Abstract
Maternal smoking in pregnancy (MSP) affects the offspring’s DNA methylation (DNAm). There is a lack of knowledge regarding individual differences in susceptibility to exposure to MSP. Glutathione S-transferase (GST) genes are involved in protection against harmful oxidants such as those found in cigarette smoke. This study aimed to test whether polymorphisms in GST genes influence the effect of MSP on offspring DNAm. Using data from the Isle of Wight birth cohort, we assessed the association of MSP and offspring DNAm in 493 mother-child dyads (251 male, 242 female) with the effect-modifying role of GST gene polymorphism (at rs506008, rs574344, rs12736389, rs3768490, rs1537234, and rs1695). MSP was assessed by levels of nicotine and its downstream metabolites (cotinine, norcotinine, and hydroxycotinine) in maternal sera. In males, associations of hydroxycotinine with DNAm at cg18473733, cg25949550, cg11647108, and cg01952185 and norcotinine with DNAm at cg09935388 were modified by GST gene polymorphisms (p-values < 0.05). In females, associations of hydroxycotinine with DNAm at cg12160087 and norcotinine with DNAm at cg18473733 were modified by GST gene polymorphisms (p-values < 0.05). Our study emphasizes the role of genetic polymorphism in GST genes in DNAm’s susceptibility to MSP.
1. Introduction
Despite being associated with a multitude of adverse health outcomes both for the mother and the offspring, maternal cigarette smoking in pregnancy (MSP) is prevalent around the world [1]. According to a global meta-analysis, the prevalence of MSP exceeded 10% in 29 of 174 countries and exceeded 20% in 12 other countries [1]. In Western countries, the estimated prevalence of MSP is 8%, showing significant variation between countries: 16% in France, 12% in the UK, 9% in Germany, and 7% in the US, respectively [2].
Epidemiological studies have shown that offspring exposed to in-utero cigarette smoking are at increased risk of adverse development of the respiratory system [3]. MSP is a leading preventable cause of suboptimal in-utero development of the lungs, leading to a future risk of decreased lung function parameters, wheezing, asthma, and respiratory infections [3]. Awareness of the newborn’s prenatal exposure to cigarette smoking is critical to identifying those at risk of developing adverse health outcomes [4].
Cigarette smoke is composed of a wide variety of toxic chemicals such as ammonia, benzene, acrolein, carcinogenic nitrosamines, quinones, polycyclic aromatic hydrocarbons [5], and nicotine [6]. Nicotine is a major addictive ingredient in tobacco plant leaves that impedes smokers’ efforts to quit [7]. After inhalation of cigarette smoke, nicotine is absorbed from the lungs’ alveoli and reaches the bloodstream. It is then metabolized by the liver enzyme CYP2A6 to cotinine, a downstream metabolite [7]. Nicotine and its downstream metabolites such as cotinine, norcotinine, and hydroxycotinine cross the placenta and reach the fetal bloodstream [8]. Nicotine and its metabolites have been used as biomarkers of smoking exposure. Since nicotine has a short half-life (1–2 h) and is quickly removed from the bloodstream, its downstream metabolites such as cotinine, norcotinine, and hydroxycotinine might provide more reliable biomarkers (half-life: 13–19 h) [8].
Although epidemiological studies have shown adverse effects of MSP on offspring health, the underlying mechanisms remain unclear. One potential mechanism is through changes in DNA methylation (DNAm) [9]. This involves transferring methyl groups to cytosine. Changes in DNAm can be detected on cytosines preceding a guanine nucleotide, called ‘CpG sites’ [10]. These C-G rich segments of the DNA influence gene expression through their levels of methylation [11]. In addition, DNAm has been shown to have an impact on gene expression by impeding the binding of transcription factors to the DNA and on alternative splicing by marking exons through hypermethylation [12]. Methylation of DNA can also occur in the bodies of genes such as the hydrocarbon receptor repressor (AHRR) gene involved in the metabolism of toxic substances, including those in cigarette smoke [13], although the functional consequences of this methylation are still unknown. Through these mechanisms, DNAm leads to variations in metabolic processes and cellular behavior [14].
It is assumed that during the formation of the zygote, DNAm marks are removed [15]. Subsequently, DNAm is reestablished during embryonic development [16]. DNAm is critical for the differentiation of cells into distinct lineages from a single zygote [16]. Additionally, DNAm has been linked to the development of a range of diseases and other health conditions [16]. The establishment of DNAm is highly influenced by environmental factors to which the growing embryo is exposed [17].
Prenatal smoking exposure has been shown to affect offspring DNAm in several studies [2]. In a meta-analysis by Joubert et al., over 6000 differentially methylated CpG sites were identified to be associated with MSP [18]. Given the effect of prenatal smoking exposure on the offspring’s DNAm and the potential association of differential DNAm with health conditions, Joubert et al. suggested that influencing DNAm could be a potential mechanism by which prenatal smoking exposure affects the risk of future adverse health outcomes [18].
However, one limitation of prior studies is the lack of knowledge on the impact of individual genetic differences on the association of MPS with DNAm. For instance, Glutathione S-transferase (GST) represents a gene family that encodes several proteins involved in the detoxification of environmental pollutants and protection against reactive oxygen species (ROS) [19]. Studies have shown that some GST genes, such as GSTA1, GSTP1, and GSTM1, are highly expressed in the fetal liver and are responsible for mitigating the effects of ROS produced as a result of exposure to toxic substances [20,21]. This study aimed to test whether the effects of MSP on offspring DNAm are influenced by their GST genetic polymorphism. We used data from the Isle of Wight birth cohort (IoWBC) to examine whether GST gene polymorphisms have a modifying role in the association between MSP and DNAm.
2. Materials and Methods
2.1. Study Population
The IoWBC is a British birth cohort established in 1989 to investigate asthma and allergic disorders on the Isle of Wight, UK [22]. The birth cohort has been approved by both the local research ethics committee, specifically the NRES Committee of South Central—Hampshire B, UK, and the University of Memphis IRB (Institutional Review Board) in Memphis, U.S. (STUDY number: 2423). All individuals participating in recruitment and follow-ups gave parental or personal written consent.
A total of 1536 children born between 1 January 1989 and 28 February 1990, were identified on the Isle of Wight. After excluding those who declined to participate, perinatal deaths, and adoptions, 1456 remained in the study (F1 generation). Parents of the cohort members (F1 generation) were included as the F0 generation. Mother-child dyads (F0 mother-F1 child) were included in this study.
2.2. Assessment of Exposure: MSP
Information on MSP was obtained during pregnancy using questionnaires. In addition, nicotine and its metabolites were measured from maternal blood serum drawn at delivery.
