Comparative Plastomes of Curcuma alismatifolia (Zingiberaceae) Reveal Diversified Patterns among 56 Different Cut-Flower Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction, Sequencing
2.2. Plastome Sequencing, Assembly, and Annotation
2.3. Comparative Genomic Analysis
2.4. Phylogenetic Analysis
3. Results
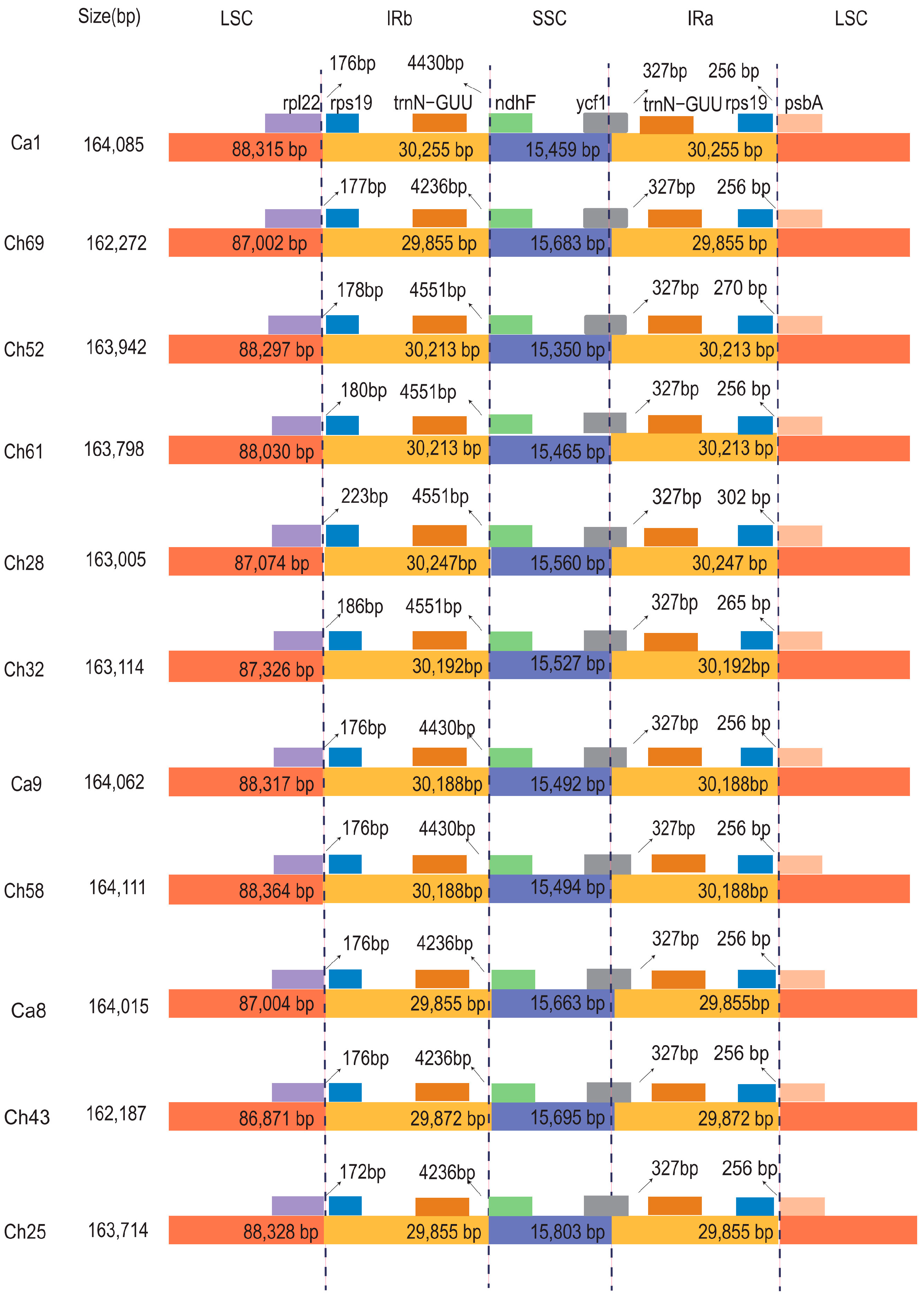
3.1. General Features of C. alismatifolia Plastomes
3.2. Contraction and Expansion of IRs
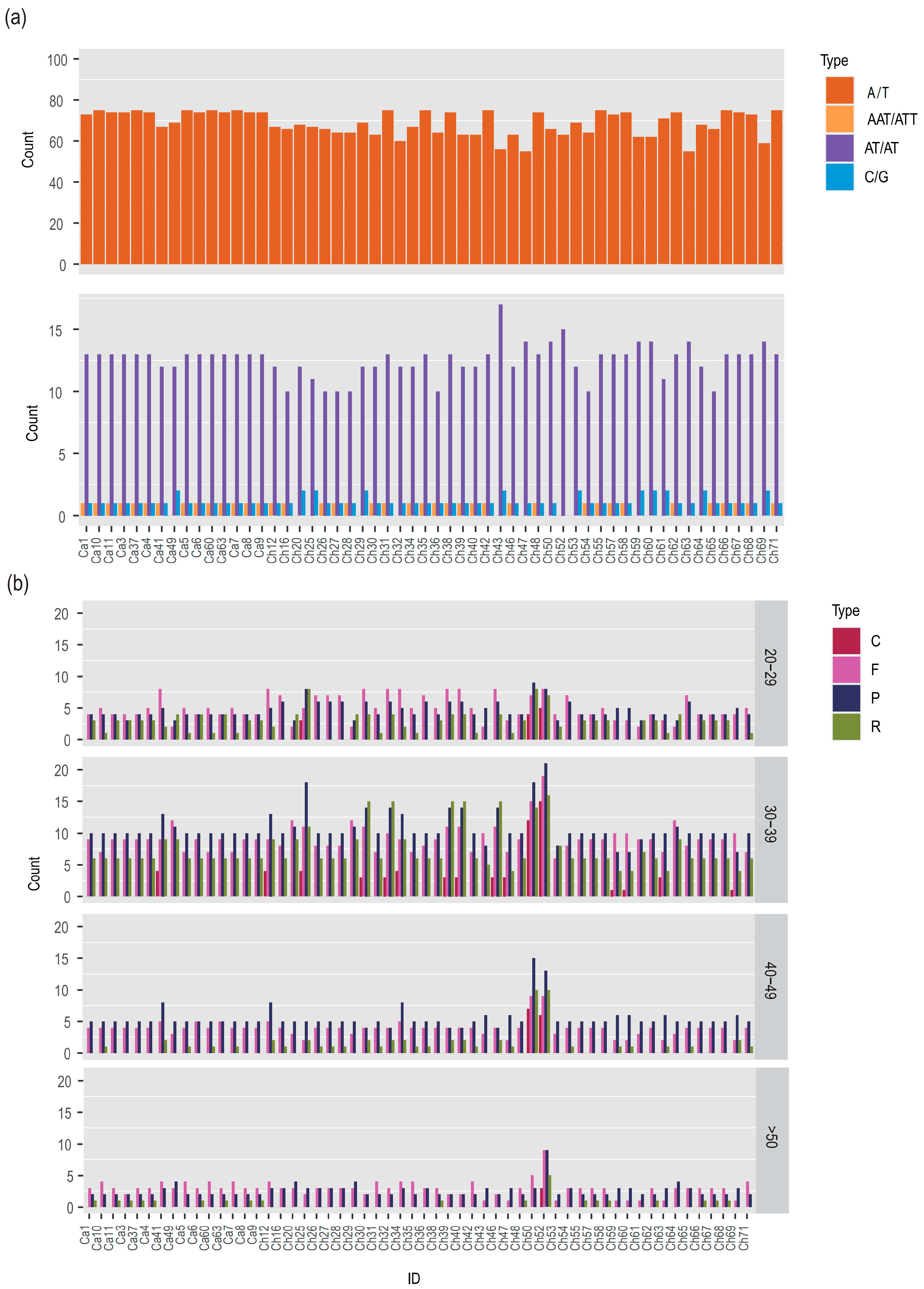
3.3. Sequence Repeats in the Complete Plastome
3.4. Evolutionary Rates among Protein Coding Genes
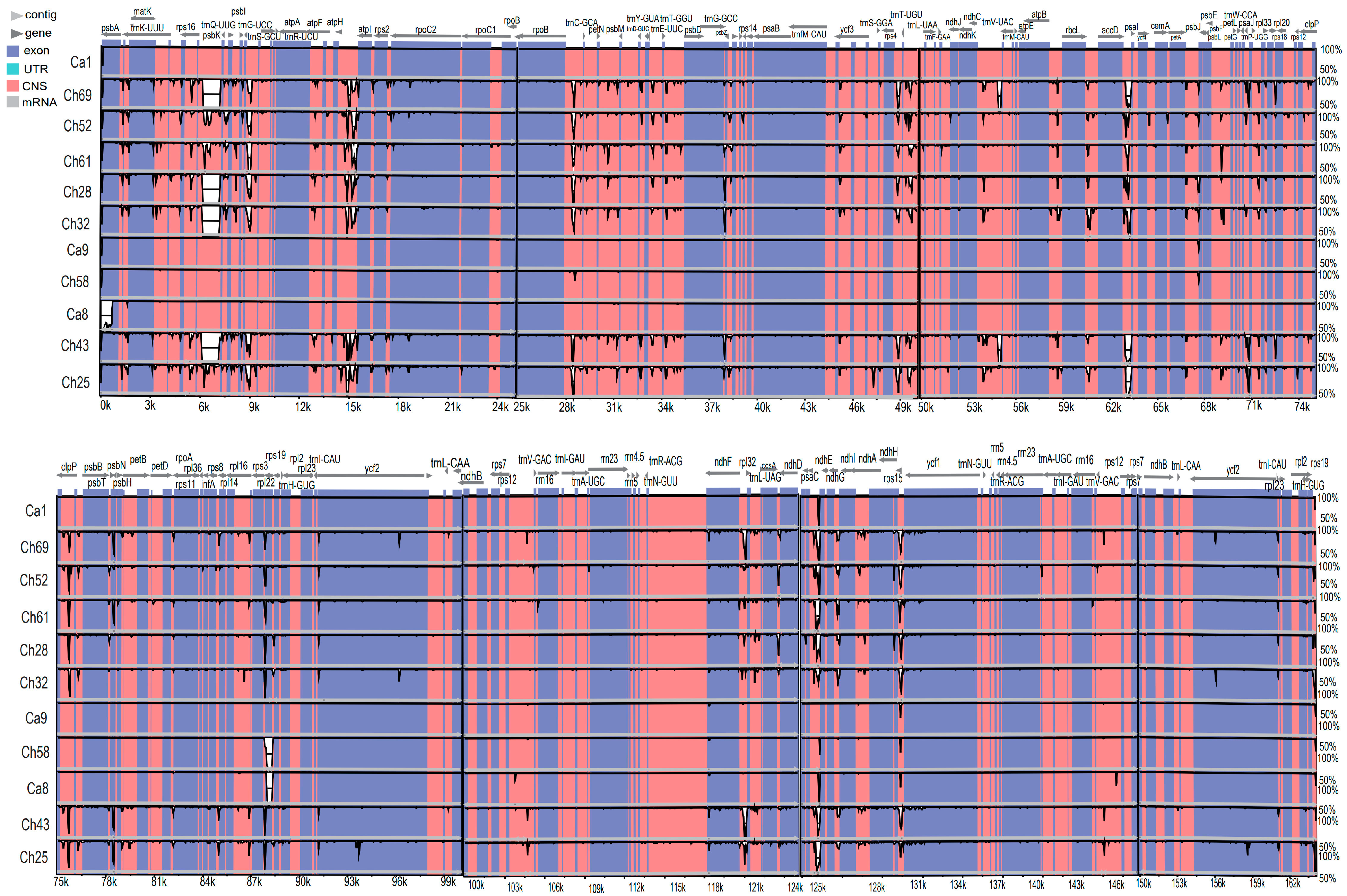
3.5. Genome Sequence Divergence
3.6. Phylogenetic Analyses and Molecular Marker Identification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taheri, S.; Abdullah, T.L.; Noor, Y.M.; Padil, H.M.; Sahebi, M.; Azizi, P. Data of the first de novo transcriptome assembly of the inflorescence of Curcuma alismatifolia. Data Brief 2018, 19, 2452–2454. [Google Scholar] [PubMed]
- Paisooksantivatana, Y.; Kako, S.; Seko, H. Genetic diversity of Curcuma alismatifolia Gagnep. (Zingiberaceae) in Thailand as revealed by allozyme polymorphism. Genet. Resour. Crop Evol. 2001, 48, 459–465. [Google Scholar]
- Ghani, A.; Haque, S.; Alam, M.A.; Hossain, M.M.; Majumder, M.M.; Siddiqua, S.A.; Hasan, S.M.R.; Akter, R. Evaluation of Analgesic and Antioxidant Potential of the Leaves of Curcuma alismatifolia Gagnep. Stamford J. Pharm. Sci. 1970, 1, 3–9. [Google Scholar]
- Taheri, S.; Abdullah, T.L.; Rafii, M.Y.; Harikrishna, J.A.; Werbrouck, S.P.O.; Teo, C.H.; Sahebi, M.; Azizi, P. De novo assembly of transcriptomes, mining, and development of novel EST-SSR markers in Curcuma alismatifolia (Zingiberaceae family) through Illumina sequencing. Sci. Rep. 2019, 9, 3047. [Google Scholar] [PubMed]
