1. Introduction
The microbiota of the gastrointestinal tract comprises a vast number of microorganisms [
1]. The constitution of its bacterial population is crucial, since its stability and proper composition are beneficial for the maintenance of the host’s health. These microorganisms participate in several metabolic pathways, such as the production of short-chain fatty acids and secondary bile acids, which are important for the immune system, maintenance of the intestinal barrier, and resistance to colonisation by pathogenic bacteria [
2]. Age, diet, genetics, and many other environmental factors can play a significant role in maintaining a healthy microbiome; however, the changes that these factors may cause are low when compared to those seen in diseased animals. Acute or chronic gastrointestinal inflammation often leads to dysbiosis, which is characterised by significant reductions in microbial diversity compared to healthy animals [
3]. Dysbiosis is marked by broad changes in the composition of microbial communities, decreased species diversity, alterations in the relative abundance of specific organisms, and consequent shifts in the production of metabolites. However, the possibility that a healthy animal may present an unstable microbiota should not be overlooked [
3,
4].
If dysbiosis occurs, it is necessary to control the instability of the intestinal microbiota. Diet, prebiotics, probiotics, symbiotics, and antimicrobials are frequently used with this purpose, but in some cases, these treatments may not be effective, or, in the case of antimicrobials, their administration may be associated with increased antimicrobial resistance or increased dysbiosis [
5]. Faecal Microbiota Transplantation (FMT) aims to restore intestinal stability by introducing a healthy “microbial ecosystem” in the host [
1]. FMT consists of transferring faecal material from a healthy donor into the gut of a non-healthy recipient to restore its gut microbiota. The beneficial effects of this alternative method have not yet been clarified. However, it is known to contribute to the enrichment of the microbiome and to the alteration of microbial profiles [
2].
In veterinary medicine, FMT has been considered a possible treatment in cases of giardiasis refractory to treatment, chronic enteropathy, parvovirus infection, and other types of acute diarrhoea, such as haemorrhagic gastroenteritis [
5,
6,
7,
8,
9]. To date, no serious adverse effects associated with FMT have been reported, which is probably due to limited data availability [
10]. It should be noted that currently, both in human and veterinary medicine, there is still no consensus on the methods of faecal material preparation and storage, donor selection criteria, doses to be administered, time interval between administrations, and the frequency and route of administration [
2].
The goal of this pilot study was to evaluate the influence of the long-term administration of freeze-dried faecal capsules via oral route on the composition of the intestinal microbiota of dogs and their effectiveness in correcting animals’ faecal consistency.
2. Materials and Methods
2.1. Animals in the Study
For this study, five animals were selected from an official rescue institution. All animals were cared for according to the rules given by the current EU (Directive 2010/63/EC) and national (DL 276/2001 and DL 113/2013) legislation and by the competent authority (Direção Geral de Alimentação e Veterinária, DGAV) in Portugal. The ethical guidelines of the official rescue centre were followed in this study, and a written protocol was established to perform the study. All procedures were performed by trained veterinarians, and only non-invasive procedures were used.
From the five study dogs, two presented a normal faecal consistency and were selected as the positive (PC—subjected to capsules administration) and negative (NC—to which no capsules were administered) controls; the other three animals presented chronic diarrhoea (over or equal to 3 weeks [
11]) and constituted the target animals of the study (treatment group).
The selected dogs were housed in a kennel in separate outdoor covered boxes (except for Animal 1 and the NC, which were together in the same box), shared a common environment (including water and air quality and cleaning procedures), and were fed with the same diet. These dogs exhibited the characteristics outlined in
Table 1, which includes the classification of their initial faecal consistency according to the Bristol Stool Scale [
12]. The use of this scale was due to its simplicity, not being exclusive to human medicine [
13].
