Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.4. Statistical Methods
3. Results
3.1. Main Characteristics of Study Population
3.2. Relative mRNA Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in PBMCs from Controls and Patients with MI Six Months after MI
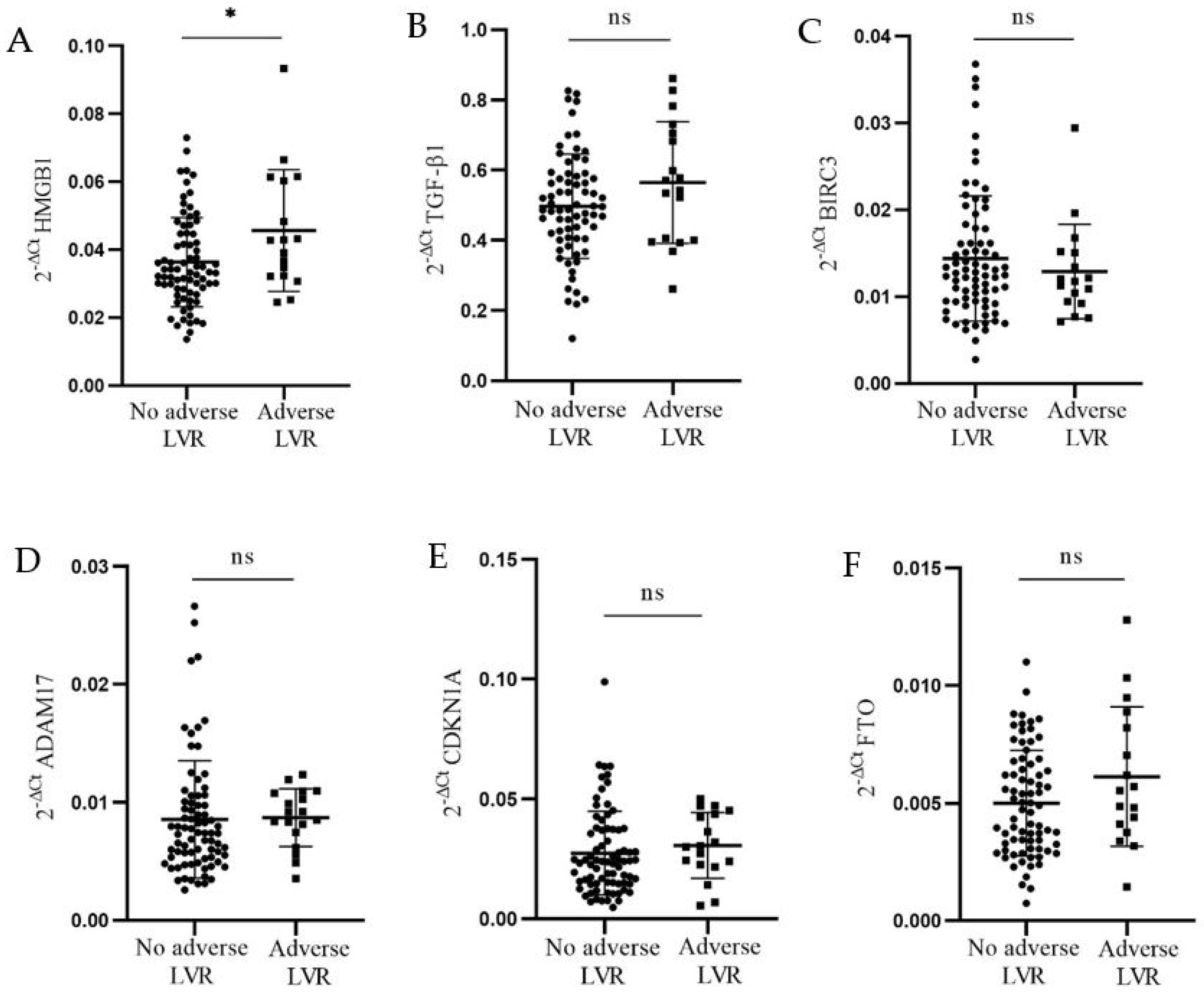
3.3. Relative mRNA Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Association with Adverse LV Remodeling and Normal Reference Intervals for LV Dimensions, Volumes, and Systolic Function
3.4. Relative mRNA Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Correlation with Left Ventricular Structure and Function Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Agrawal, D.K. Infarct Zone: A Novel Platform for Exosome Trade in Cardiac Tissue Regeneration. J. Cardiovasc. Transl. Res. 2020, 13, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Galli, A.; Lombardi, F. Postinfarct Left Ventricular Remodelling: A Prevailing Cause of Heart Failure. Cardiol. Res. Pract. 2016, 2016, 2579832. [Google Scholar] [CrossRef]
- Bulluck, H.; Go, Y.Y.; Crimi, G.; Ludman, A.J.; Rosmini, S.; Abdel-Gadir, A.; Bhuva, A.N.; Treibel, T.A.; Fontana, M.; Pica, S.; et al. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 26. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: From physics to clinical practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Foglio, E.; Pellegrini, L.; Russo, M.A.; Limana, F. HMGB1-Mediated Activation of the Inflammatory-Reparative Response Following Myocardial Infarction. Cells 2022, 11, 216. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, J.; Zhang, J.; Xiao, W. MiR-708-3p Alleviates Inflammation and Myocardial Injury After Myocardial Infarction by Suppressing ADAM17 Expression. Inflammation 2021, 44, 1083–1095. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Wang, J.; Hong, S.; Han, F.; Chen, J.; Chen, G. HMGB1 induces lung fibroblast to myofibroblast differentiation through NF-κB-mediated TGF-β1 release. Mol. Med. Rep. 2017, 15, 3062–3068. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Adamiak, M.; Mayourian, J.; Sassi, Y.; Liang, Y.; Agarwal, N.; Jha, D.; Zhang, S.; Kohlbrenner, E.; Chepurko, E.; et al. FTO-Dependent N6-Methyladenosine Regulates Cardiac Function During Remodeling and Repair. Circulation 2019, 139, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Guo, R.; Zhao, L.; Yan, J.; Gao, C. Comprehensive Analysis of N6-Methyladenosine RNA Methylation Regulators in the Diagnosis and Subtype Classification of Acute Myocardial Infarction. J. Immunol. Res. 2022, 2022, 5173761. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Cao, S.; Tian, Y.; Liu, D.; Chen, L.; Chai, Q.; Wei, M.; Sun, S.; Wang, L.; Xin, S.; et al. Potential diagnostic biomarkers: 6 cuproptosis- and ferroptosis-related genes linking immune infiltration in acute myocardial infarction. Genes Immun. 2023, 24, 159–170. [Google Scholar] [CrossRef]