- Liao, X.; Ye, Y.; Zhang, X.; Peng, D.; Hou, M.; Fu, G.; Tan, J.; Zhao, J.; Jiang, R.; Xu, Y.; et al. The genomic and bulked segregant analysis of Curcuma alismatifolia revealed its diverse bract pigmentation. aBiotech 2022, 3, 178–196. [Google Scholar] [PubMed]
- Gui, L.; Jiang, S.; Xie, D.; Yu, L.; Huang, Y.; Zhang, Z.; Liu, Y. Analysis of complete chloroplast genomes of Curcuma and the contribution to phylogeny and adaptive evolution. Gene 2020, 732, 144355. [Google Scholar]
- Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar]
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar]
- Deng, J.; Liang, H.; Zhang, L.; Zhang, W.; Zhang, G.; Luo, X.; Yang, R.; Shafique Ahmad, K. Evaluation on genetic relationships among China’s endemic Curcuma herbs by SRAP markers. Plant 2021, 9, 16. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Y.; Deng, J.; Gao, G.; Ding, C.; Zhang, L.; Yang, R. The complete chloroplast genome sequences of 14 Curcuma species: Insights into genome evolution and phylogenetic relationships within Zingiberales. Front. Genet. 2020, 11, 802. [Google Scholar]
- Gao, C.; Wu, C.; Zhang, Q.; Zhao, X.; Wu, M.; Chen, R.; Zhao, Y.; Li, Z. Characterization of chloroplast genomes from two salvia medicinal plants and gene transfer among their mitochondrial and chloroplast genomes. Front. Genet. 2020, 11, 574962. [Google Scholar] [PubMed]
- Li, H.T.; Yi, T.S.; Gao, L.M.; Ma, P.F.; Zhang, T.; Yang, J.B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y.; et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 2019, 5, 461–470. [Google Scholar] [PubMed]