The animals were of indeterminate breed, aged 2 years or older and with a body weight of more than 9 kg and less than or equal to 27 kg. The selected dogs did not receive any type of medication in the six months before the study, excluding internal and external dewormers. Animals’ vaccination status was regularised, except for Animal 2, which had only recently entered the kennel. Animals presented no gross signs of disease apart from the alteration in faecal consistency in Animal 1, Animal 2, and Animal 3.
2.2. Faecal Microbiota Transplantation
Oral FMT was performed through the administration of oral capsules commercialised since 2016 by
AnimalBiome® (Oakland, CA, USA). According to the information available at the
AnimalBiome® website, capsules are produced through the lyophilisation of faecal material from a rigorously selected canine donor, aiming to exclude the presence of pathogens or multidrug-resistant bacteria within the transplanted faecal material. Donor animals are preferably aged between 1 and 10 years, with optimal body condition and normal faecal consistency, and sound health upon physical examination. Moreover, with the absence of antimicrobial usage within the last 3, 6, or 12 months, up-to-date vaccinations and empirical deworming with broad-spectrum drugs are further prerequisites. Laboratory screenings demand normal haematology and biochemistry, negative faecal flotation results, and negative results for pathogens such as Giardia and diseases like parvovirus and distemper [
2,
14,
15]. In this case, considering that it involved a commercial product, no information was obtained regarding the faecal donor of the capsules utilised during the study.
Regarding the composition of the capsules, in addition to the faecal material, these also contain inactive ingredients such as glycerol and the enclosing capsule, which confers resistance to enzymatic action within the gastric and intestinal compartments.
2.3. Experimental Design
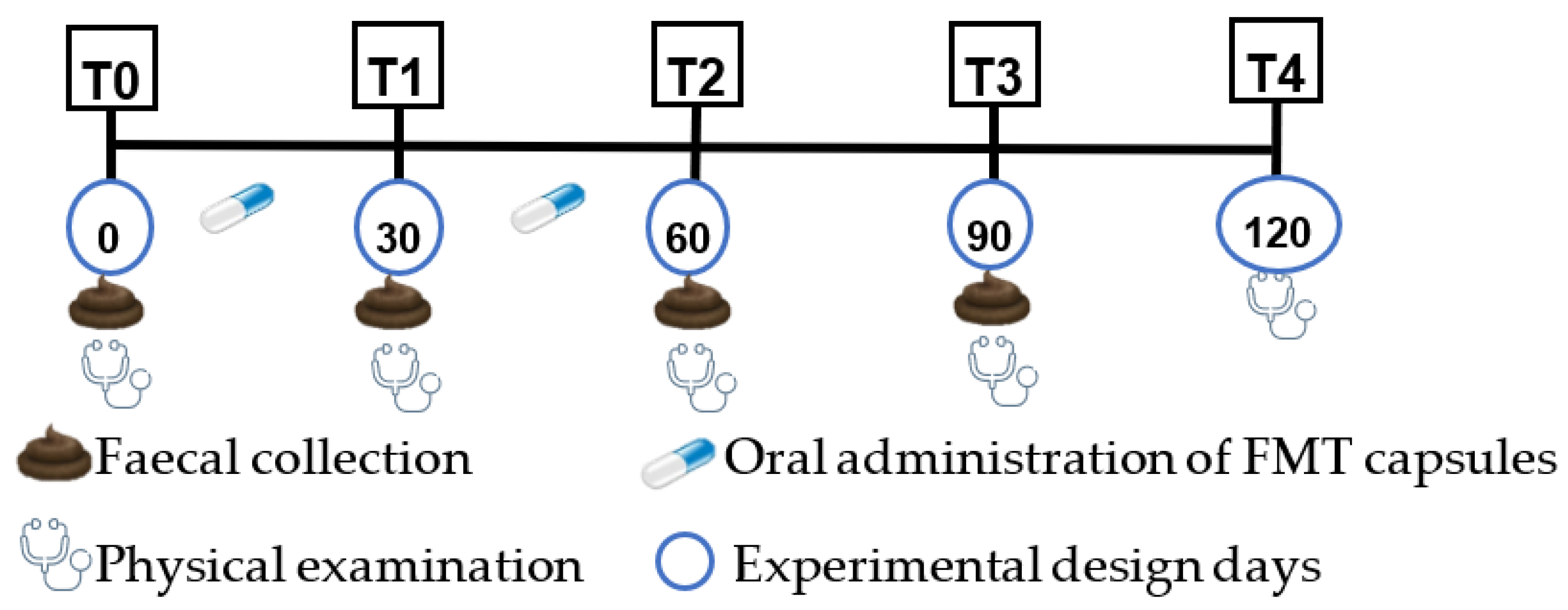
This pilot clinical trial was a controlled study that included one dog as a negative control (with normal faecal consistency and no treatment) and two test groups: one with a single animal showing normal faecal consistency (positive control) and another group with three animals demonstrating altered faecal consistency (treatment group). At the beginning of the trial, and before any capsule’s administration, samples were collected from each animal that participated in the study, which were used for comparison along the clinical trial. Considering that, these samples were used as controls of the animals from which they were originated.