- Alonso, J.; Galán, M.; Martí-Pàmies, I.; Romero, J.M.; Camacho, M.; Rodríguez, C.; Martínez-González, J. NOR-1/NR4A3 regulates the cellular inhibitor of apoptosis 2 (cIAP2) in vascular cells: Role in the survival response to hypoxic stress. Sci. Rep. 2016, 6, 34056. [Google Scholar] [CrossRef] [PubMed]
- Philip, L.; Shivakumar, K. cIAP-2 protects cardiac fibroblasts from oxidative damage: An obligate regulatory role for ERK1/2 MAPK and NF-κB. J. Mol. Cell. Cardiol. 2013, 62, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bruyninckx, R.; Aertgeerts, B.; Bruyninckx, P.; Buntinx, F. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: A diagnostic meta-analysis. Br. J. Gen. Pract. 2008, 58, 105–111. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Dabhadkar, K.C.; Agarwal, S.; Aronow, W.S.; Timmermans, R.; Jain, D.; Cooper, H.A.; Frishman, W.H.; Menon, V.; et al. Trends in Coronary Angiography, Revascularization, and Outcomes of Cardiogenic Shock Complicating Non-ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2016, 117, 1–9. [Google Scholar] [CrossRef]
- Jobs, A.; Mehta, S.R.; Montalescot, G.; Vicaut, E.; Van’t Hof, A.W.J.; Badings, E.A.; Neumann, F.J.; Kastrati, A.; Sciahbasi, A.; Reuter, P.G.; et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: A meta-analysis of randomised trials. Lancet 2017, 390, 737–746. [Google Scholar] [CrossRef]
- Djordjevic, A.; Dekleva, M.; Zivkovic, M.; Stankovic, A.; Markovic Nikolic, N.; Alavantic, D.; Djuric, T. Left ventricular remodeling after the first myocardial infarction in association with LGALS-3 neighbouring variants rs2274273 and rs17128183 and its relative mRNA expression: A prospective study. Mol. Biol. Rep. 2018, 45, 2227–2236. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.; Huang, H.; Chen, W.; Liu, L.; Wang, B.; Lai, W.; Yi, S.; Ying, M.; Tang, R.; et al. Dilated Left Ventricular End-Diastolic Diameter Is a New Risk Factor of Acute Kidney Injury Following Coronary Angiography. Front. Cardiovasc. Med. 2022, 9, 827524. [Google Scholar] [CrossRef] [PubMed]
