Triplication of the PCDH19 Gene as a Novel Disease Mechanism Leading to Epileptic Encephalopathy Resembling Loss-of-Function Pathogenic Variants
Abstract
1. Introduction
2. Materials and Methods
3. Results
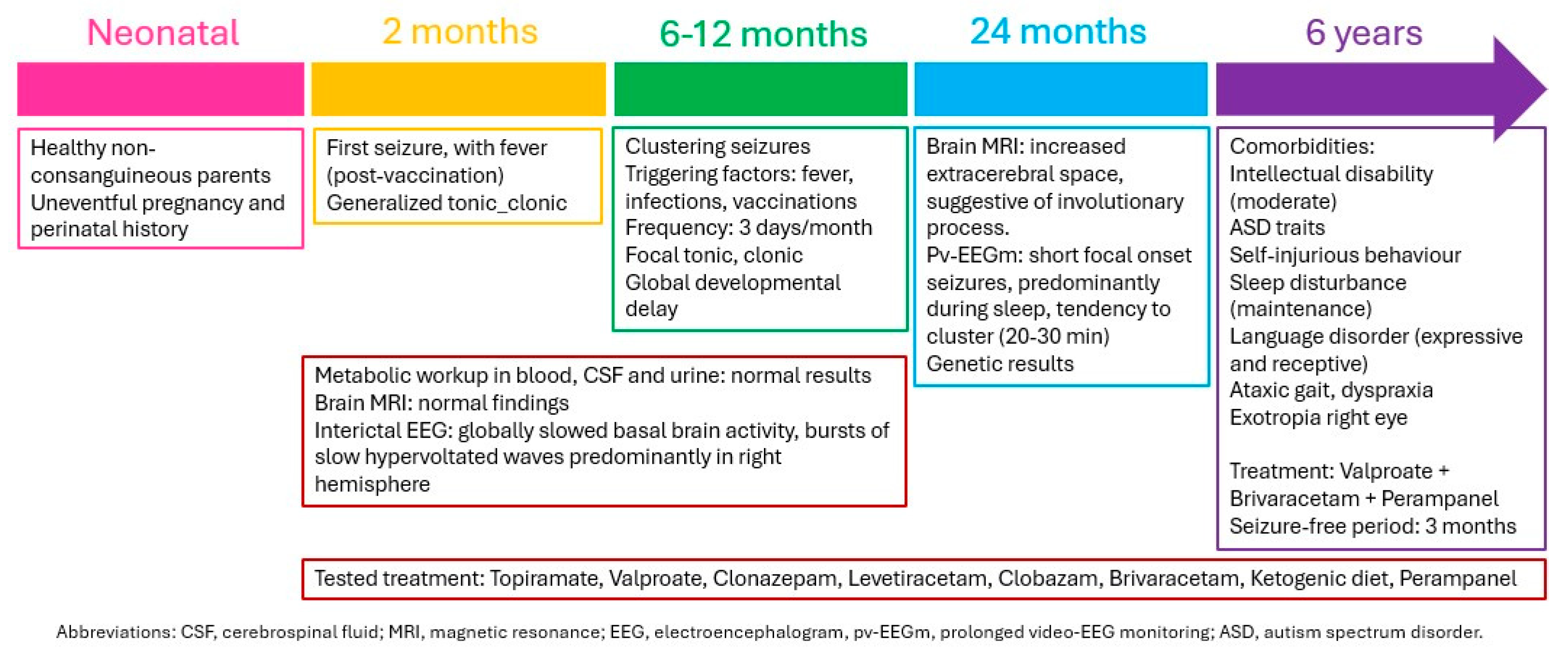
3.1. Case Reports
3.1.1. Patient 1
3.1.2. Patient 2
3.2. Genetic Studies
3.2.1. Patient 1
3.2.2. Patient 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samanta, D. PCDH19-Related Epilepsy Syndrome: A Comprehensive Clinical Review. Pediatr. Neurol. 2020, 105, 3–9. [Google Scholar] [CrossRef] [PubMed]
- de Nys, R.; Kumar, R.; Gecz, J. Protocadherin 19 clustering epilepsy and neurosteroids: Opportunities for intervention. Int. J. Mol. Sci. 2021, 22, 9769. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, G.B.; Vinti, V.; Fattorusso, A.; Tascini, G.; Mencaroni, E.; Di Cara, G.; Striano, P.; Verrotti, A. The broad clinical spectrum of epilepsies associated with Protocadherin 19 gene mutation. Front. Neurol. 2022, 12, 780053. [Google Scholar] [CrossRef]
- Mincheva-Tasheva, S.; Nieto Guil, A.F.; Homan, C.C.; Gecz, J.; Thomas, P.Q. Disrupted Excitatory Synaptic Contacts and Altered Neuronal Network Activity Underpins the Neurological Phenotype in PCDH19-Clustering Epilepsy (PCDH19-CE). Mol. Neurobiol. 2021, 58, 2005–2018. [Google Scholar] [CrossRef]
- Moncayo, J.A.; Ayala, I.N.; Argudo, J.M.; Aguirre, A.S.; Parwani, J.; Pachano, A.; Ojeda, D.; Cordova, S.; Mora, M.G.; Tapia, C.M.; et al. Understanding Protein Protocadherin-19 (PCDH19) Syndrome: A Literature Review of the Pathophysiology. Cureus 2022, 19, 12–16. [Google Scholar] [CrossRef]
- Gecz, J.; Thomas, P.Q. Disentangling the paradox of the PCDH19 clustering epilepsy, a disorder of cellular mosaics. Curr. Opin. Genet. Dev. 2020, 65, 169–175. [Google Scholar] [CrossRef]
- Pederick, D.T.; Richards, K.L.; Piltz, S.G.; Kumar, R.; Mincheva-Tasheva, S.; Mandelstam, S.A.; Dale, R.C.; Scheffer, I.E.; Gecz, J.; Petrou, S.; et al. Abnormal Cell Sorting Underlies the Unique X-Linked Inheritance of PCDH19 Epilepsy. Neuron 2018, 97, 59–66.e5. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, D.; Richieri-Costa, A.; Guion-Almeida, M.L.; Cohen, M.M. Craniofrontonasal syndrome: Study of 41 patients. Am. J. Med. Genet. 1996, 61, 147–151. [Google Scholar] [CrossRef]
- Motosugi, N.; Sugiyama, A.; Otomo, A.; Sakata, Y.; Araki, T.; Hadano, S.; Kumasaka, N.; Fukuda, A. Effect of PCDH19 missense mutations on cell-to-cell proximity and neuronal development under heterotypic conditions. PNAS Nexus 2024, 3, pgae060. [Google Scholar] [CrossRef]
