Stakeholder Perception of the Implementation of Genetic Risk Testing for Twelve Multifactorial Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Participant Characteristics
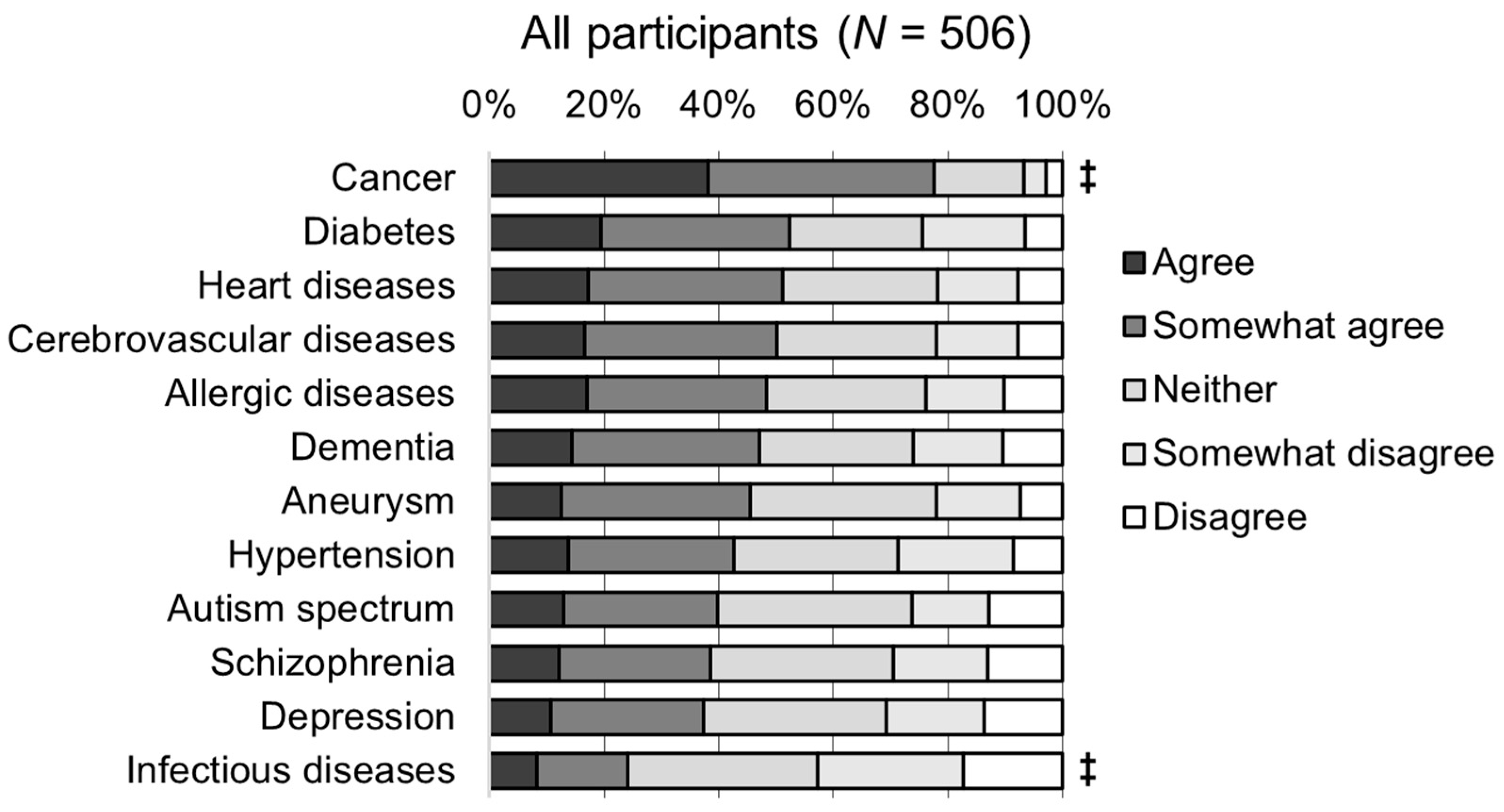
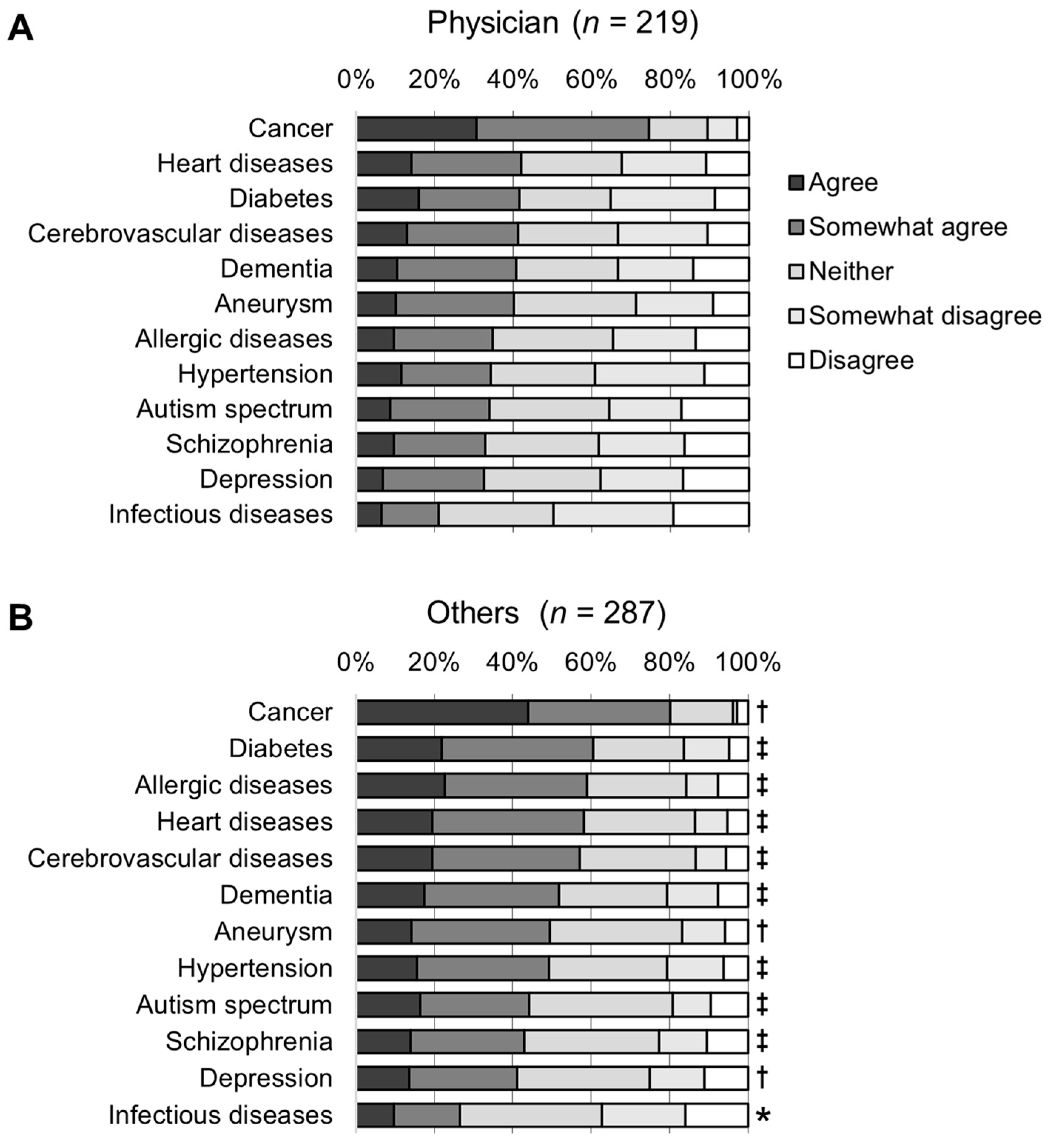
3.2. Awareness of Genetic Testing for Risk Prediction
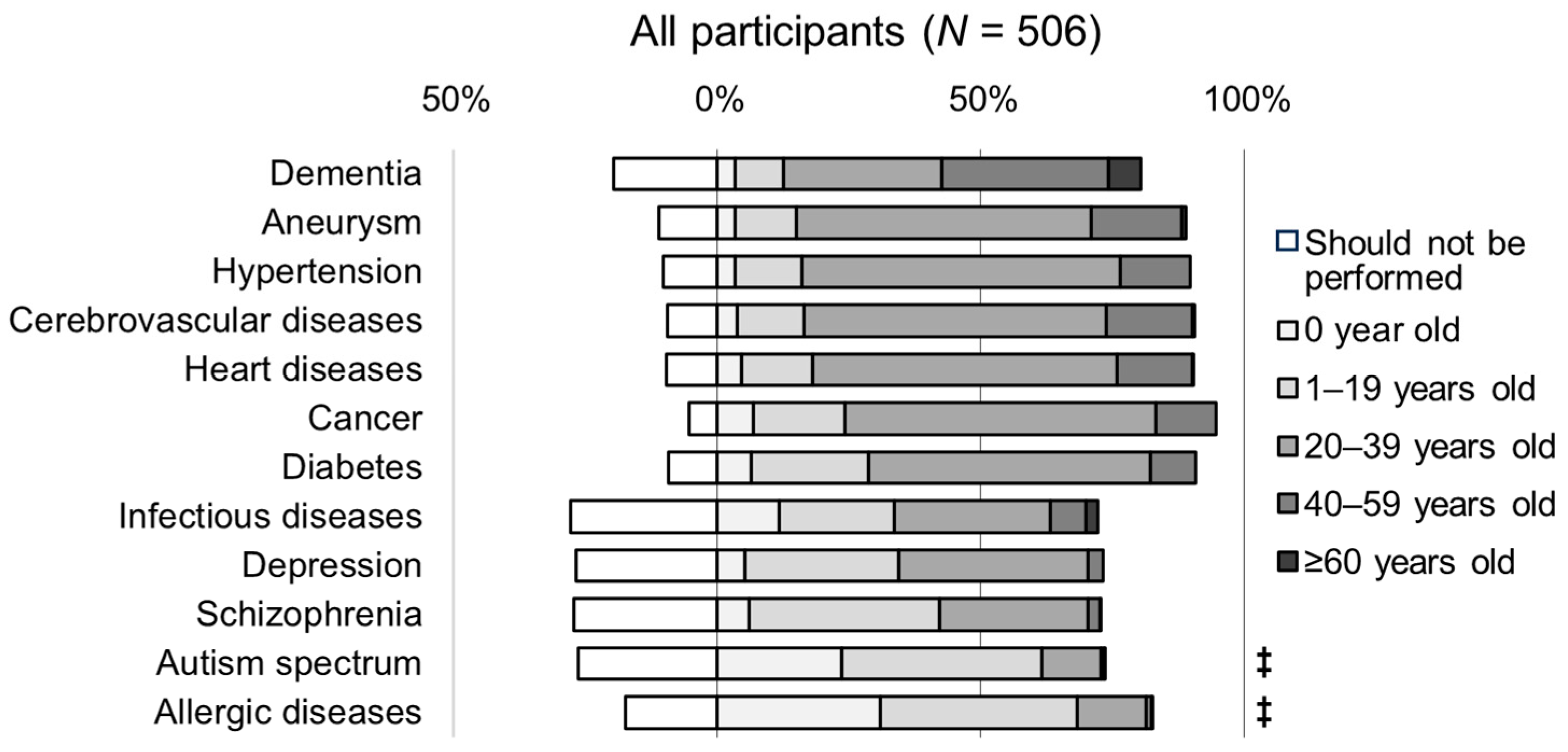
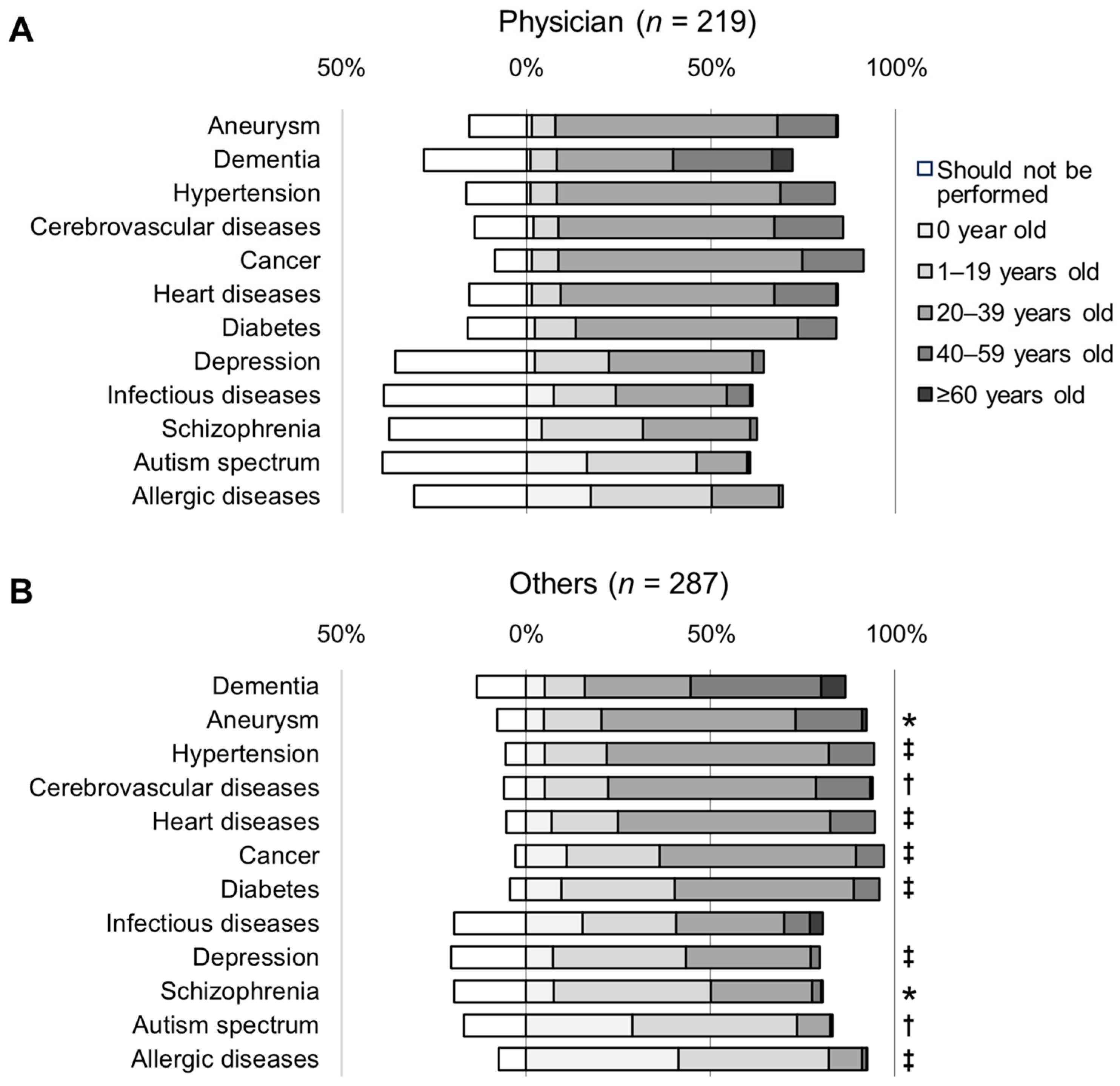
3.3. Awareness of the Appropriate Age for Genetic Testing
3.4. Awareness of Diseases Related to Participants’ Practice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013; pp. 7–13. ISBN 978-92-4-150623-6. Available online: https://www.who.int/southeastasia/publications-detail/9789241506236 (accessed on 25 November 2023).
