasitic wasps infected the silkworms, the gut microbiota structure of the silkworms changed significantly, resulting in a decrease in the efficiency of feed digestion and absorption, a slowdown in growth, and a decrease in feed conversion efficiency. This may be because the parasitic wasps inhibited the growth of beneficial flora in the silkworm’s intestine by some means while promoting the proliferation of potentially harmful flora, which disrupted the original intestinal microbial equilibrium. Experiments comparing the differences between bacterial Drosophila and normal Drosophila carrying gut microbiota when ingesting the same food revealed that gut bacteria facilitate the absorption of specific nutrients such as proteins and fats in Drosophila [64]. The important findings of this series of studies indicate that the impacts of gut bacteria on host nutrient absorption are closely related to their regulation of the dynamic balance of the gut microbiota community, further emphasising the centrality of gut microbiota–host interactions in the nutrient absorption process and their potential applications.
4.5.4. Effects on the Host’s Olfactory System
Several studies have revealed that insect gut microbiota may influence the host olfactory system by modulating the expression of host olfactory receptor genes [56,65,66]. These microbes can influence host foraging behaviour directly or indirectly through modulation of brain function by mediating the supply of essential amino acids or by producing a variety of compounds that interfere with insect feeding preferences [67]. Gut microbiota not only guide food choice by modulating amino acid metabolism but also produce a variety of chemicals that intervene in insect feeding behaviour. Gut-derived compounds such as short-chain fatty acids, bile acids, methylamines, amino acid derivatives and microbe-associated molecular patterns stimulate energy metabolism and regulate food intake through signalling mechanisms [68]. The gut microbiota shape host foraging behaviour to some extent through metabolic activities, and these metabolic pathways alter the effectiveness of nutrients (and their derivatives) recognised by the central nervous system. In addition, bacterial tryptophan metabolism is one of the key pathways by which gut microbiota influence host behaviour [69], as tryptophan, a major regulatory molecule for the synthesis of the central neurotransmitter 5-hydroxytryptamine (5-HT), has been shown to drive foraging behaviour and dietary choices in several experimental studies [70].
Some microorganisms can also produce chemical signals that insects can perceive, thus influencing their selection and localisation of host plants. Volatile organic compounds (VOCs), as a chemical signal transmitted through the air, have a significant effect on the olfactory perception and feeding behaviour of insects [71,72]. Studies have shown that volatile organic compounds produced by gut microbiota can alter the ability to perceive food odours, affecting insect food preferences [56,73]. For example, specific VOCs may direct insects to prefer foods rich in particular nutrients. Gut bacteria of the genus Citrobacter in Drosophila orientacea release 3-hexenyl acetate (3-HA), which attracts other female fruit flies that choose to lay their eggs on the same host fruit [74]. On the other hand, the gut bacteria of the bark beetle Dendroctonus valens produce a multifunctional pheromone called verbenone that helps the beetle determine whether pine trees are suitable for settlement [75]. The volatile organic compounds produced by gut bacteria are used for mutual attraction between conspecifics and to build reciprocal relationships between different organisms. Plants release specific plant-induced volatiles (HIPVs) when fed on by herbivorous insects. This process has a direct effect on herbivorous insects and also provides indirect defence by attracting natural enemies [76]. Insect gut bacteria also influence this indirect defence mechanism. For example, VOCs released from leaves act as attractants for natural enemies in the indirect defence response triggered by the rice brown planthopper (Nilaparvata lugens; BPH), while the symbiotic bacterium Hamiltonella defensa has been shown to reduce the recruitment rate of parasitic wasps by reducing the number of volatile compounds produced by the plant, thereby increasing the recruitment rate of pea aphid (Acyrthosiphon pisum) and survival adaptations. Despite extensive research on the impact of gut microbiota on host insect behaviour, the studies have primarily focused on a limited range of insect species. Additionally, laboratory conditions fail to fully replicate the complex interactions present in natural environments, which may render the applicability of the findings to real-world scenarios somewhat limited [77].