- Harkness, A.; Ring, L.; Augustine, D.X.; Oxborough, D.; Robinson, S.; Sharma, V.; Education Committee of the British Society of Echocardiography. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G1–G18, Erratum in Echo Res. Pract. 2020, 7, X1. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.H.; Zhang, M.; Yin, L.X.; Zhang, C.; Xu, M.J.; Deng, Y.; Liu, Y.; Deng, Y.B.; Ren, W.D.; Li, Z.A.; et al. Doppler Echocardiographic Measurements in Normal Chinese Adults (EMINCA): A prospective, nationwide, and multicentre study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 512–522. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef]
- Savoye, C.; Equine, O.; Tricot, O.; Nugue, O.; Segrestin, B.; Sautière, K.; Elkohen, M.; Pretorian, E.M.; Taghipour, K.; Philias, A.; et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am. J. Cardiol. 2006, 98, 1144–1149. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369; quiz 453–455. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012, 14, 803–869. [Google Scholar] [CrossRef]
- New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels; Little, Brown: Boston, MA, USA, 1994. [Google Scholar]
- Anzai, A.; Ko, S.; Fukuda, K. Immune and Inflammatory Networks in Myocardial Infarction: Current Research and Its Potential Implications for the Clinic. Int. J. Mol. Sci. 2022, 23, 5214. [Google Scholar] [CrossRef]
- Wahid, A.; Wen, J.; Yang, Q.; Zhang, Z.; Zhao, X.; Tang, X. Serum HMGB1 is a biomarker for acute myocardial infarction with or without heart failure. Clin. Transl. Sci. 2023, 16, 2299–2309. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Rosas-Ballina, M.; Huston, J.M.; Czura, C.J.; Lee, D.C.; Ward, M.F.; Bruchfeld, A.N.; Wang, H.; et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 2006, 25, 571–574. [Google Scholar] [CrossRef]
- Kohno, T.; Anzai, T.; Naito, K.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; Maekawa, Y.; Takahashi, T.; Yoshikawa, T.; et al. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc. Res. 2009, 81, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Guerra, F.; Pizzi, C.; Merlo, M.; Zilio, F.; Bianco, F.; Mancone, M.; Zaffalon, D.; Gioscia, R.; Bergamaschi, L.; et al. Characteristics of patients with recurrent acute myocardial infarction after MINOCA. Prog. Cardiovasc. Dis. 2023, 81, 42–47. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Greenberg, B.H.; Kopelen, H.A.; Koilpillai, C.; Limacher, M.C.; Shindler, D.M.; Shelton, B.J.; Weiner, D.H. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: Significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2000, 35, 1237–1244. [Google Scholar] [CrossRef]
- Andrassy, M.; Volz, H.C.; Riedle, N.; Gitsioudis, G.; Seidel, C.; Laohachewin, D.; Zankl, A.R.; Kaya, Z.; Bierhaus, A.; Giannitsis, E.; et al. HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. J. Intern. Med. 2011, 270, 245–253. [Google Scholar] [CrossRef]