- Biscetti, F.; Straface, G.; Giovannini, S.; Santoliquido, A.; Angelini, F.; Santoro, L.; Porreca, C.F.; Pecorini, G.; Ghirlanda, G.; Flex, A. Association between TNFRSF11B gene polymorphisms and history of ischemic stroke in Italian diabetic patients. Hum. Genet. 2013, 132, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Naji, D.H.; Wang, B.; Zhao, Y.; Wang, J.; Wang, D.; Zhang, Y.; Li, S.; Chen, S.; Huang, Y.; et al. Significant Association between OPG/TNFRSF11B Variant and Common Complex Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Läll, K.; Mägi, R.; Morris, A.; Metspalu, A.; Fischer, K. Personalized risk prediction for type 2 diabetes: The potential of genetic risk scores. Genet. Med. 2017, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Abraham, G.; Nelson, C.P.; Wood, A.M.; Sweeting, M.J.; Dudbridge, F.; Lai, F.Y.; Kaptoge, S.; Brozynska, M.; Wang, T.; et al. Genomic risk prediction of coronary artery disease in 480,000 adults: Implications for primary prevention. J. Am. Coll. Cardiol. 2018, 72, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Bodinier, B.; Bond, T.A.; Chadeau-Hyam, M.; Evangelou, E.; Moons, K.G.M.; Dehghan, A.; Muller, D.C.; Elliott, P.; Tzoulaki, I. Predictive accuracy of a polygenic risk score-enhanced prediction model vs a clinical risk score for coronary artery disease. JAMA 2020, 323, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Hata, J.; Hirakawa, Y.; Yoshida, D.; Furuta, Y.; Kitazono, T.; Shimizu, A.; Ninomiya, T. Genome-wide polygenic score and the risk of ischemic stroke in a prospective cohort: The Hisayama study. Stroke 2020, 51, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.F.; Green, R.C. Polygenic risk scores in the clinic: New perspectives needed on familiar ethical issues. Genome Med. 2021, 13, 14. [Google Scholar] [CrossRef]
- Prime Minister of Japan and His Cabinet. Multi-Layered and Integrated Promotion of Genomic Medicine and Precision Medicine. Available online: https://www.kantei.go.jp/jp/singi/kenkouiryou/genome/genome_dai1/siryou3.pdf (accessed on 25 November 2023). (In Japanese).