5. Conclusions
Significant breakthroughs have been made in studying the influence of gut microbiota on the feeding behaviour of insect hosts. Current scientific evidence suggests that microorganisms in insects play a crucial role in shaping their food preferences and feeding strategies by optimising their nutrient metabolism, interfering with neurophysiological signalling mechanisms, and regulating immune responses and overall physiological status. With the development and application of techniques for the study of gut microbiota, more research has been conducted on gut microbiota. However, more in-depth studies are needed to investigate how gut microbiota can adjust and transform the feeding behaviour of the host. Future research pathways could focus on the following directions: firstly, to clarify the causal links between specific gut bacterial species and their metabolites and host-specific eating habits and to identify key strains or metabolites with significant regulatory effects. Second, the molecular mechanisms behind microbe–host interactions should be systematically analysed using integrated multi-omics analyses (including transcriptomics, proteomics, and metabolomics). In addition, it is necessary to explore how environmental factors (e.g., food quality, temperature changes, pollutant exposure, etc.) affect the gut microbiota and change the feeding behaviour of the host and to assess the importance of such dynamic interactions between micro-ecology and macro-ecology in terms of the ecological adaptive capacity of the species and the geographical distribution pattern. In the long run, these research results are expected to be applied to integrated pest management, effective use of biological resources, and environmental protection. By precisely regulating insect gut microbiota, we can improve insect population management strategies and promote the development of sustainable agricultural practices while safeguarding ecosystem health and stability. Some gut bacteria help pests adapt to host plants and degrade phytotoxins. Thus, the symbiotic relationships between gut bacteria and insect pests have partly contributed to the success and diversification of insects but have also increased the difficulty of pest control. Interestingly, some studies have found that insect gut bacteria can potentially be manipulated to increase or decrease insect fitness [73]. Gut bacteria have great potential as biocontrol agents for pest management. In addition, biological control agents cause less pollution to the environment compared to chemical insecticides. However, considering that most enteric bacteria are not culturable and other species may be present in insect–plant interactions in the field, laboratory studies may have limitations, making future research difficult.
Author Contributions
Writing—original draft preparation, Y.W., L.W. (Liang Wang), D.L., Z.C. and Y.L.; writing—review and editing, J.Z., B.L., R.Y., H.L. and L.W. (Lingjun Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation Project of China, grant number 82060374; The Science and Technology Foundation of Guizhou Province, grant number QKHJC-ZK-[2023]-YB517; Open Project Fund of Key Laboratory of Parasite and Vector Biology, NHC, grant number NHCKFKT2023-10.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, J.; Wu, J.; Deng, X.Y.; Lin, L.B.; Liu, S.; Li, J.L. Advances in research on insect gut microbiota and their functions. Chin. J. Appl. Entomol. 2015, 52, 1345–1352. [Google Scholar]
- Gould, A.L.; Zhang, V.; Lamberti, L.; Jones, E.W.; Obadia, B.; Korasidis, N.; Gavryushkin, A.; Carlson, J.M.; Beerenwinkel, N.; Ludington, W.B. Microbiome interactions shape host fitness. Proc. Natl. Acad. Sci. USA 2018, 115, E11951–E16190. [Google Scholar] [CrossRef]
- Basile, E.J.; Launico, M.V.; Sheer, A.J. Physiology, Nutrient Absorption. 2023. Available online: https://europepmc.org/article/NBK/nbk597379 (accessed on 20 December 2023).