- Bobik, A. Transforming growth factor-betas and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1712–1720. [Google Scholar] [CrossRef]
- Rainer, P.P.; Hao, S.; Vanhoutte, D.; Lee, D.I.; Koitabashi, N.; Molkentin, J.D.; Kass, D.A. Cardiomyocyte-specific transforming growth factor β suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ. Res. 2014, 114, 1246–1257. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Werner, F.; Jain, M.K.; Feinberg, M.W.; Sibinga, N.E.; Pellacani, A.; Wiesel, P.; Chin, M.T.; Topper, J.N.; Perrella, M.A.; Lee, M.E. Transforming growth factor-β 1 inhibition of macrophage activation is mediated via Smad3. J. Biol. Chem. 2000, 275, 36653–36658. [Google Scholar] [CrossRef]
- Farbehi, N.; Patrick, R.; Dorison, A.; Xaymardan, M.; Janbandhu, V.; Wystub-Lis, K.; Ho, J.W.; Nordon, R.E.; Harvey, R.P. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife 2019, 8, e43882. [Google Scholar] [CrossRef]
- Ser, Ö.S.; Çetinkal, G.; Kiliçarslan, O.; Dalgıç, Y.; Batit, S.; Keskin, K.; Özkara, G.; Aslan, E.I.; Aydoğan, H.Y.; Yıldız, A.; et al. The comparison of serum TGF-β levels and associated polymorphisms in patients with coronary artery ectasia and normal coronary artery. Egypt. Heart J. 2021, 73, 32. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Saar, K.; Nazari-Jahantigh, M.; Gogiraju, R.; Wiedenroth, C.B.; Münzel, T.; Mayer, E.; Fink, L.; Schober, A.; Hübner, N.; et al. Endothelial Overexpression of TGF-β-Induced Protein Impairs Venous Thrombus Resolution: Possible Role in CTEPH. JACC Basic. Transl. Sci. 2023, 9, 100–116. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Takemura, G.; Kanoh, M.; Li, Y.; Koda, M.; Kawase, Y.; Maruyama, R.; Okada, H.; Minatoguchi, S.; Fujiwara, T.; et al. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation 2003, 108, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Porter, K.E. Function and fate of myofibroblasts after myocardial infarction. Fibrogenes. Tissue Repair. 2013, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Scheubel, R.J.; Bartling, B.; Simm, A.; Silber, R.E.; Drogaris, K.; Darmer, D.; Holtz, J. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: Fragile balance of myocyte survival? J. Am. Coll. Cardiol. 2002, 39, 481–488. [Google Scholar] [CrossRef]
- Shimoda, Y.; Satoh, M.; Nakamura, M.; Akatsu, T.; Hiramori, K. Activated tumour necrosis factor-α shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin. Sci. 2005, 108, 339–347. [Google Scholar] [CrossRef]