Assessment of Nicotine and Its Downstream Metabolites in Maternal Sera
Nicotine and its downstream metabolites, cotinine, norcotinine, and hydroxycotinine, were assessed in maternal sera as markers of cigarette smoking during pregnancy. Processing and analysis of maternal serum specimens were carried out in random order. Maternal sera at delivery (20 µL aliquots) were obtained using a modified version of the Matyash protocol [23] after addition of 25 pmol cotinine-d3 as an internal standard and metabolites partitioned into water- and organic-soluble fractions. After separation of the fractions, a SpeedVac vacuum centrifuge was used without applying heat to evaporate solvent from the lower (polar) fraction. The collected residues were dissolved in 200 µL of acetonitrile/water and subsequently transferred to an auto-sampler vial with a 200-microliter insert.
Polar fraction metabolites were profiled using LC/high resolution MS on a QExactive mass spectrometer (Thermo Electron North America LLC, Madison, WI, USA) with a Thermo Vanquish Flex binary pump. An auto-sampler equipped with an Acquity BEH Amide column (measures: 10 cm × 1.0 mm, 1.7 µm, Waters, Milford, MA, USA) for hydrophilic interaction liquid chromatography (HILIC) separation was used. Analyses were performed in positive-ion mode with full scan/all-ion fragmentation. Chromatographic separations were carried out using a gradient based on solvent A (100 mM ammonium acetate + 0.4% ammonium hydroxide, pH 9.0 before mixing) in acetonitrile/water (1:1 v/v) and Solvent B (containing 100 mM ammonium acetate + 0.04% ammonium hydroxide, adjusted to pH 9.0 before mixing) in acetonitrile/water (9:1 v/v). Gradient was 0.0–1.0 min (99% B); 7.0–10.0 min (50% B); 10.01 min (99% B); hold until 15 min. The process of aligning, detecting, and normalizing serum constituents was carried out using Progenesis QI v2.4 software provided by Waters, Nonlinear Dynamics, located in Newcastle upon Tyne, NE1 2JE, UK. For metabolite annotation, a search of the extracted spectra was carried out using Compound Discoverer software (Thermo) and the mzCloud database (Thermo). Fragment ions presence characteristics in the high collision-energy mass spectra were manually verified. From the Progenesis software, peak areas were exported. Furthermore, the dataset was filtered to remove signals with a high number of blanks and those with RMD (relative mass defect) > 1200 ppm [24,25], which are generally inorganic salts. Peaks after export were normalized based on areas of internal standard cotinine-d3. They were scaled by multiplying by 1 × 104. Due to the similar properties of nicotine and its downstream metabolites, their levels were all normalized by multiplication by 0.125 in order to convert the signals to nM concentrations in the sera.
Nicotine and some of its metabolites exhibited a relatively high percentage of zero values, exceeding 30%. These zeros could be attributed to either technical factors, such as values falling below the limit of detection or accidental errors in detecting peak or thresholding, or biological factors, such as zero or near-zero abundance in non-smokers. To handle the substantial number of values below the detection limit while minimizing the impact on relatively rare exposure markers, we could have employed a QRILC approach (quantile regression imputation of left-censored data) [26] for the imputation of missing values. This approach, however, would introduce a challenge. The metabolite levels exhibit strong right-skewness due to the presence of severe outliers. If we treat measurements of metabolites as continuous data, it would be necessary to apply a log transformation before conducting any statistical analyses that depend on normality assumptions. However, if a significant proportion of zeros (>30%) were imputed with small random values, the log transformation can amplify the influence of these artificially imputed values, potentially biasing the estimation of parameters in subsequent analyses. Rather than imputing the excessive zeros (>30%), we decided to rank nicotine and its metabolites based on signal abundances using the PROC RANK in SAS, allowing for up to five ranks (0/1/2/3/4). This conservative approach also helps minimize the impact of outliers [27]. To control for the variation among batches of nicotine and its metabolites, analyses were adjusted by a batch variable.
2.3. Assessment of the Outcome: DNAm of the F1 Offspring
DNA from the F1 generation was extracted from dried blood spots obtained from heel prick tests collected on Guthrie cards after birth following the procedure by Beyan et al. [28]. Briefly, QIAamp DNA Investigator kits (Qiagen Inc., Germantown, MD, USA) were utilized for DNA extraction from three 6mm samples that were punched from Guthrie cards following the manufacturing company’s protocol. The measurement of DNA concentration was performed using a Qubit spectrophotometer. Samples with a concentration equal to or higher than 1.14 ng/uL were chosen for further processing. Infinium Methylation EPIC BeadChip arrays (Illumina Inc., San Diego, CA, USA) were used for DNAm measurements, which provided about 850,000 DNAm sites. There were eight total batches from the F1 generation’s DNAm data. DNAm intensities underwent quality control and pre-quantile normalization following the CPACOR pipeline [29]. ComBat [30] was used to remove the batch effect. In addition, we excluded CpG sites containing probe-SNPs within ten base pairs and those CpGs that had a MAF (minor allele frequency) less than 0.007. Finally, 551,710 CpGs remained for further analysis. DNAm levels in β values were assigned to each CpG locus based on the BeadStudio software methylation module. β values (β = methylated/(methylated + unmethylated + c)) signify the proportions of methylated on the total methylated and unmethylated CpG loci. Herein, c is a constant to avoid division by zero [28]. M values were calculated as the logit-transformed β-values of DNAm (M = log2 (β/1 − β)). In regression analyses, M values were used to alleviate severe heteroscedasticity in β values [31].
Because of the different compositions of cell-type populations, analyses were adjusted for proportions of cell types to remove their confounding effect on DNAm assessment [32]. For estimating cell-type proportions of blood, the R-package “minfi” was used [33]. Eosinophils’ proportions were further estimated indirectly using a DNAm profile based on the Houseman approach [34] incorporated into the “minfi” R package. We adjusted for estimations of cell-type proportions including CD4+ T, B-cells, monocytes, natural killer cells, neutrophils, and eosinophils in statistical models using DNAm data.
This investigation focuses on DNAm CpG sites known to be associated with maternal smoking. Hence, CpGs were extracted from the meta-analysis by Joubert et al. [18]. Based on the meta-analysis of data from 13 cohorts using the Illumina 450 k methylation array, Joubert et al. identified 6073 CpG sites that had a significant association with MSP, of which 568 survived Bonferroni adjustments [18]. Among the 551,710 CpGs in the IoWBC, 460 of the 568 CpGs were available and included in our analyses.
2.4. Assessment of Covariates
Due to sex-specific differences in DNAm [35,36], analyses were conducted with a sex-stratified approach. Covariates used in all models include maternal age at delivery, maternal body mass index (BMI), and the child’s socioeconomic status (SES) during childhood/adolescence. The weight and height of the mothers were assessed during pregnancy and used to calculate BMI (BMI = weight (kg)/height2 (m)). Hospital records were used to obtain mothers’ ages at delivery and the gender of their offspring. SES was derived from three variables: British socioeconomic classes determined by the occupation of the parents (1–6), the total income of the family, and the number of children residing in the bedroom of the index child. SNPs of the GST gene were evaluated as potential effect modifiers of the association between nicotine and its metabolites and offspring DNAm.