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Lu, D.H.; Huang, Y.M.; Kong, Y.; Tao, T.; Zhu, X. Gut microecology: Why our microbes could be key to our health. Biomed. Pharmacother. 2020, 131, 110784. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in agricultural pest management and their environmental risks: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10507–10532. [Google Scholar] [CrossRef]
- Wornell, K.; Pardesi, B.; Lee, K.; Boycheva, S.; Roberton, A.M.; White, W.L. High-throughput Method for novel medium development for culture of anaerobic gut bacteria. Curr. Protoc. 2022, 2, e463. [Google Scholar] [CrossRef]
- Geng, J.; Sui, Z.X.; Dou, W.H.; Miao, Y.H.; Wang, T.; Wei, X.F.; Chen, S.C.; Zhang, Z.Q.; Xiao, J.H.; Huang, D.W. 16S rRNA gene sequencing reveals specific gut microbes common to medicinal insects. Front. Microbiol. 2022, 13, 892767. [Google Scholar] [CrossRef]
- Cao, L.; Ning, K. Metagenomics of insect gut: New borders of microbial big data. Acta Microbiol. Sin. 2018, 58, 964–984. [Google Scholar]
- Choubey, J.; Choudhari, J.K.; Sahariah, B.P.; Verma, M.K.; Banerjee, A. Chapter 25—Molecular Tools: Advance Approaches to Analyze Diversity of Microbial Community; Elsevier: Amsterdam, The Netherlands, 2021; pp. 507–520. [Google Scholar]
- Deutscher, A.T.; Burke, C.M.; Darling, A.E.; Riegler, M.; Reynolds, O.L.; Chapman, T.A. Near full-length 16S rRNA gene next-generation sequencing revealed Asaia as a common midgut bacterium of wild and domesticated Queensland fruit fly larvae. Microbiome 2018, 6, 85. [Google Scholar] [CrossRef]
- Hosokawa, M.; Endoh, T.; Kamata, K.; Arikawa, K.; Nishikawa, Y.; Kogawa, M.; Saeki, T.; Yoda, T.; Takeyama, H. Strain-level profiling of viable microbial community by selective single-cell genome sequencing. Sci. Rep. 2022, 12, 4443. [Google Scholar] [CrossRef]
- Diao, Z.L.; Li, J.M. Metagenomic Next-generation Sequencing: Current Status, Challenges and Prospects of Clinical Application. Med. J. Peking Union Med. Coll. Hosp. 2023, 14, 905–910. [Google Scholar]
- Wang, D.Q.; He, P.S.; Wang, Z.J.; Li, G.Y.; Majed, N.; Gu, A.Z. Advances in single cell Raman spectroscopy technologies for biological and environmental applications. Curr. Opin. Biotech. 2020, 64, 218–229. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Waltmann, A.; Willcox, A.C.; Balasubramanian, S.; Borrini Mayori, K.; Mendoza Guerrero, S.; Salazar Sanchez, R.S.; Roach, J.; Condori Pino, C.; Gilman, R.H.; Bern, C. Hindgut microbiota in laboratory-reared and wild Triatoma infestans. PLoS Negl. Trop. Dis. 2019, 13, e0007383. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Chapman, T.A.; Shuttleworth, L.A.; Riegler, M.; Reynolds, O.L. Tephritid-microbial interactions to enhance fruit fly performance in sterile insect technique programs. BMC Microbiol. 2019, 19, 287. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.G.; Xu, H.X.; Liu, Y.H.; Lu, Z.X. Effects of host plant and insect generation on shaping of the gut microbiota in the rice leaffolder, Cnaphalocrocis medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef]
- Wu, X.L.; Xia, X.F.; Chen, J.H.; Gurr, G.M.; You, M.S. Effects of different diets on the diversity of larval gut bacteria of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2019, 62, 1172–1185. [Google Scholar]
- Huerta-García, A.; Álvarez-Cervantes, J. The gut microbiota of insects: A potential source of bacteria and metabolites. Int. J. Trop. Insect Sci. 2024, 44, 13–30. [Google Scholar] [CrossRef]
- Miyazawa, H.; Aulehla, A. Revisiting the role of metabolism during development. Development 2018, 145, dev131110. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Watanabe, H.; Tokuda, G. Cellulolytic systems in insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012, 109, 11002–11007. [Google Scholar] [CrossRef]