- Li, H.T.; Luo, Y.; Gan, L.; Ma, P.F.; Gao, L.M.; Yang, J.B.; Cai, J.; Gitzendanner, M.A.; Fritsch, P.W.; Zhang, T.; et al. Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biol. 2021, 19, 232. [Google Scholar]
- Jansen, R.K.; Ruhlman, T.A. Plastid Genomes of Seed Plants. In Genomics of Chloroplasts and Mitochondria; Springer: Berlin, Germany, 2012; pp. 103–126. [Google Scholar]
- Rogalski, M.; do Nascimento Vieira, L.; Fraga, H.P.; Guerra, M.P. Plastid genomics in horticultural species: Importance and applications for plant population genetics, evolution, and biotechnology. Front. Plant Sci. 2015, 6, 586. [Google Scholar]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar]
- Ruhlman, T.A.; Zhang, J.; Blazier, J.C.; Sabir, J.S.M.; Jansen, R.K. Recombination-dependent replication and gene conversion homogenize repeat sequences and diversify plastid genome structure. Am. J. Bot. 2017, 104, 559–572. [Google Scholar] [PubMed]
- Zhou, J.; Zhang, S.; Wang, J.; Shen, H.; Ai, B.; Gao, W.; Zhang, C.; Fei, Q.; Yuan, D.; Wu, Z.; et al. Chloroplast genomes in Populus (Salicaceae): Comparisons from an intensively sampled genus reveal dynamic patterns of evolution. Sci. Rep. 2021, 11, 9471. [Google Scholar]
- Alwadani, K.G.; Janes, J.K.; Andrew, R.L. Chloroplast genome analysis of box-ironbark Eucalyptus. Mol. Phylogenet. Evol. 2019, 136, 76–86. [Google Scholar]
- Gitzendanner, M.A.; Soltis, P.S.; Wong, G.K.S.; Ruhfel, B.R.; Soltis, D.E. Plastid phylogenomic analysis of green plants: A billion years of evolutionary history. Am. J. Bot. 2018, 105, 291–301. [Google Scholar] [PubMed]
- Yang, Z.; Ma, W.; Yang, X.; Wang, L.; Zhao, T.; Liang, L.; Wang, G.; Ma, Q. Plastome phylogenomics provide new perspective into the phylogeny and evolution of Betulaceae (Fagales). BMC Plant Biol. 2022, 22, 611. [Google Scholar]