2.5. Assessment of Effect Modifiers (GST Gene Polymorphisms)
DNA was extracted from the whole blood or saliva of IoWBC participants for genotyping (N = 1211), as previously described [37]. Single nucleotide Polymorphisms (SNPs) in GST genes were genotyped using Illumina GoldenGate assays [38]. GST SNPs with available data were rs929166, rs10735234, rs11807, rs12024479, rs12736389, rs1537234, rs1537236, rs1695, rs366631, rs3768490, rs506008, rs560018, rs574344, rs638820, and rs7483.
To decrease the number of multiple tests, particularly for correlated SNPs, the number of SNPs was reduced to haplotype blocks based on linkage disequilibrium (LD) [39]. To identify haplotype blocks, Haploview [40] was used. The GST SNPs were statistically grouped into six blocks of LD (Table 1). SNPs used in the analysis are underlined. The SNP rs1695 did not belong to any block but represented the GSTP1 gene and was analyzed accordingly.

Table 1.
Blocks of linkage disequilibrium (LD) between genetic variants of GST genes.
2.6. Statistical Analysis
All analyses were carried out using the F0 (mothers) and F1 (offspring) generations of the IoWBC using SAS (version 9.0) and Haploview [40]. To show the differences in the methylation proportion for different levels of the nicotine metabolites, we compared the DNAm of the lowest with the highest rank of the metabolites.
The association of nicotine and its metabolites with offspring DNAm was assessed using linear regression models with nicotine and its metabolites in maternal sera as the predictors, the offspring GST SNP as a predictor and potential effect modifier, and the DNAm of the offspring as the outcome. The linear regression models were adjusted with the following covariates: maternal age, BMI, SES, and a variable presenting batch groups of nicotine metabolites.
To assess the goodness of fit of the regression models, the explained variance (R2) was calculated for three models. Model 1 included nicotine and its metabolites and confounders. Model 2 additionally addressed the GST SNPs. Model 3 included nicotine metabolites, GST SNPs, and the interaction between nicotine metabolites and GST SNPs. Model 1 explains how much variability in the offspring’s DNAm is related to exposure to nicotine and its metabolites. Comparing models 1 and 2 shows whether GST polymorphisms add to the explanatory power of the model. Comparing models 3 and 2 shows the role of interaction terms in explaining a part of the DNAm variability.
The False Discovery Rate (FDR) method [25] was utilized to account for multiple testing during the assessment of the association between nicotine metabolites and offspring DNAm [32]. A significance level of p < 0.05 was employed to determine statistical significance.
3. Results
After excluding those with missing information on biomarkers of MSP or offspring DNAm, 493 mother-child dyads from F0–F1 generations were included in the study. There was no statistical difference in characteristics between the analyzed sample and the total cohort for males and females (Table 2).

Table 2.
Characteristics of the total Isle of Wight birth cohort and the analyzed samples.
The SNP rs506008 has three genotype groups: AA, AC, and CC (Table 3). Since AA was a rare occurrence (3 in 233 males and 4 in 227 females, respectively), it will cause a violation of the 5% rule for the chi-square test or a reduced sample size for regression analysis. Due to the rarity of individuals with the AA genotype, we combined AA with AC.

Table 3.
GST genotypes of the total Isle of Wight birth cohort and the analyzed samples.
Similarly, two other GST SNPs (rs574344 and rs12736389) had sparse data for one of their variants. For rs574344, the AA genotype was combined with AT and compared to TT. For rs12736389, the CC genotype was added to CG and compared to GG.
To check if combining the rare genotype affects the results of the regression analysis, we further assessed the three regression models with and without the combination of the rare variant and excluding the rare variant. For example, in males, the rs12736389 polymorphism (genotypes: CC, CG, and GG) is an effect modifier of the association between maternal serum norcotinine and offspring DNAm level at cg09935388. Three regression models were run with (a) CC separated from CG, (b) CC combined with CG, and (c) CC removed from the dataset. The effect estimates and p values of the CG group compared to the reference group (GG) were (a) β = 0.334, p = 0.098; (b) β = 0.335, p = 0.096; and (c) β = 0.334, p = 0.098. The effect estimates and p values of the interaction term were (a) β = −0.174, p = 0.04; (b) β = −0.176, p = 0.04; and (c) β = −0.174, p = 0.04. Since combining the CC genotype with the CG genotype did not change the statistics, we took the approach explained above.
GST gene polymorphisms did not affect the concentrations of nicotine metabolites in maternal serum for any of the blocks (Supplementary Table S1), nor was there any interaction of haplotype blocks with MSP for the concentration of nicotine metabolites in maternal serum. These findings simplified the analyses on whether (b) GST gene polymorphism of the offspring modifies the association of exposure to nicotine and its metabolites with offspring DNAm since there is no need to account for the impact of GST SNPs on nicotine metabolites in the analysis.
Next, a potential effect modification of GST gene polymorphism on the association between nicotine metabolites in maternal serum (exposure) and offspring DNAm (outcome) was assessed, adjusting for effect confounders and controlling for multiple testing (FDR). From the meta-analysis by Joubert et al. [19], 460 CpGs were available in our DNAm dataset (850 k), which were used for further analysis.
Table 4 shows the CpG sites whose methylation levels were associated with MSP and with effect modification of GST gene polymorphisms (after FDR adjustment) for male and female offspring, respectively (only statistically significant associations are shown).

Table 4.
Multiple linear regression with norcotinine and hydroxycotinine, GST SNPs, and their interaction on CpG sites whose methylation levels are associated with nicotine and its metabolites in male and female offspring.
Nicotine levels were not associated with differential DNAm at any of the 460 CpG sites. However, cotinine, norcotinine, and hydroxycotinine levels were associated with different levels of offspring DNAm; however, only norcotinine and hydroxycotinine showed significant interactions with GST SNPs on DNAm. For male offspring, the associations of hydroxycotinine with DNAm at cg18473733 (rs574344; GSTM2), cg25949550 (rs574344; GSTM2), cg11647108 (rs1695; GSTP1), cg01952185 (rs1695; GSTP1), and maternal norcotinine with DNAm at cg09935388 (rs12736389; GSTM5) were significantly modified by GST SNPs. For female offspring, the associations of hydroxycotinine with DNAm at cg12160087 (rs506008; GSTM4, rs1537234; GSTM3) and norcotinine with DNAm at cg18473733 (rs506008; GSTM4) were significantly modified by GST SNPs.
The following graphs (Figure 1 and Figure 2) compare the difference in DNAm (β-values or proportion of methylation) levels in the highest versus lowest ranks of hydroxycotinine and norcotinine for different SNPs and genotypes. The mean difference in DNAm levels and their 95% confidence interval comparing the highest rank (4) vs. the lowest rank (0) for the interaction of the nicotine metabolite groups with GST genes were calculated.