The animal of the positive control and the treatment group were submitted to the oral administration of one capsule of
AnimalBiome® per day for two months (60 days). The negative control animal was not submitted to any treatment or placebo. The study period comprised a total of four months and included the monthly collection of a faecal sample per animal (
Figure 1). The first sampling was carried out at the beginning of the study (T0) without any capsule being administered to the animals, as previously mentioned. The second faecal sampling was performed 30 days after the first administration of the freeze-dried capsules (T1). The third sampling was performed 30 days after T1, at the end of the FMT administration (T2). The last sampling (T3) was performed one month after T2, aiming to assess the long-term efficacy of the faecal capsule’s administration.
At each sampling moment, a physical examination of each one of the animals was performed, including the general observation of the animal, faecal consistency evaluation, measurement of rectal temperature, respiratory and heart rate, cardiac and respiratory auscultation, evaluation of the eyes, ears, nose, mouth, skin for any abnormalities, observation and palpation of lymph nodes, abdominal palpation and testicles, and observation of the mucous membranes. In addition, two months after the end of the capsule administration, a new physical evaluation was performed (T4).
In addition to the monthly visits, weekly telephone calls were made to ascertain the state of health of the animals and the presence or absence of side effects.
2.4. Collection, Packaging and Storage of Faecal Samples
Faecal samples were collected using sterile plastic cups after defecation either during the animal’s daily walk or after their feeding period. The samples were temporarily stored in a Styrofoam box with freeze plates and later moved to a −20 °C freezer for long-term storage within 24 h.
2.5. Quantification of the Bacterial Population Present in the Capsules
First, 1 mg of the faecal material present inside a capsule was diluted in 1 mL of saline solution. Afterwards, the quantification of the total number of viable bacteria present in the lyophilised capsules was performed by inoculating 100 µL of serial 10-fold dilutions up to the 10−10 dilution onto the surface of a Trypticase Soy Agar (TSA) plate, in duplicate. All plates were incubated aerobically at 37 °C for 24 h, after which the number of colonies present on each plate was quantified.
2.6. Characterisation of Capsules and Faecal Samples Microbiome
To determine the composition of the microbiome present in the capsules and faecal samples, DNA extraction was performed using the QIAamp
® PowerFecal
® Pro DNA kit [
16]. The DNA was then amplified by PCR using a Biometra T1 Thermocycler T-1 Thermoblock. The composition of the master mix used was the following: 60 mM Tris-SO
4, 20 mM (NH
4) 2SO
4, 2 mM MgSO
4, 0.3 mM dNTPs, 3% Glycerol, 0.06% IGEPAL
® CA-630, 0.05% Tween20, 125 units/mL LongAmp Taq DNA Polymerase; pH 9.1 at 25 °C and 1.5 µL of each primer, 27f and 1492r, targeting the 16S rRNA. The amplification protocol included 35 cycles as follows: denaturation (95 °C, 10–30 s), annealing (55 °C, 15–60 s) and replication (65 °C, 50 s). Subsequently, amplification products were visualised through gel electrophoresis and purified using the Solid-Phase Reversible Immobilisation (SPRI) technique with magnetic beads [
17,
18]. After, a DNA library was built. The samples were then quantified using the Qubit
® fluorometer, and the adapters were fixed for subsequent genomic sequencing, which was performed using GridION X5, commercialised by Oxford Nanopore Technologies
® [
19].