- Satoh, M.; Ishikawa, Y.; Itoh, T.; Minami, Y.; Takahashi, Y.; Nakamura, M. The expression of TNF-α converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2008, 38, 97–105. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Shen, M.; Wang, W.; Wang, X.; Basu, R.; Oudit, G.Y.; Kassiri, Z. Cardiomyocyte A Disintegrin and Metalloproteinase 17 (ADAM17) Is Essential in Post-Myocardial Infarction Repair by Regulating Angiogenesis. Circ. Heart Fail. 2015, 8, 970–979. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Zhao, J.; Yang, J.M.; Wang, M.; Zhang, X.T. Enhanced ADAM17 expression is associated with cardiac remodeling in rats with acute myocardial infarction. Life Sci. 2016, 151, 61–69. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Bie, B.; Zhao, B.; Zhang, Y.; Fang, S.; Li, S.; Zhang, Y. P38 MAPK activated ADAM17 mediates ACE2 shedding and promotes cardiac remodeling and heart failure after myocardial infarction. Cell Commun. Signal. 2023, 21, 73. [Google Scholar] [CrossRef]
- Rodríguez, I.; Coto, E.; Reguero, J.R.; González, P.; Andrés, V.; Lozano, I.; Martín, M.; Alvarez, V.; Morís, C. Role of the CDKN1A/p21, CDKN1C/p57, and CDKN2A/p16 genes in the risk of atherosclerosis and myocardial infarction. Cell Cycle 2007, 6, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, L. Functional analysis of keratinocyte and fibroblast gene expression in skin and keloid scar tissue based on deviation analysis of dynamic capabilities. Exp. Ther. Med. 2016, 12, 3633–3641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, K.; Li, Z.; Ma, W.; Sun, Y.; Liu, X.; Qian, L.; Hong, J.; Lu, D.; Zhang, J.; Xu, D. Construction of miRNA-mRNA network reveals crucial miRNAs and genes in acute myocardial infarction. J. Biomed. Res. 2021, 35, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Han, X.; He, L.; Li, Y.; Huang, X.; Zhang, M.; Lv, Z.; Yu, W.; Wang, Q.D.; Cai, D.; et al. A genetic system for tissue-specific inhibition of cell proliferation. Development 2020, 147, dev183830. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.T.; Sharp, P.A.; et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell. 2014, 54, 777–790. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y. A New linc(-RNA) Between NFAT/MEF2 and Cardiac Hypertrophy. Circ. Res. 2024, 135, 450–452. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Cui, X.; Jiang, H.; Luo, W.; Weng, X.; Wang, Y.; Zhao, Y.; Sun, A.; Ge, J. Alteration of m6A RNA Methylation in Heart Failure with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 647806. [Google Scholar] [CrossRef]


| Variable | Controls, N = 24 | MI Patients, N = 95 | p |