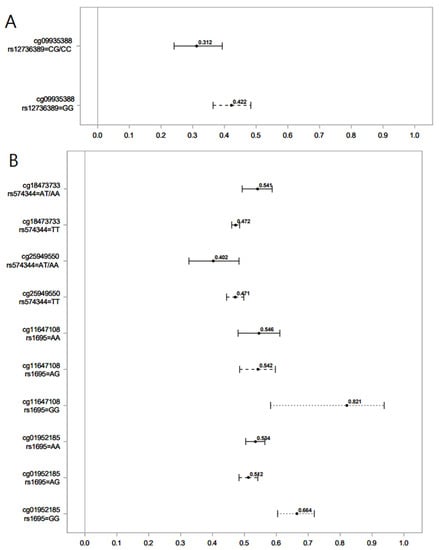
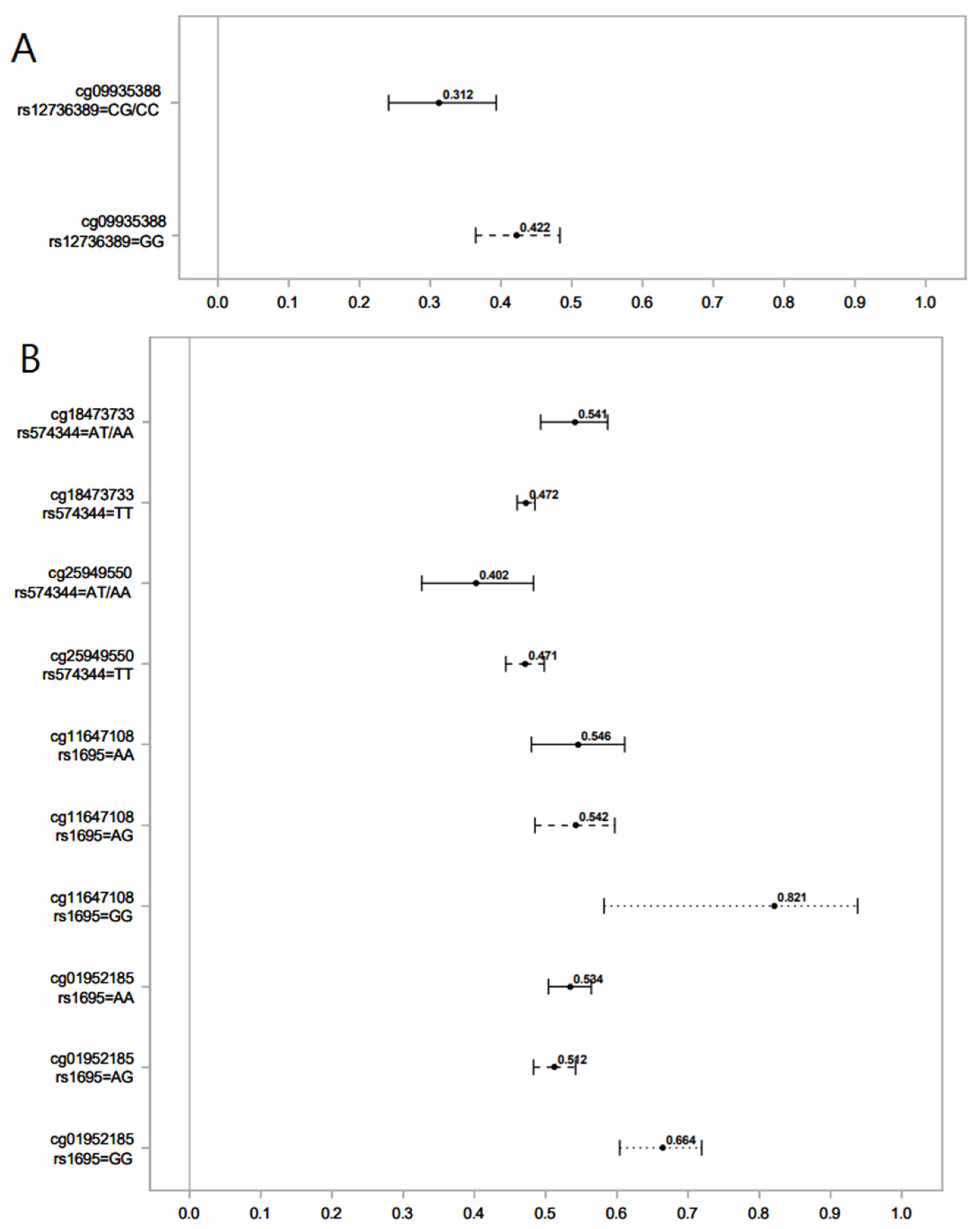
Figure 1.
Association of serum levels of norcotinine (A) and hydroxycotinine (B) in maternal sera and male offspring DNAm for different SNPs of the GST gene. Lines show the proportion of methylation and their 95% CI for the highest vs. lowest rank of hydroxycotinine. Statistically important differential DNAm levels were identified using M-values; however, to present them as proportions, we used β values in the graphs. Due to the rarity of the AA genotype for rs574344, for analysis, individuals with this genotype were combined with those with the AT genotype. Due to the rarity of the CC genotype for rs12736389, individuals with this genotype were combined with those with the CG genotype.