2.7. Statistical Analysis and Data Handling
Samples were analysed using a custom analytical pipeline developed by BioISIGenomics® to obtain a highly accurate taxonomic classification. Initially, for each sample, operational taxonomic units (OTUs) were identified and, subsequently, a heatmap was established to compare the bacterial composition of the samples. In addition, each sample was analysed for its α diversity based on Shannon’s diversity index and between samples for their β diversity based on the Bray–Curtis dissimilarity index. The β diversity was expressed using Principal Coordinates Analysis (PCoA). The Kruskal–Wallis non-parametric statistical test was applied in multiple paired comparisons to compare the α diversity of each sample (p-value = 0.05).
4. Discussion
This pilot study aimed to assess the influence of long-term Faecal Microbiota Transplantation in animals with chronic diarrhoea; therefore, the collection of the faecal samples to be analysed was performed at a later stage after transplantation, enabling the long-term evaluation of the intestinal microbiota adaptation process to this new stimulus.
Animals to be included lived in a kennel, which allowed their continuous vigilance by veterinary medical trained personnel throughout the assay. Furthermore, being a study about the gut microbiome, the selection of animals that shared the same environment, diet and veterinary care aimed at minimising the variables present [
21] and standardising the sample collection and storage procedures. Together with the small sample size, distinctive characteristics presented by the animals, such as age, body condition and vaccination status, can be considered as limitations, as they can influence the constitution of the gut microbiota [
21,
22,
23]. For example, the absence of information on the vaccination status of Animal 2 should be noted, since its samples had different results from the others. The absence of a detailed clinical history and of a diagnosis of the underlying cause of the change in faecal consistency of the animals under study should also be noted [
11] as well as the characterisation of a possible chronic enteropathy [
24]. Nevertheless, this study only aimed to be a preliminary evaluation of the influence of FMT based on the oral administration of lyophilised faecal capsules on the faecal consistency and on the composition of the intestinal bacterial population of the dogs under study.
If rigorous screening of faecal material is performed, side effects associated with FMT are rarely seen [
6,
25], which was also evidenced in this study. Regarding faecal consistency, the improvement of this parameter was evident in Animals 1 and 3 as well as the maintenance of the values in the long-term evaluation (T4). Also, Animal 2 showed a positive evaluation regarding this parameter during the FMT period, but in the long-term assessment, the faecal consistency of its samples relapsed, showing faecal consistency values similar to the one observed at T0. This animal was characterised as being very anxious and nervous [
26] and, in addition, it was the youngest animal enrolled for the study and the one with an unknown vaccination status.