|---|---|---|---|
| Age, years | 48.5 ± 8.5 | 55.2 ± 7.7 | 0.001 § |
| Gender, f/m, % | 48.0/52.0 | 22.1/77.9 | 0.01 |
| BMI, kg/m2 | 26.07 ± 3.28 | 27.15 ± 3.81 | ns § |
| TC, mmol/L | 5.92 ± 0.97 | 5.46 ± 1.04 | ns ¥ |
| HDLC, mmol/L | 1.52 ± 0.31 | 1.08 ± 0.27 | <0.001 § |
| LDLC, mmol/L | 3.81 ± 0.88 | 3.52 ± 0.98 | ns ¥ |
| TG, mmol/L | 1.47 ± 0.90 | 1.86 ± 1.20 | 0.05 § |
| T2DM, % | 0 | 34.7 | N/A |
| Hypertension, % | 0 | 48.4 | N/A |
| Current smokers, % | 39.1 | 63.2 | 0.04 |
| Variable | Patients without Adverse LVR | Patients with Adverse LVR | p-Value |
|---|---|---|---|
| N = 77 | N = 18 | ||
| Demographic characteristics, laboratory data and risk factors (baseline values) | |||
| Age, years | 55.4 ± 7.8 | 54.3 ± 7.7 | 0.57 |
| Gender, f/m, % | 20.8/79.2 | 27.8/72.2 | 0.52 |
| BMI, kg/m2 | 27.04 ± 3.61 | 26.83 ± 4.29 | 0.64 |
| TC, mmol/L | 5.43 ± 1.04 | 5.61 ± 1.08 | 0.52 ¥ |
| HDLC, mmol/L | 1.07 ± 0.25 | 1.14 ± 0.33 | 0.39 |
| LDLC, mmol/L | 3.45 ± 0.97 | 3.79 ± 0.99 | 0.19 ¥ |
| TG, mmol/L | 1.94 ± 1.28 | 1.49 ± 0.70 | 0.16 |
| T2DM, % | 37.7 | 22.2 | 0.21 |
| Hypertension, % | 49.3 | 44.4 | 0.71 |
| Current smokers, % | 61.0 | 72.2 | 0.38 |
| Glucose, mmol/L | 9.67 ± 5.32 | 8.22 ± 4.52 | 0.18 |
| CKmax, U/L | 1773.77 ± 1618.62 | 2955.11 ± 2347.95 | 0.05 |
| CK-MBmax, U/L | 159.29 ± 136.30 | 247.65 ± 202.03 | 0.08 |
| Tnmax, U/L | 207.80 ± 842.54 | 116.85 ± 63.17 | 0.19 |
| CRP, mg/L | 28.30 ± 38.64 | 22.06 ± 36.38 | 0.55 |
| HR, beats per minute | 75.8 ± 18.1 | 77.5 ± 12.4 | 0.29 |
| Systolic blood pressure, mm Hg | 129.7 ± 28.2 | 125.6 ± 24.5 | 0.77 |
| Diastolic blood pressure, mm Hg | 80.5 ± 16.3 | 76.6 ± 13.1 | 0.22 |
| Echocardiography (six-month follow-up point) | |||
| LVEDD, mm | 54.2 ± 5.8 | 58.6 ± 8.8 | 0.01 ¥ |
| LVESD, mm | 39.4 ± 6.5 | 43.8 ± 9.7 | 0.06 |
| LVEF, % | 46.3 ± 9.0 | 42.2 ± 9.9 | 0.1 ¥ |
| SV, mL | 75.7 ± 13.8 | 80.7 ± 22.1 | 0.23 ¥ |
| LVEDVi, mL/m2 | 55.2 ± 14.0 | 69.6 ± 21.2 | 0.01 |
| LVESVi, mL/m2 | 31.8 ± 11.7 | 42.9 ± 18.3 | 0.01 |
| LVMi, g/m2 | 109.8 ± 26.2 | 126.0 ± 27.1 | 0.02 ¥ |
| ∆LVEDD, mm | 0.1 ± 4.2 | 3.7 ± 5.0 | 0.002 ¥ |
| ∆LVESD, mm | −0.2 ± 4.9 | 0.9 ± 7.9 | 0.31 |
| ∆LVEF, % | 1.5 ± 7.3 | −1.1 ± 7.4 | 0.18 ¥ |
| ∆LVEDVi, mL | −2.4 ± 10.1 | 19.9 ± 8.4 | <0.001 |
| ∆LVESVi, mL | −1.1 ± 7.1 | 13.2 ± 8.9 | <0.001 |
| ∆SV, mL | 3.7 ± 16.5 | 4.6 ± 26.4 | 0.86 ¥ |
| ∆LVMi, g/m2 | −5.2 ± 23.6 | 12.8 ± 22.1 | 0.01 ¥ |
| Advanced HF, % | 4.2 | 12.5 | 0.19 |
| LVEF < 55% | 77.9 | 94.4 | 0.11 |
| Severe systolic dysfunction, % | 10.4 | 22.2 | 0.17 |
| LVEDD dilated, % | 37.7 | 66.7 | 0.02 |