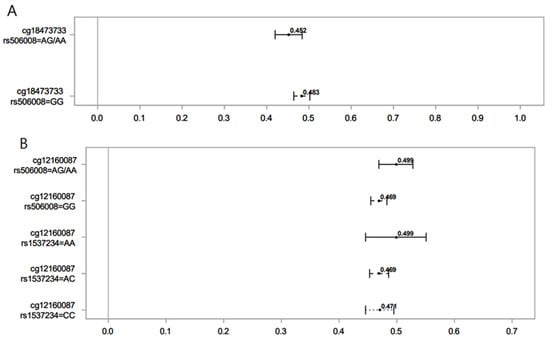
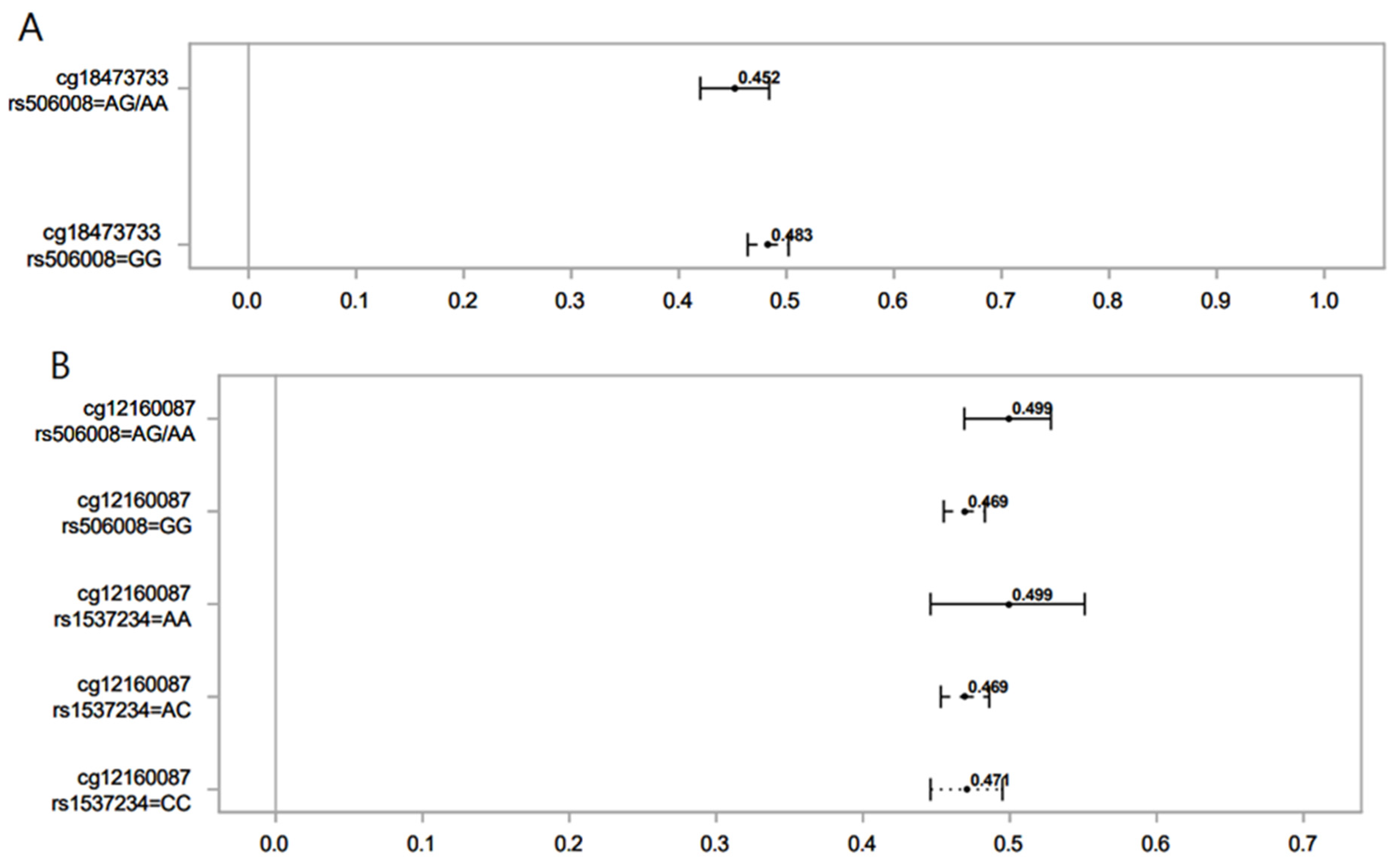
Figure 2.
Association of norcotinine (A) and hydroxycotinine (B) in maternal sera and female offspring DNAm for different SNPs of the GST gene. Lines show the proportion of methylation and their 95% CI for the highest vs. lowest rank of hydroxycotinine. Statistically important differential DNAm levels were identified using M-values; however, to present them as proportions, we used β values in the graphs. Due to the rarity of the AA genotype for rs506008, individuals with the AA genotype were combined with the AG genotype.
The explained variance (R square) of the DNAm was calculated for regression models with and without GST SNP and the interaction term between nicotine metabolite and GST SNP (Table 5).

Table 5.
R square for different multiple linear regressions on nicotine downstream metabolites and offspring DNAm with and without GST SNPs and the interaction terms between nicotine metabolites and GST SNPs.
In regression models with norcotinine or hydroxycotinine as predictors and DANm as outcome, the addition of GST polymorphism increased R2. The addition of the interaction term between nicotine metabolite and GST SNP further increased R2 for both male and female offspring. For example, in males, the addition of the rs1695 SNP and the interaction term with hydroxycotinine increased the R2 by 39.6% (from 0.182 to 0.254) for DNAm at cg11647108. In females, the addition of the rs1537234 SNP and the interaction term with hydroxycotinine increased R2 by 27.5% (from 0.167 to 0.213) for DNAm at cg12160087.
4. Discussion
Since the concentrations of the four smoking metabolites in maternal sera were not affected by the GST genotypes of the offspring, associations between nicotine metabolites, GST genotypes, and their interactions on DNAm do not need adjustment for varying levels of nicotine metabolites due to GST enzyme activity. This is not particularly surprising in that GSTs have not been documented to play important direct roles in nicotine metabolism but are likely involved in the metabolism of other tobacco smoke chemicals.
The results suggest that associations of nicotine metabolites in maternal sera with offspring DNAm are partially influenced by GST gene polymorphism and their interaction with tobacco constituent metabolites. Effect modification was observed for several members of the GST gene family. Hence, the role of the enzymes encoded by the GST gene family in the protection of the developing fetus from oxidative stress suggests individual susceptibility to MSP based on genetic polymorphism.
We found that the interaction term improved the explained variance (R2) for all five CpGs in males and two CpGs in females, respectively. This implies that for some CpGs, the addition of interaction terms from GST gene polymorphisms with nicotine metabolites improves the explanation.
We observed that the effect of modifications of GST SNPs on the association between nicotine metabolites and offspring DNAm is sex-specific. This might be explained by differences in the response of the placenta to oxidative stress based on the offspring’s sex [41]. Research has indicated that when exposed to unfavorable maternal conditions characterized by oxidative stress, the male placenta tends to exhibit a more prominent response compared to the female placenta [42]. In rodents, high oxidative stress conditions caused sexually dimorphic changes in placental morphology, gene expression, and enzymes involved in DNAm [41]. Furthermore, glutathione metabolism is reported to be different in male and female offspring [43]. O’Shaughnessy et al. have shown that MSP affects the transcription of some GST genes in the fetal liver (GSTA1, GSTP1, and GSTM1) in a sex-dependent manner [44].
We found that in male offspring, the AT genotype of rs574344 (GSTM2 gene) attenuated the decrease in DNAm at cg18473733 by hydroxycotinine, but it amplified the decrease in DNAm at cg25949550 by hydroxycotinine. Genetic polymorphisms of the GSTM2 gene at different loci, including rs574344, have been studied in association with offspring lung function after exposure to MSP [45]. No study, however, has investigated the modifying role of GST SNPs on the association between MSP and offspring DNAm. The CpGs cg18473733 and cg25949550 belong to the genes KLF2 and CNTNAP2, respectively. The Krüppel-like factor 2 (KLF2) gene codes for a transcription factor that plays a crucial role during the development of embryonic vasculature [46]. The Contactin-Associated Protein 2 (CNTNAP2) gene is involved in nervous system development and has been implicated in disorders such as autism and intellectual disability in association with exposure to MSP [47]. The potential effect of GSTM2 polymorphism on health outcomes in offspring exposed to MSP by altering specific DNAm remains to be addressed by future studies.