According to
AnimalBiome® [
27], the tested capsules contain 20 ×
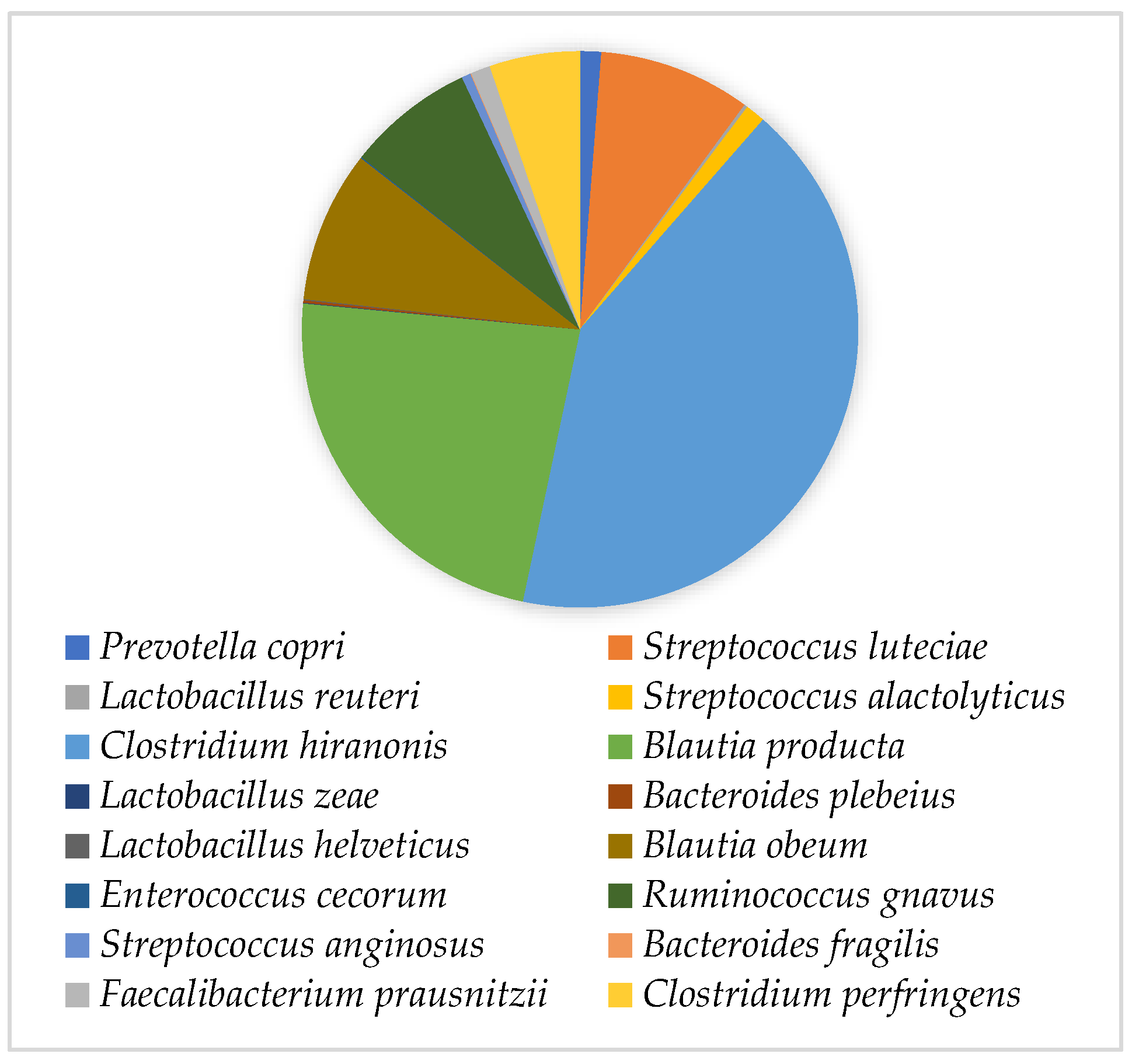
CFU per gram of faeces. The quantification performed in this study showed only a 100 CFU/g difference, revealing that the capsules’ manufacturing procedure supports the conservation of the bacterial population. Regarding capsules constitution, the results showed that the families
Lachnospiraceae,
Clostridiaceae and
Peptostreptococcaceae of the class
Clostridia, phylum
Firmicutes, were the most abundant. However, the value corresponding to this phylum was higher than expected, resulting in extremely low concentrations of the remaining phyla [
10]. In addition, the higher concentration of Actinobacteria is not in accordance with the study by Pereira and Clemente [
28], who stated that this phylum is generally present in a lower concentration. The reduced concentration of Proteobacteria agreed with previous studies, which indicate that this phylum only increases in diseased animals [
29]. Regarding the species present, the predominant one was
C. hiranonis, the main bacterium responsible for the conversion of bile acids, with bacteria belonging to the genera
Blautia and
Ruminococcus, which also have a beneficial role in maintaining the host homeostasis, also presenting high concentrations.