- Clinicaltrials.gov. NCT03583983, NCT04291157, NCT04331535, NCT04604353. Available online: https://clinicaltrials.gov (accessed on 25 November 2023).
- GOV.UK. Advancing Our Health: Prevention in the 2020s—Consultation Document. Available online: https://www.gov.uk/government/consultations/advancing-our-health-prevention-in-the-2020s/advancing-our-health-prevention-in-the-2020s-consultation-document (accessed on 25 November 2023).
- Esserman, L.J.; WISDOM Study and Athena Investigators. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017, 3, 34. [Google Scholar] [CrossRef]
- Linder, J.E.; Allworth, A.; Bland, H.T.; Caraballo, P.J.; Chisholm, R.L.; Clayton, E.W.; Crosslin, D.R.; Dikilitas, O.; DiVietro, A.; Esplin, E.D.; et al. Returning integrated genomic risk and clinical recommendations: The eMERGE study. Genet. Med. 2023, 25, 100006. [Google Scholar] [CrossRef]
- Kuriyama, S.; Yaegashi, N.; Nagami, F.; Arai, T.; Kawaguchi, Y.; Osumi, N.; Sakaida, M.; Suzuki, Y.; Nakayama, K.; Hashizume, H.; et al. The Tohoku Medical Megabank Project: Design and mission. J. Epidemiol. 2016, 26, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Kawame, H.; Fukushima, A.; Fuse, N.; Nagami, F.; Suzuki, Y.; Sakurai-Yageta, M.; Yasuda, J.; Yamaguchi-Kabata, Y.; Kinoshita, K.; Ogishima, S.; et al. The return of individual genomic results to research participants: Design and pilot study of Tohoku Medical Megabank Project. J. Hum. Genet. 2022, 67, 9–17. [Google Scholar] [CrossRef]
- Ohneda, K.; Hamanaka, Y.; Kawame, H.; Fuse, N.; Nagami, F.; Suzuki, Y.; Yamaguchi-Kabata, Y.; Shimada, M.; Masamune, A.; Aoki, Y.; et al. Returning individual genomic results to population-based cohort study participants with BRCA1/2 pathogenic variants. Breast Cancer 2023, 30, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ohneda, K.; Hiratsuka, M.; Kawame, H.; Nagami, F.; Suzuki, Y.; Suzuki, K.; Uruno, A.; Sakurai-Yageta, M.; Hamanaka, Y.; Taira, M.; et al. A pilot study for return of individual pharmacogenomic results to population-based cohort study participants. JMA J. 2022, 5, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Greendale, K.; Pyeritz, R.E. Empowering primary care health professionals in medical genetics: How soon? How fast? How far? Am. J. Med. Genet. 2001, 106, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher, A.E.; Jenkins, J.; Uhlmann, W.R. Genomic medicine: Who will practice it? A call to open arms. Am. J. Med. Genet. 2001, 106, 216–222. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dent, T.; Pashayan, N.; Hall, A.; Lyratzopoulos, G.; Hallowell, N.; Hall, P.; Pharoah, P.; Burton, H. Incorporating genomics into breast and prostate cancer screening: Assessing the implications. Genet. Med. 2013, 15, 423–432. [Google Scholar] [CrossRef]
