A Novel Homozygous Loss-of-Function Variant in SPRED2 Causes Autosomal Recessive Noonan-like Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Evaluation
2.2. Sample Collection
2.3. Whole Exome Sequencing
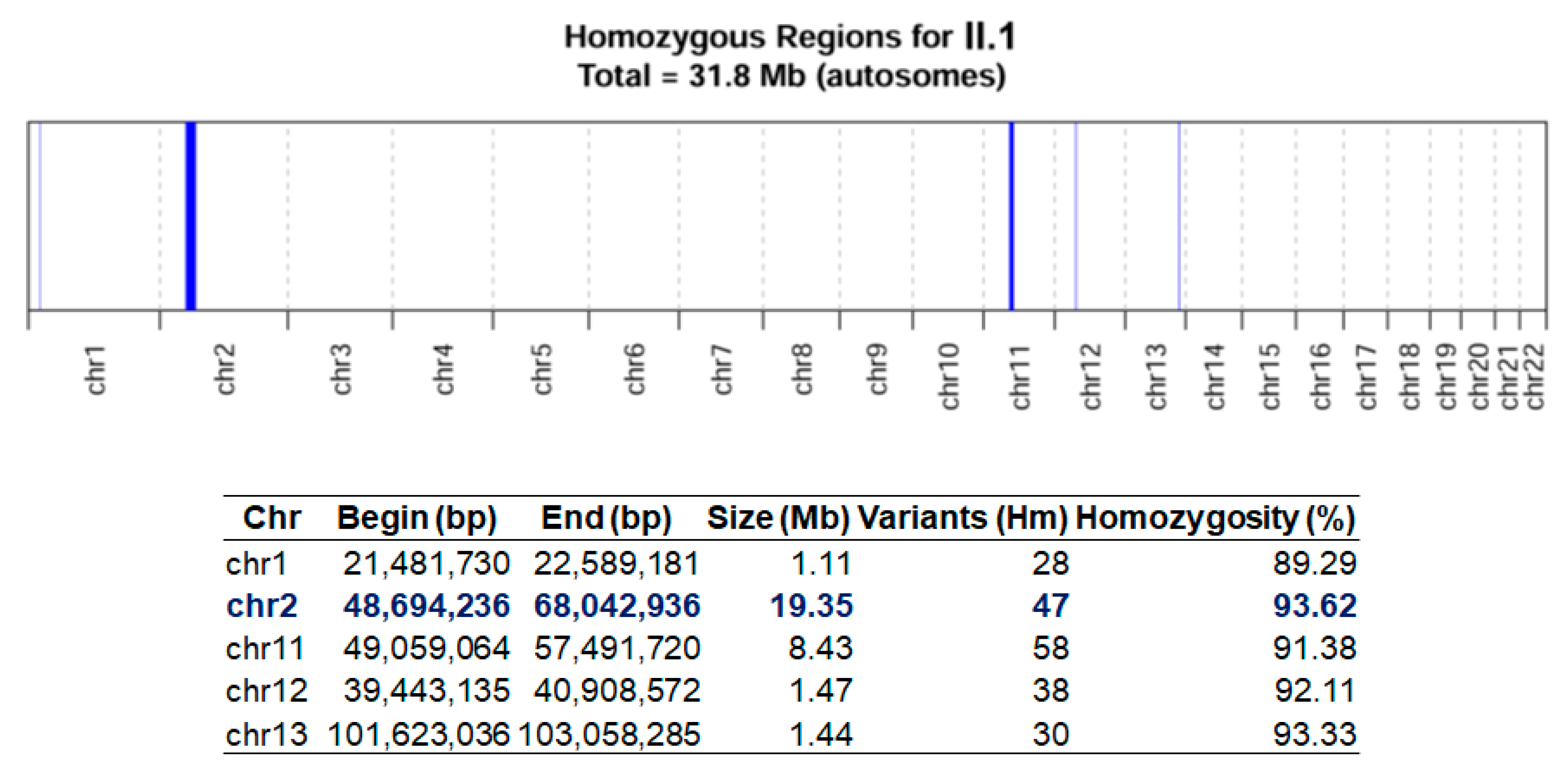
2.4. Homozygosity Mapping
2.5. Variant Validation and Segregation Analysis
3. Results
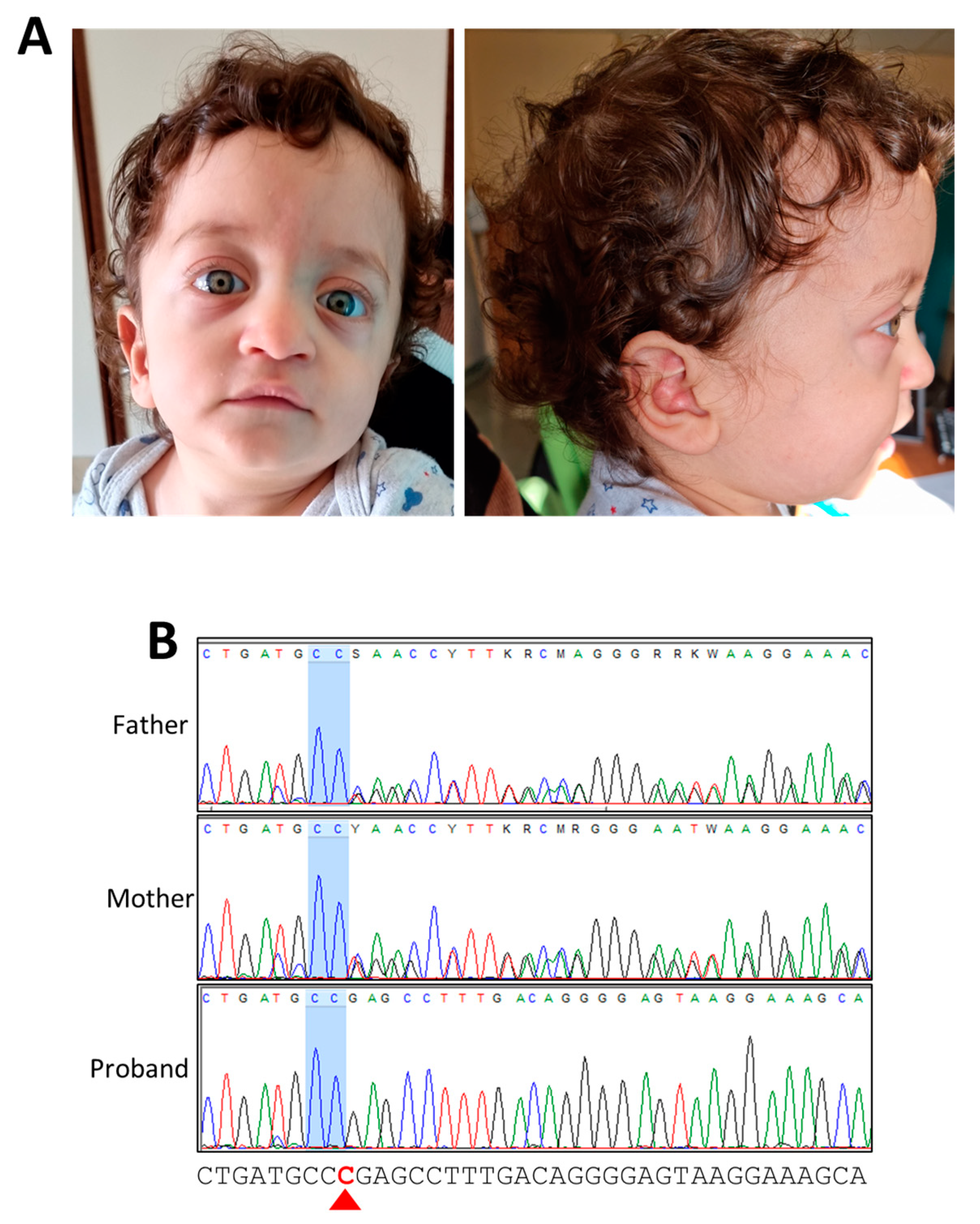
3.1. Case Presentation
3.2. Molecular Diagnosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tartaglia, M.; Aoki, Y.; Gelb, B.D. The molecular genetics of RASopathies: An update on novel disease genes and new disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Riller, Q.; Rieux-Laucat, F. RASopathies: From germline mutations to somatic and multigenic diseases. Biomed. J. 2021, 44, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Paccoud, R.; Branka, S.; Edouard, T.; Yart, A. The RASopathy Family: Consequences of Germline Activation of the RAS/MAPK Pathway. Endocr. Rev. 2018, 39, 676–700. [Google Scholar] [CrossRef] [PubMed]
- Hilal, N.; Chen, Z.; Chen, M.H.; Choudhury, S. RASopathies and cardiac manifestations. Front. Cardiovasc. Med. 2023, 10, 1176828. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E. Noonan Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/20301303/ (accessed on 1 November 2023).