S. luteciae was also present at a high concentration, which is not in accordance with available publications, as the highest concentration of this bacterial group is usually associated with disease—particularly with chronic enteropathy [
3,
30].
C. perfringens concentration depicted the commensal presence of this bacterial species, not being indicative of gastrointestinal disease [
10,
31]. However, this species was present at a higher concentration than
F. prausnitzii, which, according to Honneffer et al. [
30], when present at a low concentration can be considered a marker of faecal dysbiosis.
In the faecal samples of the control animals, the phyla concentrations were in accordance with previous studies [
10,
28] except for the reduced concentration of Fusobacteria since, according to Niina et al. [
29], a low proportion of this Phylum and an increase in Proteobacteria may be suggestive of an enteropathy. However, these animals did not show an increase in Proteobacteria or any clinical signs suggestive of a gastrointestinal disorder. Of note is the high concentration of Bacilli, more specifically of
Lactobacillus, which is generally increased in the case of disease [
2,
32]. According to Aboim [
33], the fact that the animals under study were housed in a kennel is a possible justification for this result. Additionally,
Clostridiaceae,
Peptostreptococcaceae and
Lachnospiraceae were only at high concentrations in the NC animal at T1, which does not corroborate the presence of a microbiota in equilibrium [
4]. However, most samples showed a low concentration of
Enterobacteriaceae and
Streptococcus spp. and a high concentration of
C. hiranonis and
Blautia spp., which are characteristic of a healthy microbiota [
3]. As observed in the capsule’s composition,
F. prausnitzii was also found in reduced quantities, and the commensal presence of
C. perfringens was also detected. Regarding the T1 sample of the negative control, despite the animal’s normal physical exam and constant faecal consistency during the study, an increase in bacterial diversity was observed. However, it is important to say that healthy animals may present an unstable microbiota, which may justify this result [