In males, the CG genotype of rs12736389 (GSTM5) amplified the decrease in DNAm at cg09935388 by norcotinine. This CpG belongs to the growth factor independent 1 transcriptional repressor (GFI1) gene and plays a role in developmental processes, including hematopoiesis [48]. DNAm at cg09935388 has been reported to mediate the effects of exposure to MSP on offspring birth weight [49]. The genetic polymorphism of GST5 has been associated with lung function measures (FEV1 and FVC) in interaction with MSP exposure [38].
The AA and AG genotypes of rs1695 (GSTP1) attenuated the increase in DNAm at cg11647108 (ANXA11) and cg01952185 (TIFAB) by hydroxycotinine. The gene ANXA11 codes for Annexin A11, a member of Annexins, i.e., phospholipid-binding proteins regulated by calcium with significant involvement in various cellular processes such as the cell life cycle, exocytosis, and apoptosis [49]. TRAF-interacting protein with a forkhead-associated domain B (TIFAB) is another protein-coding gene implicated in several cellular signaling pathways involved in hematopoiesis [50]. Structural polymorphisms in the GSTP1 gene have been reported to affect the association between MSP and offspring health outcomes by altering the encoded enzyme activity [51]. The genotypes AA and AG of rs1695 (GSTP1) have been shown to increase the risk of early life wheezing in children exposed to MSP [51].
In female offspring, hydroxycotinine decreased DNAm at cg12160087. However, offspring with an AG genotype of rs506008 (GSTM4) had higher DNAm levels compared to GG. Those with AA and AC SNPs of rs1537234 (GSTM3) had higher and lower DNAm levels compared to CC, respectively. DNAm levels at cg12160087 in the latter group (AC genotype of rs1537234) were not significantly different from the CC genotype of rs1537234. The CpG site cg12160087 is associated with the Coiled-coil domain containing 64 (CCDC64), a protein-coding gene involved in cellular transport and nervous system development [52]. DNAm at cg12160087 has been positively associated with offspring birth weight [53]. Previous research shows that genetic polymorphisms of the GSTM3 gene affect lung function (FEV1 and FVC) by interacting with MSP [38].
Norcotinine was associated with decreased DNAm levels at cg18473733 (KLF2). The AG genotype of rs506008 (GSTM4) further lowered the levels of DNAm compared to the GG SNP. This CpG site has also been observed to be significantly associated with MSP in male offspring. GSTM4 has not been well studied in association with health outcomes [45]. Variations in GSM4 have been linked to lung function measures [45] and lung cancer [54].
In this study, we focused on the CpGs whose DNAm was associated with nicotine metabolites, with a significant effect-modifying role for GST SNPs. However, there are CpGs associated with nicotine metabolites for which we did not find a significant effect-modifying role for GST polymorphism. This may imply that maternal nicotine metabolites may serve as surrogates for tobacco smoke chemicals that affect offspring DNAm through different biological mechanisms in addition to increasing oxidative stress. One of these CpGs is cg05575921 (in the body of the AHRR gene), whose lower methylation has been reported consistently as a marker of exposure to MSP [55,56]. Although significantly associated with nicotine metabolites, DNAm at cg05575921 was not influenced by GST SNPs in our study. This might further emphasize its usefulness as a smoking exposure biomarker since it is not influenced by individual differences in GST genotype.
To the best of our knowledge, our study is the first to address the role of GST gene polymorphisms in modifying the associations of nicotine metabolites with offspring DNAm. Using a prospective design and a population-based cohort are among the strengths of our study. Additionally, we used a more objective assessment for MSP, i.e., nicotine and its downstream metabolites in maternal serum.
There are some limitations in our study worth mentioning. First, from the 586 CpGs extracted from the Joubert et al. meta-analysis, we had information on 460 CpGs. Hence, some of the CpGs not included in our quality control might have been associated with nicotine metabolites through the effect-modifying role of GST SNPs. Second, our data on the GST gene was limited to different GSTM genes and one GSTP gene. Although most GST genes associated with MSP belong to these two groups, there are previous studies that suggest a potential role for GSTA, GSTT, and GSTO genes [57,58,59] in health outcomes associated with MSP.
The third limitation is the lack of information on the other toxic constituents of cigarette smoke other than nicotine that might lead to adverse consequences in the offspring. For example, cigarette smoke contains numerous toxic compounds, including polycyclic aromatic hydrocarbons (PAHs) and benzoquinone [60]. This group of compounds has been known to mediate several toxic effects of exposure to cigarette smoke, including childhood asthma and impaired lung function [61]. Fourth, we did not have data on the placenta samples and the expression of GST genes. The placenta plays a crucial role in protecting the developing fetus from harmful substances by mitigating oxidative stress [62]. GST enzymes produced by the placenta catalyze the transfer of reduced glutathione (GSH) to ROS and assorted reactive electrophilic intermediates, helping to neutralize their harmful effects by making them more water-soluble and more rapidly excreted [63].
Fifth, we focused on offspring genotypes, but maternal genotypes regarding GST genes were not available. Although offspring GST polymorphism did not seem to affect maternal levels of nicotine and its metabolites, GST enzymes in the mother might affect nicotine metabolites or other compounds [21] and modify the effect of exposure on offspring DNAm. Sixth, the analyses of the associations between nicotine metabolites and offspring DNAm only focused on DNAm measurement at birth. Future work may be necessary to investigate the long-term associations of MSP and DNAm measures at 10 and 18 years in this cohort.
5. Conclusions
The findings suggest that offspring genetic variations in GST genes modify the effect of tobacco smoke chemicals (nicotine metabolites in maternal sera) on offspring DNAm at a limited number of CpGs in a sex-specific manner. Importantly, the methylation of a specific CpG site, cg05575921 (associated with the AHRR gene), which has consistently been identified as an indicator of exposure to MSP, remained unaffected by GST polymorphisms. This further supports its value as a reliable biomarker for MSP exposure. Future studies are necessary to test the role of additional genetic polymorphisms in the association between MSP and offspring DNAm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081644/s1. Table S1: Association of offspring GST gene polymorphism with nicotine and downstream metabolites in maternal sera.
Author Contributions
Conceptualization, P.K.R. and W.K.; methodology, P.K.R., W.K., H.Z., Y.J. and S.C.; biochemical and technical aspects, A.D.J.; software, P.K.R. and R.B.; formal analysis, P.K.R. and S.E. (Shakiba Eslamimehr); data curation, S.C. and S.E. (Susan Ewart); writing—original draft preparation, P.K.R., W.K., J.W.H., S.E. (Susan Ewart) and H.A.; writing—review and editing, all authors; visualization, P.K.R.; supervision, W.K.; funding acquisition, W.K., H.A. and A.D.J. All authors have read and agreed to the published version of the manuscript.