- Monfort, S.; Orellana, C.; Oltra, S.; Rosello, M.; Caro-Llopis, A.; Martinez, F. Prevalence of pathogenic copy number variants among children conceived by donor oocyte. Sci. Rep. 2021, 11, 11–16. [Google Scholar] [CrossRef]
- Cutler Allen, R.; Zoghbi, H.Y.; Annemarie Moseley, I.B.; Rosenblatt, H.M.; Belmont, J.W. Methylation of Hpall and Hhal Sites Near the Polymorphic CAG Repeat in the Human Androgen-Receptor Gene Correlates with X Chromosome Inactivation. Am. J. Hum. Genet. 1992, 51, 1229–1239. [Google Scholar]
- Kohmoto, T.; Okamoto, N.; Naruto, T.; Murata, C.; Ouchi, Y.; Fujita, N.; Inagaki, H.; Satomura, S.; Okamoto, N.; Saito, M.; et al. A case with concurrent duplication, triplication, and uniparental isodisomy at 1q42.12-qter supporting microhomology-mediated break-induced replication model for replicative rearrangements. Mol. Cytogenet. 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.; Wang, J.C.; Elnaggar, M.M.; Sanchez-Lara, P.; Ross, L.P.; Mahon, L.W.; Hafezi, K.; Deming, A.; Hinman, L.; Bruno, Y.; et al. Concurrent triplication and uniparental isodisomy: Evidence for microhomology-mediated break-induced replication model for genomic rearrangements. Eur. J. Hum. Genet. 2015, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cianci, P.; Agosti, M.; Modena, P.; Selicorni, A. De novo Xq21.31-q21.32 duplication in intellectual disability: A new report. Clin. Dysmorphol. 2019, 28, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Malibari, O.I.; Al-Balawi, A.M.; Afzal, S.; Eldardear, A.E.M.; Ramzan, K. Xq21.31-q21.32 duplication underlies intellectual disability in a large family with five affected males. Am. J. Med. Genet. Part A 2016, 170, 87–93. [Google Scholar] [CrossRef]
- Vandewalle, J.; Van Esch, H.; Govaerts, K.; Verbeeck, J.; Zweier, C.; Madrigal, I.; Mila, M.; Pijkels, E.; Fernandez, I.; Kohlhase, J.; et al. Dosage-Dependent Severity of the Phenotype in Patients with Mental Retardation Due to a Recurrent Copy-Number Gain at Xq28 Mediated by an Unusual Recombination. Am. J. Hum. Genet. 2009, 85, 809–822. [Google Scholar] [CrossRef]
- Roll, P.; Rudolf, G.; Pereira, S.; Royer, B.; Scheffer, I.E.; Massacrier, A.; Valenti, M.P.; Roeckel-Trevisiol, N.; Jamali, S.; Beclin, C.; et al. SRPX2 mutations in disorders of language cortex and cognition. Hum. Mol. Genet. 2006, 15, 1195–1207. [Google Scholar] [CrossRef]
- Grozdanov, P.N.; Masoumzadeh, E.; Kalscheuer, V.M.; Bienvenu, T.; Billuart, P.; Delrue, M.A.; Latham, M.P.; MacDonald, C.C. A missense mutation in the CSTF2 gene that impairs the function of the RNA recognition motif and causes defects in 3′ end processing is associated with intellectual disability in humans. Nucleic Acids Res. 2020, 48, 9804–9821. [Google Scholar] [CrossRef]