- Wu, Z.Q.; Ge, S. The phylogeny of the BEP clade in grasses revisited: Evidence from the whole-genome sequences of chloroplasts. Mol. Phylogenet. Evol. 2012, 62, 573–578. [Google Scholar] [CrossRef]
- Lu, R.-S.; Yang, T.; Chen, Y.; Wang, S.-Y.; Cai, M.-Q.; Cameron, K.M.; Li, P.; Fu, C.-X. Comparative plastome genomics and phylogenetic analyses of Liliaceae. Bot. J. Linn. Soc. 2021, 196, 279–293. [Google Scholar] [CrossRef]
- Wang, J.; Fu, G.; Tembrock, L.R.; Liao, X.; Ge, S.; Wu, Z. Mutational meltdown or controlled chain reaction: The dynamics of rapid plastome evolution in the hyperdiversity of Poaceae. J. Syst. Evol. 2023, 61, 328–344. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Li, D.Z.; van der Bank, M.; Twyford, A.D. Telling plant species apart with DNA: From barcodes to genomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150338. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M.; Ou, L.; Yu, H.; Chen, R.; Zhou, Y.; Hassan, H.; Feng, B.; Taitano, N.; Van der Knaap, E.; Zou, X.; et al. Pan-plastome approach empowers the assessment of genetic variation in cultivated Capsicum species. Hortic. Res. 2019, 6, 108. [Google Scholar] [CrossRef]
- Ge, S.; Guo, Y. Evolution of genes and genomes in the genomics era. Sci. China Life Sci. 2020, 63, 602–605. [Google Scholar] [CrossRef]
- Sugiura, M. History of chloroplast genomics. Photosynth. Res. 2003, 76, 371–377. [Google Scholar] [CrossRef]
- Raubeson, L.A.; Peery, R.; Chumley, T.W.; Dziubek, C.; Fourcade, H.M.; Boore, J.L.; Jansen, R.K. Comparative chloroplast genomics: Analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genom. 2007, 8, 174. [Google Scholar] [CrossRef]