Funding
The funding for this research was provided by the National Institute of Allergy and Infectious Diseases [R01 AI091905] and the National Heart, Lung, and Blood Institute [R01 HL132321], awarded to W.K. A.D.J. acknowledges support from Michigan AgBioResearch, which is funded by the USDA National Institute of Food and Agriculture, under the Hatch project number MICL02474.
Institutional Review Board Statement
Ethics approval was obtained from the Isle of Wight Local Research Ethics Committee at the recruitment of this birth cohort born on the Isle of Wight, United Kingdom, between January 1989 and February 1990. Additional approval was acquired for year 1 and 2 follow-ups (No. 05/89; dated 22 August 1988), and the Ethics Committee approved an extension for this study to allow follow-up at 4 years (dated 17 January 1993). Subsequently, at 10 years follow-up, we obtained permission from the Isle of Wight Local Research Ethics Committee for the follow-up as well as the collection of blood for genetic studies into asthma and allergy (No. 18/98, dated 20 July 1998). For the 18-year follow-up, ethics approval was given by the Isle of Wight, Portsmouth, and SE Hampshire Local Research Ethics Committee (No. 06/Q1701/34, dated 16 June 2006). In addition, the University of Memphis Institutional Review Board in Memphis, U.S., approved the analysis of the data (Study #: 2423).
Informed Consent Statement
Written informed consent was obtained from all parents during visits.
Data Availability Statement
To ensure compliance with participants’ consent and ethical approval, data access is restricted. For inquiries regarding the data presented in this study, interested parties may contact the corresponding author.
Acknowledgments
The staff at the David Hide Asthma and Allergy Research Centre, Isle of Wight, UK, are greatly appreciated for their assistance in recruitment and collecting samples. The authors would also like to express their gratitude to all the individuals who participated in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; François, O.; Lepeule, J. Epigenetic Alterations of Maternal Tobacco Smoking during Pregnancy: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5083. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Spindel, E.R. Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef]
- Kataoka, M.C.; Carvalheira, A.P.P.; Ferrari, A.P.; Malta, M.B.; de Barros Leite Carvalhaes, M.A.; de Lima Parada, C.M.G. Smoking during pregnancy and harm reduction in birth weight: A cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 67. [Google Scholar] [CrossRef]
- Shah, G.; Bhatt, U.; Soni, V. Cigarette: An unsung anthropogenic evil in the environment. Environ. Sci. Pollut. Res. Int. 2023, 30, 59151–59162. [Google Scholar] [CrossRef]
- Morgan, J.C.; Byron, M.J.; Baig, S.A.; Stepanov, I.; Brewer, N.T. How people think about the chemicals in cigarette smoke: A systematic review. J. Behav. Med. 2017, 40, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; St Helen, G.; Dempsey, D.A.; Jacob, P., 3rd; Tyndale, R.F. Disposition kinetics and metabolism of nicotine and cotinine in African American smokers: Impact of CYP2A6 genetic variation and enzymatic activity. Pharmacogenet. Genom. 2016, 26, 340–350. [Google Scholar] [CrossRef]
- Wickström, R. Effects of nicotine during pregnancy: Human and experimental evidence. Curr. Neuropharmacol. 2007, 5, 213–222. [Google Scholar] [CrossRef]
- Wiklund, P.; Karhunen, V.; Richmond, R.C.; Parmar, P.; Rodriguez, A.; De Silva, M.; Wielscher, M.; Rezwan, F.I.; Richardson, T.G.; Veijola, J.; et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin. Epigenet. 2019, 11, 97. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Vavouri, T.; Lehner, B. Human genes with CpG island promoters have a distinct transcription-associated chromatin organization. Genome Biol. 2012, 13, R110. [Google Scholar] [CrossRef]
- Lev Maor, G.; Yearim, A.; Ast, G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015, 31, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Grieshober, L.; Graw, S.; Barnett, M.J.; Thornquist, M.D.; Goodman, G.E.; Chen, C.; Koestler, D.C.; Marsit, C.J.; Doherty, J.A. AHRR methylation in heavy smokers: Associations with smoking, lung cancer risk, and lung cancer mortality. BMC Cancer 2020, 20, 905. [Google Scholar] [CrossRef] [PubMed]
- Dhar, G.A.; Saha, S.; Mitra, P.; Nag Chaudhuri, R. DNA methylation and regulation of gene expression: Guardian of our health. Nucleus 2021, 64, 259–270. [Google Scholar] [CrossRef]
- Wang, X.; Bhandari, R.K. DNA methylation dynamics during epigenetic reprogramming of medaka embryo. Epigenetics 2019, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef]
- Das, J.; Maitra, A. Maternal DNA Methylation During Pregnancy: A Review. Reprod. Sci. 2021, 28, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; Felix, J.F.; Yousefi, P.; Bakulski, K.M.; Just, A.C.; Breton, C.; Reese, S.E.; Markunas, C.A.; Richmond, R.C.; Xu, C.J.; et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am. J. Hum. Genet. 2016, 98, 680–696. [Google Scholar] [CrossRef]
- Nebert, D.W.; Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004, 1, 460–464. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxid. Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Holloway, J.W.; Karmaus, W.; Zhang, H.; Ewart, S.; Mansfield, L.; Matthews, S.; Hodgekiss, C.; Roberts, G.; Kurukulaaratchy, R. Cohort Profile: The Isle Of Wight Whole Population Birth Cohort (IOWBC). Int. J. Epidemiol. 2018, 47, 1043–1044i. [Google Scholar] [CrossRef] [PubMed]
- Sostare, J.; Di Guida, R.; Kirwan, J.; Chalal, K.; Palmer, E.; Dunn, W.B.; Viant, M.R. Comparison of modified Matyash method to conventional solvent systems for polar metabolite and lipid extractions. Anal. Chim. Acta 2018, 1037, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Stagliano, M.C.; DeKeyser, J.G.; Omiecinski, C.J.; Jones, A.D. Bioassay-directed fractionation for discovery of bioactive neutral lipids guided by relative mass defect filtering and multiplexed collision-induced dissociation. Rapid Commun. Mass. Spectrom. 2010, 24, 3578–3584. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, E.A.; Celiz, M.D.; Jones, A.D. Relative mass defect filtering of mass spectra: A path to discovery of plant specialized metabolites. Plant Physiol. 2015, 167, 1221–1232. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef]