- Shahimi, S.; Lamri, M.F.; Mutalib, S.A.; Khalid, R.M.; Tab, M.M.; Khairuddin, F. Gene expression of microbial gelatinase activity for porcine gelatine identification. Food Chem. 2021, 355, 129586. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Raut, A.M.; Pavan, S.Z.; Banu, A.N. Diversity of micro-flora in insect gut and their potential role in insect metabolism. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 33–40. [Google Scholar]
- Cai, S.B.; Wu, G.; Dong, Z.X.; Lin, L.B.; Guo, J.; Zhang, Q.L. Colonization dynamics of the gut flora in western honey bee workers within 7-day post-emergence. Apidologie 2022, 53, 44. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect microbial symbionts: Ecology, interactions, and biological significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y.J. Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Garcia, F.C.; Moya, A.; Latorre, A. Insects potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef]
- Tobias, N.J.; Eberhard, F.E.; Guarneri, A.A. Enzymatic biosynthesis of B-complex vitamins is supplied by diverse microbiota in the Rhodnius prolixus anterior midgut following Trypanosoma cruzi infection. Comput. Struct. Biotechnol. J. 2020, 18, 3395–3401. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhang, F.; Lu, X.M. Diversity and functional roles of the gut microbiota in Lepidopteran insects. Microorganisms 2022, 10, 1234. [Google Scholar] [CrossRef]
- Li, Y.Z.; Chang, L.Y.; Xu, K.; Zhang, S.H.; Gao, F.J.; Fan, Y.S. Research progresses on the function and detection methods of insect gut microbes. Microorganisms 2023, 11, 1208. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef]
- Guilhot, R.; Xuéreb, A.; Lagmairi, A.; Olazcuaga, L.; Fellous, S. Microbiota acquisition and transmission in Drosophila flies. iScience 2023, 26, 107656. [Google Scholar] [CrossRef]
- Nikolouli, K.; Sassù, F.; Ntougias, S.; Stauffer, C.; Cáceres, C.; Bourtzis, K. Enterobacter sp. AA26 as a Protein Source in the Larval Diet of Drosophila suzukii. Insects 2021, 12, 923. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Ntougias, S.; Papadopoulos, N.T.; Bourtzis, K.; Cáceres, C. Enterobacter sp. AA26 gut symbiont as a protein source for Mediterranean fruit fly mass-rearing and sterile insect technique applications. BMC Microbiol. 2019, 19, 288. [Google Scholar] [CrossRef]
- Lü, D.; Dong, Y.; Yan, Z.; Liu, X.Y.; Zhang, Y.J.; Yang, D.B.; He, K.L.; Wang, Z.Y.; Wang, P.; Yuan, X.Q. Dynamics of gut microflora across the life cycle of Spodoptera frugiperda and its effects on the feeding and growth of larvae. Pest Manag. Sci. 2023, 79, 173–182. [Google Scholar] [CrossRef]
- Shan, H.W.; Xia, X.J.; Feng, Y.L.; Wu, W.; Li, H.J.; Sun, Z.T.; Li, J.M.; Chen, J.P. The plant-sucking insect selects assembly of the gut microbiota from environment to enhance host reproduction. npj Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef]
- Selcho, M.; Pauls, D. Linking physiological processes and feeding behaviors by octopamine. Curr. Opin. Insect Sci. 2019, 36, 125–130. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kutsuna, N.; Daubnerová, I.; Roller, L.; Žitňan, D.; Nagasawa, H.; Nagata, S. Enteroendocrine peptides regulate feeding behavior via controlling intestinal contraction of the silkworm Bombyx mori. PLoS ONE 2019, 14, e0219050. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.M.; Zhang, X.Y.; Zhang, K.X.; Li, Y.; Yin, Y.S.; Zhang, R.L.; Zhang, Z. Beneficial bacteria in the intestines of housefly larvae promote larval development and humoral phenoloxidase activity, while harmful bacteria do the opposite. Front. Immunol. 2022, 13, 938972. [Google Scholar] [CrossRef]