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; van der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Fasano, G.; Gredy, S.; Brinkmann, J.; Bonnard, A.A.; Simsek-Kiper, P.O.; Gulec, E.Y.; Essaddam, L.; Utine, G.E.; Guarnetti Prandi, I.; et al. SPRED2 loss-of-function causes a recessive Noonan syndrome-like phenotype. Am. J. Hum. Genet. 2021, 108, 2112–2129. [Google Scholar] [CrossRef] [PubMed]
- Markholt, S.; Andreasen, L.; Bjerre, J.; Gregersen, P.A.; Andersen, B.N. Autosomal recessive Noonan-like syndrome caused by homozygosity for a previously unreported variant in SPRED2. Eur. J. Med. Genet. 2023, 66, 104695. [Google Scholar] [CrossRef]
- Wakioka, T.; Sasaki, A.; Kato, R.; Shouda, T.; Matsumoto, A.; Miyoshi, K.; Tsuneoka, M.; Komiya, S.; Baron, R.; Yoshimura, A. Spred is a Sprouty-related suppressor of Ras signalling. Nature 2001, 412, 647–651. [Google Scholar] [CrossRef]
- Lorenzo, C.; McCormick, F. SPRED proteins and their roles in signal transduction, development, and malignancy. Genes Dev. 2020, 34, 1410–1421. [Google Scholar] [CrossRef]
- Lopez, J.; Bonsor, D.A.; Sale, M.J.; Urisman, A.; Mehalko, J.L.; Cabanski-Dunning, M.; Castel, P.; Simanshu, D.K.; McCormick, F. The ribosomal S6 kinase 2 (RSK2)-SPRED2 complex regulates the phosphorylation of RSK substrates and MAPK signaling. J. Biol. Chem. 2023, 299, 104789. [Google Scholar] [CrossRef]
- Hirata, Y.; Brems, H.; Suzuki, M.; Kanamori, M.; Okada, M.; Morita, R.; Llano-Rivas, I.; Ose, T.; Messiaen, L.; Legius, E.; et al. Interaction between a Domain of the Negative Regulator of the Ras-ERK Pathway, SPRED1 Protein, and the GTPase-activating Protein-related Domain of Neurofibromin Is Implicated in Legius Syndrome and Neurofibromatosis Type 1. J. Biol. Chem. 2016, 291, 3124–3134. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Markegard, E.; Dharmaiah, S.; Urisman, A.; Drew, M.; Esposito, D.; Scheffzek, K.; Nissley, D.V.; McCormick, F.; Simanshu, D.K. Structural Insights into the SPRED1-Neurofibromin-KRAS Complex and Disruption of SPRED1-Neurofibromin Interaction by Oncogenic EGFR. Cell Rep. 2020, 32, 107909. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Nonami, A.; Taketomi, T.; Wakioka, T.; Kuroiwa, A.; Matsuda, Y.; Yoshimura, A. Molecular cloning of mammalian Spred-3 which suppresses tyrosine kinase-mediated Erk activation. Biochem. Biophys. Res. Commun. 2003, 302, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Brems, H.; Chmara, M.; Sahbatou, M.; Denayer, E.; Taniguchi, K.; Kato, R.; Somers, R.; Messiaen, L.; De Schepper, S.; Fryns, J.P.; et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat. Genet. 2007, 39, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Stevenson, D. Legius Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK47312/ (accessed on 1 November 2023).