- Chowdhury, S.; Henneman, L.; Dent, T.; Hall, A.; Burton, A.; Pharoah, P.; Pashayan, N.; Burton, H. Do health professionals need additional competencies for stratified cancer prevention based on genetic risk profiling? J. Pers. Med. 2015, 5, 191–212. [Google Scholar] [CrossRef]
- Smit, A.K.; Sharman, A.R.; Espinoza, D.; Wallingford, C.; Young, M.A.; Dunlop, K.; Tiller, J.; Newson, A.J.; Meiser, B.; Cust, A.E.; et al. Knowledge, views and expectations for cancer polygenic risk testing in clinical practice: A cross-sectional survey of health professionals. Clin. Genet. 2021, 100, 430–439. [Google Scholar] [CrossRef]
- Lapointe, J.; Buron, A.C.; Mbuya-Bienge, C.; Dorval, M.; Pashayan, N.; Brooks, J.D.; Walker, M.J.; Chiquette, J.; Eloy, L.; Blackmore, K.; et al. Polygenic risk scores and risk-stratified breast cancer screening: Familiarity and perspectives of health care professionals. Genet. Med. 2022, 24, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.; Lapointe, J.; Nabi, H.; Pashayan, N. Risk-stratified breast cancer screening incorporating a polygenic risk score: A survey of UK general practitioners’ knowledge and attitudes. Genes 2023, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Cabinet Secretariat; Office of Healthcare Policy. Prime Minister of Japan and His Cabinet. Available online: https://www.kantei.go.jp/jp/singi/kenkouiryou/genome/genome_dai2/sankou2.pdf (accessed on 25 November 2023). (In Japanese).
- Cabinet Secretariat; Office of Healthcare Policy. Prime Minister of Japan and His Cabinet. Available online: https://www.kantei.go.jp/jp/singi/kenkouiryou/genome/dai6/siryou3_1.pdf (accessed on 25 November 2023). (In Japanese).
- Lambert, S.A.; Abraham, G.; Inouye, M. Towards clinical utility of polygenic risk scores. Hum. Mol. Genet. 2019, 28, R133–R142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, G.; Park, J.H.; Chatterjee, N. Estimation of complex effect-size distributions using summary-level statistics from genome-wide association studies across 32 complex traits. Nat. Genet. 2018, 50, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Vassos, E.; Di Forti, M.; Coleman, J.; Iyegbe, C.; Prata, D.; Euesden, J.; O’Reilly, P.; Curtis, C.; Kolliakou, A.; Patel, H.; et al. An examination of polygenic score risk prediction in individuals with first-episode psychosis. Biol. Psychiatry 2017, 81, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Daly, M.J.; Robinson, E.B.; Hyman, S.E.; Neale, B.M. Predicting polygenic risk of psychiatric disorders. Biol. Psychiatry 2019, 86, 97–109. [Google Scholar] [CrossRef]
- Ikeda, M.; Saito, T.; Kanazawa, T.; Iwata, N. Polygenic risk score as clinical utility in psychiatry: A clinical viewpoint. J. Hum. Genet. 2021, 66, 53–60. [Google Scholar] [CrossRef]
- Japanese Association of Medical Sciences. Guidelines for Genetic Tests and Diagnoses in Medical Practice. Available online: https://jams.med.or.jp/guideline/genetics-diagnosis_e_2022.pdf (accessed on 25 November 2023).