3,
4].
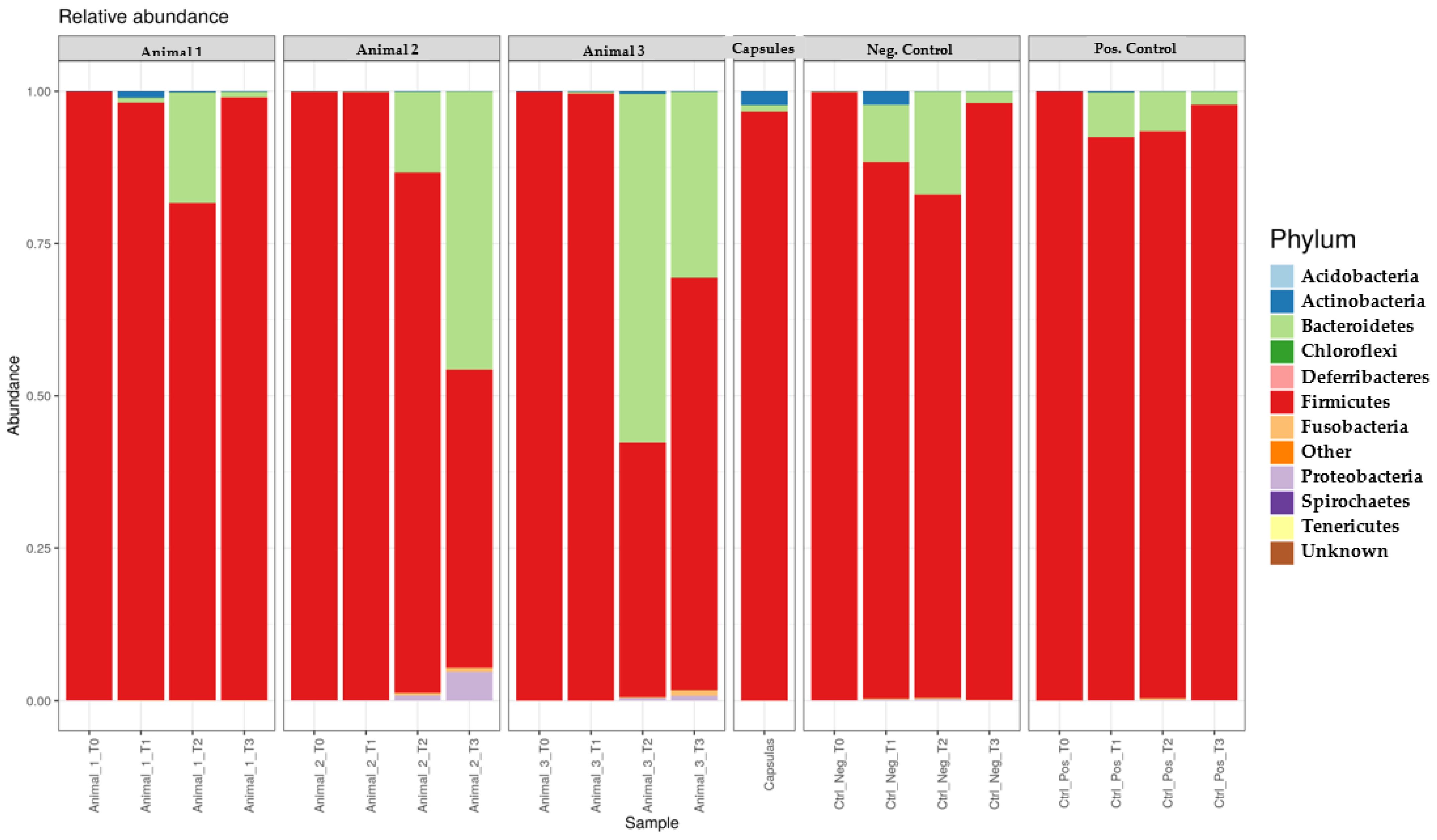
Regarding the action of the capsules on microbiome composition, although no significant differences were detected, certain trends were observed that may be a result of FMT administration [
4,
34,
35]. For example, in most faecal samples, there was an increase in Bacteroidetes and Actinobacteria and a decrease in Firmicutes, corresponding to a decrease in the predominant Phylum and an increase in the Phyla previously present in lower concentration, conferring an increase in the diversity of the bacterial population. Nevertheless, the opposite variations were observed in the samples from the PC animal at T2, which may be associated with the normalisation of the intestinal microbiota and adaptation to capsules ingestion. The slight increase in Actinobacteria in Animal 2 observed only after two months of FMT, which may reflect a higher degree of initial faecal dysbiosis, and the fact that the samples from the NC animal at T2 were the only ones that showed a lower concentration of Actinobacteria when compared to the one present in the samples collected at T0, reflect the importance of capsule administration in the increase in this phylum. The variation verified in Proteobacteria revealed an attempt to reduce these values; however, the values for this phylum were never particularly high in the animals under the study despite the association of Proteobacteria with an unbalanced microbiota [
3]. Overall, the samples collected after FMT were characterised by the opposite variation. However, when compared to T0, the effect of the capsules was associated with a decreased concentration of Firmicutes and an increased concentration of Bacteroidetes. Actinobacteria concentration did not present significant variations. Finally, it is noteworthy that Animal 2 presented the highest value concentration of Proteobacteria, which may justify the worsening of the faecal consistency.