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Quinodoz, M.; Peter, V.G.; Bedoni, N.; Royer Bertrand, B.; Cisarova, K.; Salmaninejad, A.; Sepahi, N.; Rodrigues, R.; Piran, M.; Mojarrad, M.; et al. AutoMap is a high performance homozygosity mapping tool using next-generation sequencing data. Nat. Commun. 2021, 12, 518. [Google Scholar] [CrossRef]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef]
- Sund, K.L.; Zimmerman, S.L.; Thomas, C.; Mitchell, A.L.; Prada, C.E.; Grote, L.; Bao, L.; Martin, L.J.; Smolarek, T.A. Regions of homozygosity identified by SNP microarray analysis aid in the diagnosis of autosomal recessive disease and incidentally detect parental blood relationships. Genet. Med. 2013, 15, 70–78. [Google Scholar] [CrossRef]
- Correia-Costa, G.R.; Sgardioli, I.C.; Santos, A.P.D.; Araujo, T.K.; Secolin, R.; Lopes-Cendes, I.; Gil-da-Silva-Lopes, V.L.; Vieira, T.P. Increased runs of homozygosity in the autosomal genome of Brazilian individuals with neurodevelopmental delay/intellectual disability and/or multiple congenital anomalies investigated by chromosomal microarray analysis. Genet. Mol. Biol. 2022, 45, e20200480. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, G.L.; Aguena, M.; Gos, M.; Hung, C.; Pilch, J.; Fahiminiya, S.; Abramowicz, A.; Cristian, I.; Buscarilli, M.; Naslavsky, M.S.; et al. Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome. J. Med. Genet. 2015, 52, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.J.; van der Smagt, J.J.; Rosenfeld, J.A.; Pagnamenta, A.T.; Alswaid, A.; Baker, E.H.; Blair, E.; Borck, G.; Brinkmann, J.; Craigen, W.; et al. Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genet. Med. 2018, 20, 1175–1185. [Google Scholar] [CrossRef]
- Engelhardt, C.M.; Bundschu, K.; Messerschmitt, M.; Renne, T.; Walter, U.; Reinhard, M.; Schuh, K. Expression and subcellular localization of Spred proteins in mouse and human tissues. Histochem. Cell Biol. 2004, 122, 527–538. [Google Scholar] [CrossRef]

| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Present Case | |
|---|---|---|---|---|---|---|---|
| Reference | Motta et al. [7] | Markholt et al. [8] | This report | ||||
| Ethnicity | Tunisian | Turkish | Turkish | Syrian | Italian | ||
| Parents | consanguineous | consanguineous | consanguineous | consanguineous | possible common ancestor | ||
| Sex, age at onset | F, 2 years | M, 19 mo | F, 2 years | M, n.r. | F, 3 years | F, 2 years | M, 2 mo |
| Age at last examination | 11 years 11 mo | 8 years | 14 years 2 mo | 39 years | 4 years 1 mo | 3 years 2 mo | 1 year 2 mo |
| Height (SD) | 136.5 cm (−1.50) | 122.2 cm (−1.00) | 145 cm (−2.41) | 144 cm (−4.35) | 89 cm (−3.2) | 92.4 cm (−1.2) | 71 cm (−2.44) |
| Weight (SD) | 27.5 kg (−2.30) | 22 kg (−1.09) | 42 kg (−1.02) | 56 kg (−1.29) | 15 kg (−0.8) | 13.6 kg (−0.5) | 7.85 Kg (−2.08) |
| Head circumference (SD) | 55 cm (+1.00) | 51 cm (+0.86) | 57 cm (+1.32) | 56 cm (−1.00) | 49 cm (−0.3) | 51 cm (+0.8) | 47 cm (+0.51) |
| SPRED2 Variants | |||||||
| Domain | EVH1 | EVH1 | SRP | uncharacterized regions | EVH1 | ||
| Zygosity | Hom | Hom | Hom | Hom | Hom | ||
| cDNA change (NM_181784.3) | c.187C>T | c.299T>C | c.1142_1143delTT | c.780dup | c.325del | ||
| AA change | p.Arg63* | p.Leu100Pro | p.Leu381Hisfs*95 | p.Lys261Glnfs*3 | p.Arg109Glufs*7 | ||
| Development | |||||||
| Developmental delay | no | mild | yes | yes | yes | n.r. | mild |
| Intellectual disability | yes | mild | mild | mild | yes | n.r. | too early to evaluate |
| Language delay | no | yes | yes | yes | yes | no | mild |
| Learning disorder | attention deficit | yes | yes | yes | yes | n.r. | too early to evaluate |
| Neurological features | |||||||
| Hypotonia | no | during infancy | yes | yes | no | no | yes |
| Cardiovascular features | |||||||