- Lu, R.S.; Hu, K.; Zhang, F.J.; Sun, X.Q.; Chen, M.; Zhang, Y.M. Pan-Plastome of Greater Yam (Dioscorea alata) in China: Intraspecific Genetic Variation, Comparative Genomics, and Phylogenetic Analyses. Int. J. Mol. Sci. 2023, 24, 3341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, X.; Gu, C.; Xiang, K.; Wang, J.; Li, S.; Tembrock, L.R.; Wu, Z.; He, W. The Asian lotus (Nelumbo nucifera) pan-plastome: Diversity and divergence in a living fossil grown for seed, rhizome, and aesthetics. Ornam. Plant Res. 2022, 2, 2. [Google Scholar] [CrossRef]
- Yao, J.L.; Cohen, D. Multiple gene control of plastome-genome incompatibility and plastid DNA inheritance in interspecific hybrids of Zantedeschia. Theor. Appl. Genet. 2000, 101, 400–406. [Google Scholar] [CrossRef]
- Fishman, L.; Sweigart, A.L. When Two Rights Make a Wrong: The Evolutionary Genetics of Plant Hybrid Incompatibilities. Annu. Rev. Plant Biol. 2018, 69, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Munch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Brudno, M.; Do, C.B.; Cooper, G.M.; Kim, M.F.; Davydov, E.; Program, N.C.S.; Green, E.D.; Sidow, A.; Batzoglou, S. LAGAN and Multi-LAGAN: Efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003, 13, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 1997, 13, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. TrimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Mehmood, F.; Shahzadi, I.; Waseem, S.; Mirza, B.; Ahmed, I.; Waheed, M.T. Chloroplast genome of Hibiscus rosa-sinensis (Malvaceae): Comparative analyses and identification of mutational hotspots. Genomics 2020, 112, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wei, L.; Ma, L.; Wu, Z.; Gu, C.; Chen, K. Comparative analyses of chloroplast genomes from 13 Lagerstroemia (Lythraceae) species: Identification of highly divergent regions and inference of phylogenetic relationships. Plant Mol. Biol. 2020, 102, 659–676. [Google Scholar] [CrossRef]