- Veerappa, A.M.; Saldanha, M.; Padakannaya, P.; Ramachandra, N.B. Genome-wide copy number scan identifies disruption of PCDH11X in developmental dyslexia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162, 889–897. [Google Scholar] [CrossRef]
- Pancho, A.; Mitsogiannis, M.D.; Aerts, T.; Dalla, M.; Ebert, L.K.; Geenen, L.; Noterdaeme, L.; Vanlaer, R.; Stulens, A.; Hulpiau, P.; et al. Modifying PCDH19 levels affects cortical interneuron migration. Front. Neurosci. 2022, 16, 887478. [Google Scholar] [CrossRef]
- Compagni, A.; Logan, M.; Klein, R.; Adams, R.H. Control of skeletal patterning by EphrinB1-EphB interactions. Dev. Cell 2003, 5, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Babbs, C.; Stewart, H.S.; Williams, L.J.; Connell, L.; Goriely, A.; Twigg, S.R.F.; Smith, K.; Lester, T.; Wilkie, A.O.M. Duplication of the EFNB1 gene in familial hypertelorism: Imbalance in ephrin-B1 expression and abnormal phenotypes in humans and mice. Hum. Mutat. 2011, 32, 930–938. [Google Scholar] [CrossRef] [PubMed][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabaldón-Albero, A.; Smeyers, P.; Hernández-Muela, S.; Roselló, M.; Orellana, C.; Monfort, S.; Oltra, S.; Martínez, F. Triplication of the PCDH19 Gene as a Novel Disease Mechanism Leading to Epileptic Encephalopathy Resembling Loss-of-Function Pathogenic Variants. Genes 2024, 15, 1312. https://doi.org/10.3390/genes15101312
Gabaldón-Albero A, Smeyers P, Hernández-Muela S, Roselló M, Orellana C, Monfort S, Oltra S, Martínez F. Triplication of the PCDH19 Gene as a Novel Disease Mechanism Leading to Epileptic Encephalopathy Resembling Loss-of-Function Pathogenic Variants. Genes. 2024; 15(10):1312. https://doi.org/10.3390/genes15101312
Chicago/Turabian StyleGabaldón-Albero, Alba, Patricia Smeyers, Sara Hernández-Muela, Mónica Roselló, Carmen Orellana, Sandra Monfort, Silvestre Oltra, and Francisco Martínez. 2024. "Triplication of the PCDH19 Gene as a Novel Disease Mechanism Leading to Epileptic Encephalopathy Resembling Loss-of-Function Pathogenic Variants" Genes 15, no. 10: 1312. https://doi.org/10.3390/genes15101312
APA StyleGabaldón-Albero, A., Smeyers, P., Hernández-Muela, S., Roselló, M., Orellana, C., Monfort, S., Oltra, S., & Martínez, F. (2024). Triplication of the PCDH19 Gene as a Novel Disease Mechanism Leading to Epileptic Encephalopathy Resembling Loss-of-Function Pathogenic Variants. Genes, 15(10), 1312. https://doi.org/10.3390/genes15101312