| LVESD dilated, % | 15.6 | 33.3 | 0.08 |
| LVEDVi dilated, % | 6.5 | 33.3 | 0.001 |
| LVESVi dilated, % | 53.3 | 72.2 | 0.14 |
| LVESVi severely dilated, % | 16.9 | 38.9 | 0.04 |
| LVH, % | 48.0 | 72.2 | 0.06 |
| LVE, % | 35.1 | 55.6 | 0.11 |
| Post-MI discharge medications (%) | |||
| Aspirin | 100 | 100 | 1 |
| Clopidogrel | 98.7 | 100 | 0.63 |
| LMWH | 97.4 | 94.4 | 0.52 |
| UFH | 57.1 | 77.8 | 0.11 |
| Nitrates | 96.1 | 88.9 | 0.22 |
| ACE inhibitors | 98.7 | 94.44 | 0.26 |
| β-blockers | 77.9 | 77.8 | 0.99 |
| Diuretics | 18.2 | 16.7 | 0.88 |
| Statins | 98.7 | 94.4 | 0.26 |
| LV Parameter Change (∆) | 2−ΔCt HMGB1 | 2−ΔCt TGFβ | 2−ΔCt BIRC3 | 2−ΔCt ADAM17 | 2−ΔCt CDKN1A | 2−ΔCt FTO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | |
| ∆ LVEDD, mm | 0.26 | 0.01 | 0.21 | 0.05 | 0.08 | 0.43 | 0.1 | 0.32 | 0.2 | 0.06 | 0.23 | 0.03 |
| ∆ LVESD, mm | 0.07 | 0.5 | 0.09 | 0.42 | 0.2 | 0.06 | 0.04 | 0.72 | 0.06 | 0.59 | 0.14 | 0.19 |
| ∆ LVEF, % | −0.13 | 0.22 | −0.13 | 0.23 | −0.16 | 0.12 | 0.03 | 0.73 | 0.07 | 0.48 | −0.02 | 0.87 |
| ∆ LVEDVi, mL/m2 | 0.25 | 0.02 | 0.18 | 0.09 | −0.18 | 0.08 | 0.02 | 0.85 | 0.06 | 0.58 | 0.02 | 0.84 |
| ∆ LVESVi, mL/m2 | 0.23 | 0.03 | 0.19 | 0.07 | −0.02 | 0.84 | 0.01 | 0.88 | 0.02 | 0.86 | 0.02 | 0.81 |
| ∆ SV, mL | 0.21 | 0.05 | 0.2 | 0.06 | −0.01 | 0.94 | 0.44 | 0.67 | 0.06 | 0.58 | 0.04 | 0.67 |
| ∆ LVMi, g/m2 | 0.22 | 0.05 | 0.18 | 0.1 | −0.05 | 0.63 | 0.19 | 0.08 | 0.11 | 0.31 | 0.1 | 0.35 |
| ∆ GLS, % | −0.11 | 0.28 | 0.001 | 0.99 | 0.27 | 0.01 | −0.02 | 0.84 | −0.01 | 0.92 | −0.03 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuveljic, J.; Djordjevic, A.; Zivotic, I.; Dekleva, M.; Kolakovic, A.; Zivkovic, M.; Stankovic, A.; Djuric, T. Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study. Genes 2024, 15, 1296. https://doi.org/10.3390/genes15101296
Kuveljic J, Djordjevic A, Zivotic I, Dekleva M, Kolakovic A, Zivkovic M, Stankovic A, Djuric T. Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study. Genes. 2024; 15(10):1296. https://doi.org/10.3390/genes15101296
Chicago/Turabian StyleKuveljic, Jovana, Ana Djordjevic, Ivan Zivotic, Milica Dekleva, Ana Kolakovic, Maja Zivkovic, Aleksandra Stankovic, and Tamara Djuric. 2024. "Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study" Genes 15, no. 10: 1296. https://doi.org/10.3390/genes15101296
APA StyleKuveljic, J., Djordjevic, A., Zivotic, I., Dekleva, M., Kolakovic, A., Zivkovic, M., Stankovic, A., & Djuric, T. (2024). Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study. Genes, 15(10), 1296. https://doi.org/10.3390/genes15101296