Regarding bacterial classes, in the first month, the concentration of Bacilli decreased while those from the other classes—Clostridia, Coriobacteriia, Erysipelotrichi and Bacteroidia—increased. In the second month, the variations differed between animals. After the end of FMT, Bacteroidia decreased in most animals, and the remaining classes showed divergent variation, which may be due to a possible dysbiosis which may trigger different responses in the post-FMT period. Despite this, the influence of capsules remained, since most samples showed a lower value of Bacilli and higher value of Clostridia, Erysipelotrichi and Bacteroidia when compared to T0, resembling that the ones present in a healthy microbiota [
3,
32]. Once again, Animals 2 and NC represented the exceptions, which can be justified by the differences presented by Animal 2 as well as by the fact that no capsules were administered to Animal NC. Only Coriobacteriia, like the phylum to which it belongs, did not show quite different values from those of from T0.
More specifically, during FMT, as expected [
8,
32], most faecal samples showed an increase in
Prevotellaceae,
Lachnospiraceae (
Blautia spp.),
Peptostreptococcaceae,
Clostridiaceae (
C. hiranonis) and
Faecalibacterium prausnitzii, together with a decrease in
Lactobacillaceae and
Enterobacteriaceae, with the exception of Animals 2 and NC. Particularly, a decrease in
Streptococcaceae was evident in all animals only in the second month of the FMT protocol except in Animal NC. This seems to indicate that an FMT period longer than 30 days may be necessary to cause a decrease in this family concentration.
In the month following the end of the FMT, most samples showed the opposite variation of the one observed after capsule administration. This minor relapse is consistent with the observation made by Chaitman and Gaschen [
2] that dogs with chronic diarrhoea often necessitate multiple FMT treatments to prevent relapses. However, when compared to the results from samples obtained at the beginning of the study, most animals showed higher concentrations of
Prevotellaceae,
Lachnospiraceae,
Clostridiaceae and
F. prausnitzii and lower concentrations of
Streptococcaceae and
Enterobacteriaceae, which suggest an approximation with a healthy microbiota [
10], again the exceptions being Animals 2 and NC. Moreover, minimal changes were observed regarding
Fusobacteriaceae [
10].
The β diversity revealed a high dissimilarity between the samples collected at T0 from all dogs and the capsules. At the end of the study, all animals showed a greater similarity with the samples taken at T0, which portrays, as mentioned earlier, a slight relapse. However, at T3, higher values of bacteria were considered beneficial, and lower values of disease-associated bacteria were also evident [
36].
The lack of predefined reference intervals made it impossible to define the degree of dysbiosis present in each animal at the beginning of the study, and it was also not possible to determine the faecal dysbiosis index [
37,
38] due to the small sample size.
5. Conclusions
Nowadays, FMT is not widely used in the veterinary setting; however, this procedure could become a reliable alternative to the use of antibiotics. The implementation of FMT through oral capsules administered at home would highly facilitate the wider application of this technique.
In this study, it is noteworthy that no adverse effects were demonstrated, emphasising the safety of this alternative to antibiotics. Additionally, the administered capsule period and dosage were successful in restoring the faecal consistency of Animals 1 and 3. However, a similar restorative effect was not observed in the case of Animal 2. For this particular dog, an extended administration period or a treatment strategy addressing the root cause of the diarrhoea could be necessary. Lastly, considering the diverse characteristics of the small group of dogs included in this study, caution is advised when extrapolating the results to a larger population. Nevertheless, despite these limitations, noticeable trends persist within the findings, highlighting the potential of FMT interventions in canine patients.
In the future, it would be extremely relevant to conduct a prospective experimental project with a larger number of animals with similar characteristics and with a definitive gastrointestinal diagnosis. Furthermore, it would be pertinent to assess other parameters such as the faecal dysbiosis index to define the degree of initial dysbiosis and verify its evolution throughout the FMT.
In conclusion, it is important to investigate the benefits of FMT in veterinary medicine, aiming at developing guidelines for the standardised use of this therapy.