- Botkin, J.R.; Belmont, J.W.; Berg, J.S.; Berkman, B.E.; Bombard, Y.; Holm, I.A.; Levy, H.P.; Ormond, K.E.; Saal, H.M.; Spinner, N.B.; et al. Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am. J. Hum. Genet. 2015, 97, 6–21. [Google Scholar] [CrossRef]
- European Society of Human Genetics. Genetic testing in asymptomatic minors: Recommendations of the European Society of Human Genetics. Eur. J. Hum. Genet. 2009, 17, 720–721. [Google Scholar] [CrossRef]
- Lowstuter, K.J.; Sand, S.; Blazer, K.R.; MacDonald, D.J.; Banks, K.C.; Lee, C.A.; Schwerin, B.U.; Juarez, M.; Uman, G.C.; Weitzel, J.N. Influence of genetic discrimination perceptions and knowledge on cancer genetics referral practice among clinicians. Genet. Med. 2008, 10, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.H.; Wilfond, B.S.; McBride, C.M. Effects of genetic risk information on children’s psychosocial wellbeing: A systematic review of the literature. Genet. Med. 2010, 12, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, C.E.; Hanlon, L.V.; Tucker, K.M.; Patenaude, A.F.; Signorelli, C.; McLoone, J.K.; Cohn, R.J. The psychological impact of genetic information on children: A systematic review. Genet. Med. 2016, 18, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.R.; Lantos, J.D.; Biesecker, L.G.; Childerhose, J.E.; Chung, W.K.; Holm, I.A.; Koenig, B.A.; McEwen, J.E.; Wilfond, B.S.; Brothers, K. Clinical Sequencing Exploratory Research (CSER) Consortium Pediatrics Working Group. Rethinking the “open future” argument against predictive genetic testing of children. Genet. Med. 2019, 21, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Rose, N. Biomarkers in psychiatry. Nature 2009, 460, 202–207. [Google Scholar] [CrossRef]
- Palk, A.C.; Dalvie, S.; de Vries, J.; Martin, A.R.; Stein, D.J. Potential use of clinical polygenic risk scores in psychiatry—ethical implications and communicating high polygenic risk. Philos. Ethics Humanit. Med. 2019, 14, 4. [Google Scholar] [CrossRef]
- Chapman, C.R. Ethical, legal, and social implications of genetic risk prediction for multifactorial disease: A narrative review identifying concerns about interpretation and use of polygenic scores. J. Community Genet. 2023, 14, 441–452. [Google Scholar] [CrossRef]

| Characteristic | Number (%) | |
|---|---|---|
| Gender | ||
| Female | 267 (52.8) | |
| Male | 224 (44.3) | |
| Do not wish to disclose | 15 (3.0) | |
| Profession | ||
| Physician | 219 (43.3) | |
| Nurse | 112 (22.1) | |
| Pharmacist | 107 (21.1) | |
| Registered dietitian | 68 (13.4) | |
| Age | ||
| 20–29 | 74 (14.6) | |
| 30–39 | 167 (33.0) | |
| 40–49 | 158 (31.2) | |
| 50–59 | 76 (15.0) | |
| 60–69 | 30 (5.9) | |
| ≥70 | 1 (0.2) | |
| Years of experience | ||
| 0–10 | 177 (35.0) | |
| 11–20 | 165 (32.6) | |
| 21–30 | 118 (23.3) | |
| 31–40 | 40 (7.9) | |
| ≥41 | 6 (1.2) | |
| Diseases related to their practice (multiple answers) | ||
| Cancer | 308 (60.9) | |
| Diabetes | 195 (38.5) | |
| Hypertension | 167 (33.0) | |
| Heart diseases | 131 (25.9) | |
| Infectious diseases | 120 (23.7) | |
| Cerebrovascular diseases | 93 (18.4) | |
| Dementia | 77 (15.2) | |
| Allergic diseases | 73 (14.4) | |
| Depression | 59 (11.7) | |
| Schizophrenia | 50 (9.9) | |
| Autism spectrum | 46 (9.1) | |
| Aneurysm | 32 (6.3) | |
| Not applicable | 48 (9.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokutomi, T.; Yoshida, A.; Fukushima, A.; Nagami, F.; Minoura, Y.; Sasaki, M. Stakeholder Perception of the Implementation of Genetic Risk Testing for Twelve Multifactorial Diseases. Genes 2024, 15, 49. https://doi.org/10.3390/genes15010049
Tokutomi T, Yoshida A, Fukushima A, Nagami F, Minoura Y, Sasaki M. Stakeholder Perception of the Implementation of Genetic Risk Testing for Twelve Multifactorial Diseases. Genes. 2024; 15(1):49. https://doi.org/10.3390/genes15010049
Chicago/Turabian StyleTokutomi, Tomoharu, Akiko Yoshida, Akimune Fukushima, Fuji Nagami, Yuko Minoura, and Makoto Sasaki. 2024. "Stakeholder Perception of the Implementation of Genetic Risk Testing for Twelve Multifactorial Diseases" Genes 15, no. 1: 49. https://doi.org/10.3390/genes15010049
APA StyleTokutomi, T., Yoshida, A., Fukushima, A., Nagami, F., Minoura, Y., & Sasaki, M. (2024). Stakeholder Perception of the Implementation of Genetic Risk Testing for Twelve Multifactorial Diseases. Genes, 15(1), 49. https://doi.org/10.3390/genes15010049