| Congenital heart defects | PVS | PVS, pulmonary balloon valvuloplasty, small secundum ASD | mild aortic insufficiency, mitral valve prolapse | no | yes | yes | mild pulmonary stenosis |
| Hypertrophic cardiomyopathy | no | asymmetrical hypertrophy of the interventricular septum | focal interventricular septum hypertrophy | yes | no | no | yes |
| Skeletal features | |||||||
| Chest | pectus excavatum | superior pectus carinatum, inferior pectus excavatum | pectus excavatum | pectus excavatum | pectus excavatum | no | pectus carinatum |
| Hyperlaxity | yes | yes | yes | no | yes | no | yes |
| Other skeletal anomalies | kyphosis, clinodactyly, abnormal toe position | limited extension of elbows, cubitus valgus, winged shoulder blades, kyphosis, mild pes valgus and pes planus | cubitus valgus | no | mild kyphosis, mild pes valgus and pes planus, no clinodactyly | mild kyphosis, no clinodactyly | no |
| Skin features | |||||||
| Café-au-lait spots | no | no | no | no | no | no | no |
| Freckling | no | no | no | no | no | no | no |
| Other skin/ectodermal features | hyperhidrosis, deep palmar creases | sparse and curly hair, sparse and thin eyebrows and eyelashes, scaly and dry skin, eczematous skin, loose and thick skin, deep palmar creases | nevi | no | n.r. | n.r. | sparse and thin eyebrows and eyelashes, loose skin, deep palmar creases |
| Facial features | |||||||
| Bitemporal narrowing | yes | yes | yes | yes | yes | yes | yes |
| Hypertelorism | yes | yes | yes | no | no | no | yes |
| Low-set and/or posteriorly rotated ears | yes | yes | yes | yes | yes | yes | yes |
| Prominent nasal bridge | yes | yes | yes | yes | yes | yes | yes |
| Low posterior hairline | yes | yes | yes | yes | yes | yes | yes |
| Short/webbed neck | yes | yes | yes | yes | yes | yes | yes |
| Other dysmorphism or clinical features | high cranial vault, triangular and coarse face, downward slanted palpebral fissures, ptosis, prominent philtrum, large mouth, thick lips, micrognathia, high arched/narrow palate | triangular coarse face, sparse eyebrows, sparse eyelashes, downward slanted palpebral fissures, epicanthus, nasolacrimal duct stenosis, prominent nasolabial sulci, pointed receding chin | helix folding anomaly, dysmorphic ear lobe | downward slanted palpebral fissures, prominent nasolabial folds, long philtrum | downward slanted palpebral fissures, unilateral ptosis | downward slanted palpebral fissures, unilateral ptosis | high forehead, prominent eyes, downward slanted palpebral fissures, thick helix, deeply grooved philtrum, large mouth, thin lips, pointed chin, micrognathia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onore, M.E.; Caiazza, M.; Farina, A.; Scarano, G.; Budillon, A.; Borrelli, R.N.; Limongelli, G.; Nigro, V.; Piluso, G. A Novel Homozygous Loss-of-Function Variant in SPRED2 Causes Autosomal Recessive Noonan-like Syndrome. Genes 2024, 15, 32. https://doi.org/10.3390/genes15010032
Onore ME, Caiazza M, Farina A, Scarano G, Budillon A, Borrelli RN, Limongelli G, Nigro V, Piluso G. A Novel Homozygous Loss-of-Function Variant in SPRED2 Causes Autosomal Recessive Noonan-like Syndrome. Genes. 2024; 15(1):32. https://doi.org/10.3390/genes15010032
Chicago/Turabian StyleOnore, Maria Elena, Martina Caiazza, Antonella Farina, Gioacchino Scarano, Alberto Budillon, Rossella Nicoletta Borrelli, Giuseppe Limongelli, Vincenzo Nigro, and Giulio Piluso. 2024. "A Novel Homozygous Loss-of-Function Variant in SPRED2 Causes Autosomal Recessive Noonan-like Syndrome" Genes 15, no. 1: 32. https://doi.org/10.3390/genes15010032
APA StyleOnore, M. E., Caiazza, M., Farina, A., Scarano, G., Budillon, A., Borrelli, R. N., Limongelli, G., Nigro, V., & Piluso, G. (2024). A Novel Homozygous Loss-of-Function Variant in SPRED2 Causes Autosomal Recessive Noonan-like Syndrome. Genes, 15(1), 32. https://doi.org/10.3390/genes15010032