- Leliaert, F.; Verbruggen, H.; Vanormelingen, P.; Steen, F.; López Bautista, J.M.; Zuccarello, G.C.; De Clerck, O. DNA-based species delimitation in algae. Eur. J. Phycol. 2014, 49, 179–196. [Google Scholar] [CrossRef]
- Weng, M.L.; Blazier, J.C.; Govindu, M.; Jansen, R.K. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol. Biol. Evol. 2014, 31, 645–659. [Google Scholar] [CrossRef]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016, 209, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.G.; Srikant, T.; Carbonell Bejerano, P.; Becker, C.; Lensink, M.; Exposito Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 2022, 602, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Barthet, M.M.; Pierpont, C.L.; Tavernier, E.K. Unraveling the role of the enigmatic matk maturase in chloroplast group IIA intron excision. Plant Direct 2020, 4, e00208. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Friso, G.; van Wijk, K.J.; Sloan, D.B. Extreme variation in rates of evolution in the plastid Clp protease complex. Plant J. 2019, 98, 243–259. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Liao, X.; Ma, J.; Gao, W.; Wang, H.; Wu, D.; Tembrock, L.R.; Wu, Z.; Gu, C. Phylogeny, molecular evolution, and dating of divergences in Lagerstroemia using plastome sequences. Hortic. Plant J. 2023, 9, 345–355. [Google Scholar] [CrossRef]
- Abdel Ghany, S.E.; LaManna, L.M.; Harroun, H.T.; Maliga, P.; Sloan, D.B. Rapid sequence evolution is associated with genetic incompatibilities in the plastid Clp complex. Plant Mol. Biol. 2022, 108, 277–287. [Google Scholar] [CrossRef]