- Zeng, T.; Jaffar, S.; Xu, Y.J.; Qi, Y.X. The intestinal immune defense system in insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.M.; Zhang, K.X.; Yin, Y.S.; Zhang, X.Y.; Zhang, Q.; Kong, X.X.; Tang, L.Y.; Zhang, R.; Zhang, Z. Serratia marcescens in the intestine of housefly larvae inhibits host growth by interfering with gut microbiota. Parasites Vectors 2023, 16, 196. [Google Scholar] [CrossRef]
- Mason, C.J.; Shikano, I. Hotter days, stronger immunity? Exploring the impact of rising temperatures on insect gut health and microbial relationships. Curr. Opin. Insect Sci. 2023, 59, 101096. [Google Scholar] [CrossRef]
- Lemoine, M.M.; Engl, T.; Kaltenpoth, M. Microbial symbionts expanding or constraining abiotic niche space in insects. Curr. Opin. Insect Sci. 2020, 39, 14–20. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, L.Y.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef]
- Jia, Y.C.; Jin, S.; Hu, K.K.; Geng, L.; Han, C.H.; Kang, R.X.; Pang, Y.X.; Ling, E.J.; Tan, E.K.; Pan, Y.F. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef]
- Shi, Z.H.; Hou, Y.M. Current understanding on the mechanism of the interactions between insects and gut microbiota and its implications in the pest control. J. Enviromrntal Entomol. 2020, 42, 798–805. [Google Scholar]
- Kang, X.L.; Zhang, J.Y.; Wang, D.; Zhao, Y.M.; Han, X.L.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. 2019, 15, e1008331. [Google Scholar] [CrossRef]
- Petruccelli, E.; Lark, A.; Mrkvicka, J.A.; Kitamoto, T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J. Neurogenet. 2020, 34, 55–68. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.A.; Yao, Z.C.; Awan, U.A.; Zhang, Z.Y.; Zheng, W.W.; Zhang, H.Y. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Wu, G. Potential effects of gut microbiota on host cancers: Focus on immunity, DNA damage, cellular pathways, and anticancer therapy. ISME J. 2023, 17, 1535–1551. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Q.; Dai, M.; Shu, Q.L.; Li, X.; Guo, X.Q.; Wang, Y.F.; Wei, J.; Liu, W.; Dai, Y. Tachinid parasitoid Exorista japonica affects the utilization of diet by changing gut microbial composition in the silkworm, Bombyx mori. Arch. Insect Biochem. Physiol. 2023, 113, e22011. [Google Scholar] [CrossRef]
- Lesperance, D.N.A.; Broderick, N.A. Gut bacteria mediate nutrient availability in drosophila diets. Appl. Environ. Microbiol. 2020, 87, e01401-20. [Google Scholar] [CrossRef]
- Bai, S.A.; Yao, Z.C.; Raza, M.F.; Cai, Z.H.; Zhang, H.Y. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef]
- Liberti, J.; Engel, P. The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13. [Google Scholar] [CrossRef]
- Trevelline, B.K.; Kohl, K.D. The gut microbiome influences host diet selection behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2117537119. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Harrold, J.A.; Dovey, T.M.; Blundell, J.E.; Halford, J.C.G. CNS regulation of appetite. Neuropharmacology 2012, 63, 3–17. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.C. Volatile organic compounds (VOCs) as environmental pollutants: Occurrence and mitigation using nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2021, 44, 1030–1043. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, S.K.; Xu, L.T. The pivotal roles of gut microbiota in insect plant interactions for sustainable pest management. npj Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, H.; Yang, X.; Gao, Y.; Lu, Y.Y.; Cheng, D.F. Gut bacteria induce oviposition preference through ovipositor recognition in fruit fly. Commun. Biol. 2022, 5, 973. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Du, Y.W.; Shi, X.B.; Zhao, L.C.; Yuan, G.G.; Zhao, W.W.; Huang, G.H.; Chen, G. Chinese cabbage changes its release of volatiles to defend against spodoptera litura. Insects 2022, 13, 73. [Google Scholar] [CrossRef]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.J.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).