- Williams, A.M.; Carter, O.G.; Forsythe, E.S.; Mendoza, H.K.; Sloan, D.B. Gene duplication and rate variation in the evolution of plastid ACCase and Clp genes in angiosperms. Mol. Phylogen. Evol. 2022, 168, 107395. [Google Scholar] [CrossRef]


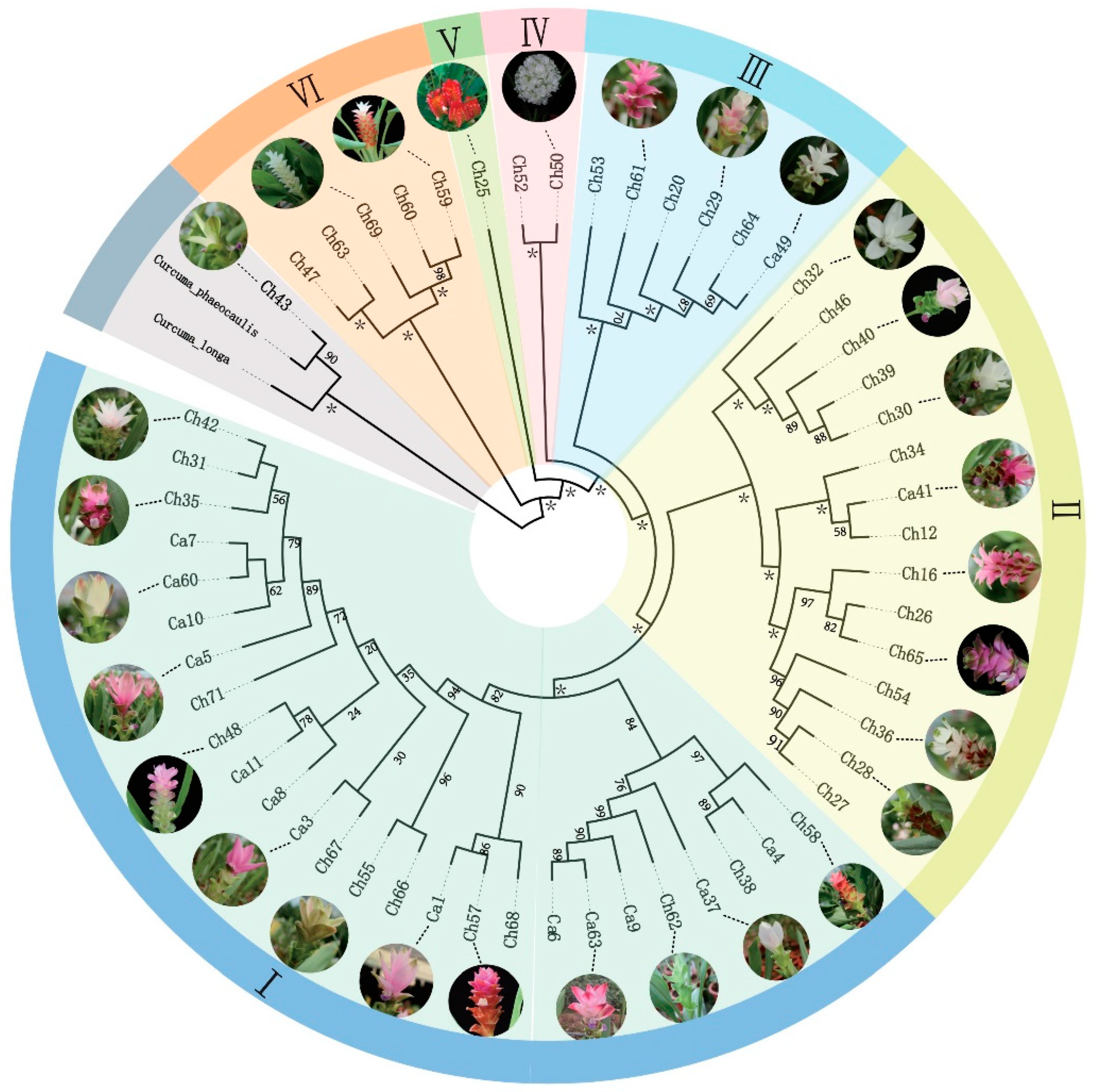
| Group | INDELs | SNPs |
|---|---|---|
| I | 55 | 100 |
| II | 33 | 54 |
| III | 49 | 70 |
| IV | 50 | 111 |
| V | 69 | 145 |
| VI | 6 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liao, X.; Li, Y.; Ye, Y.; Xing, G.; Kan, S.; Nie, L.; Li, S.; Tembrock, L.R.; Wu, Z. Comparative Plastomes of Curcuma alismatifolia (Zingiberaceae) Reveal Diversified Patterns among 56 Different Cut-Flower Cultivars. Genes 2023, 14, 1743. https://doi.org/10.3390/genes14091743
Wang J, Liao X, Li Y, Ye Y, Xing G, Kan S, Nie L, Li S, Tembrock LR, Wu Z. Comparative Plastomes of Curcuma alismatifolia (Zingiberaceae) Reveal Diversified Patterns among 56 Different Cut-Flower Cultivars. Genes. 2023; 14(9):1743. https://doi.org/10.3390/genes14091743
Chicago/Turabian StyleWang, Jie, Xuezhu Liao, Yongyao Li, Yuanjun Ye, Guoming Xing, Shenglong Kan, Liyun Nie, Sen Li, Luke R. Tembrock, and Zhiqiang Wu. 2023. "Comparative Plastomes of Curcuma alismatifolia (Zingiberaceae) Reveal Diversified Patterns among 56 Different Cut-Flower Cultivars" Genes 14, no. 9: 1743. https://doi.org/10.3390/genes14091743
APA StyleWang, J., Liao, X., Li, Y., Ye, Y., Xing, G., Kan, S., Nie, L., Li, S., Tembrock, L. R., & Wu, Z. (2023). Comparative Plastomes of Curcuma alismatifolia (Zingiberaceae) Reveal Diversified Patterns among 56 Different Cut-Flower Cultivars. Genes, 14(9), 1743. https://doi.org/10.3390/genes14091743