- Karmaus, W.; Kheirkhah Rahimabad, P.; Pham, N.; Mukherjee, N.; Chen, S.; Anthony, T.M.; Arshad, H.S.; Rathod, A.; Sultana, N.; Jones, A.D. Association of Metabolites, Nutrients, and Toxins in Maternal and Cord Serum with Asthma, IgE, SPT, FeNO, and Lung Function in Offspring. Metabolites 2023, 13, 737. [Google Scholar] [CrossRef]
- Beyan, H.; Down, T.A.; Ramagopalan, S.V.; Uvebrant, K.; Nilsson, A.; Holland, M.L.; Gemma, C.; Giovannoni, G.; Boehm, B.O.; Ebers, G.C.; et al. Guthrie card methylomics identifies temporally stable epialleles that are present at birth in humans. Genome Res. 2012, 22, 2138–2145. [Google Scholar] [CrossRef]
- Lehne, B.; Drong, A.W.; Loh, M.; Zhang, W.; Scott, W.R.; Tan, S.-T.; Afzal, U.; Scott, J.; Jarvelin, M.-R.; Elliott, P.; et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 2015, 16, 37. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Du, P.; Zhang, X.; Huang, C.C.; Jafari, N.; Kibbe, W.A.; Hou, L.; Lin, S.M. Comparison of β-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform. 2010, 11, 587. [Google Scholar] [CrossRef]
- Koestler, D.C.; Christensen, B.; Karagas, M.R.; Marsit, C.J.; Langevin, S.M.; Kelsey, K.T.; Wiencke, J.K.; Houseman, E.A. Blood-based profiles of DNA methylation predict the underlying distribution of cell types: A validation analysis. Epigenetics 2013, 8, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Kim, S.; Kelsey, K.T.; Wiencke, J.K. DNA Methylation in Whole Blood: Uses and Challenges. Curr. Environ. Health Rep. 2015, 2, 145–154. [Google Scholar] [CrossRef] [PubMed]
- El-Maarri, O.; Becker, T.; Junen, J.; Manzoor, S.S.; Diaz-Lacava, A.; Schwaab, R.; Wienker, T.; Oldenburg, J. Gender specific differences in levels of DNA methylation at selected loci from human total blood: A tendency toward higher methylation levels in males. Hum. Genet. 2007, 122, 505–514. [Google Scholar] [CrossRef]
- Grant, O.A.; Wang, Y.; Kumari, M.; Zabet, N.R.; Schalkwyk, L. Characterising sex differences of autosomal DNA methylation in whole blood using the Illumina EPIC array. Clin. Epigenet. 2022, 14, 62. [Google Scholar] [CrossRef]
- Mukherjee, N.; Lockett, G.A.; Merid, S.K.; Melén, E.; Pershagen, G.; Holloway, J.W.; Arshad, S.H.; Ewart, S.; Zhang, H.; Karmaus, W. DNA methylation and genetic polymorphisms of the Leptin gene interact to influence lung function outcomes and asthma at 18 years of age. Int. J. Mol. Epidemiol. Genet. 2016, 7, 1–17. [Google Scholar]
- Alexander, M.; Karmaus, W.; Holloway, J.W.; Zhang, H.; Roberts, G.; Kurukulaaratchy, R.J.; Arshad, S.H.; Ewart, S. Effect of GSTM2-5 polymorphisms in relation to tobacco smoke exposures on lung function growth: A birth cohort study. BMC Pulm. Med. 2013, 13, 56. [Google Scholar] [CrossRef]
- Wang, T.; Jacob, H.; Ghosh, S.; Wang, X.; Zeng, Z.B. A joint association test for multiple SNPs in genetic case-control studies. Genet. Epidemiol. 2009, 33, 151–163. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Ganguly, E.; Aljunaidy, M.M.; Kirschenman, R.; Spaans, F.; Morton, J.S.; Phillips, T.E.; Case, C.P.; Cooke, C.-L.M.; Davidge, S.T. Sex-specific effects of nanoparticle-encapsulated MitoQ (nMitoQ) delivery to the placenta in a rat model of fetal hypoxia. Front. Physiol. 2019, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.C.; Tremblay, A. Sex-Specificity of Oxidative Stress in Newborns Leading to a Personalized Antioxidant Nutritive Strategy. Antioxidants 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J.; Monteiro, A.; Bhattacharya, S.; Fowler, P.A. Maternal Smoking and Fetal Sex Significantly Affect Metabolic Enzyme Expression in the Human Fetal Liver. J. Clin. Endocrinol. Metab. 2011, 96, 2851–2860. [Google Scholar] [CrossRef]
- Breton, C.V.; Vora, H.; Salam, M.T.; Islam, T.; Wenten, M.; Gauderman, W.J.; Van den Berg, D.; Berhane, K.; Peters, J.M.; Gilliland, F.D. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am. J. Respir. Crit. Care Med. 2009, 179, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Chiplunkar, A.R.; Curtis, B.C.; Eades, G.L.; Kane, M.S.; Fox, S.J.; Haar, J.L.; Lloyd, J.A. The Krüppel-like factor 2 and Krüppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC Dev. Biol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Rzehak, P.; Saffery, R.; Reischl, E.; Covic, M.; Wahl, S.; Grote, V.; Xhonneux, A.; Langhendries, J.P.; Ferre, N.; Closa-Monasterolo, R.; et al. Maternal Smoking during Pregnancy and DNA-Methylation in Children at Age 5.5 Years: Epigenome-Wide-Analysis in the European Childhood Obesity Project (CHOP)-Study. PLoS ONE 2016, 11, e0155554. [Google Scholar] [CrossRef]
- Phelan, J.D.; Shroyer, N.F.; Cook, T.; Gebelein, B.; Grimes, H.L. Gfi1-cells and circuits: Unraveling transcriptional networks of development and disease. Curr. Opin. Hematol. 2010, 17, 300–307. [Google Scholar] [CrossRef]
- Küpers, L.K.; Xu, X.; Jankipersadsing, S.A.; Vaez, A.; La Bastide-van Gemert, S.; Scholtens, S.; Nolte, I.M.; Richmond, R.C.; Relton, C.L.; Felix, J.F.; et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int. J. Epidemiol. 2015, 44, 1224–1237. [Google Scholar] [CrossRef]
- Niederkorn, M.; Hueneman, K.; Choi, K.; Varney, M.E.; Romano, L.; Pujato, M.A.; Greis, K.D.; Inoue, J.I.; Meetei, R.; Starczynowski, D.T. TIFAB Regulates USP15-Mediated p53 Signaling during Stressed and Malignant Hematopoiesis. Cell Rep. 2020, 30, 2776–2790.e2776. [Google Scholar] [CrossRef]
- Wu, J.; Hankinson, J.; Kopec-Harding, K.; Custovic, A.; Simpson, A. Interaction between glutathione S-transferase variants, maternal smoking and childhood wheezing changes with age. Pediatr. Allergy Immunol. 2013, 24, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Terenzio, M.; Schiavo, G. The more, the better: The BICD family gets bigger. EMBO J. 2010, 29, 1625–1626. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Andrade, E.; Gomes, G.M.C.; Collison, A.; Grehan, J.; Murphy, V.E.; Gibson, P.; Mattes, J.; Karmaus, W. Variation of DNA Methylation in Newborns Associated with Exhaled Carbon Monoxide during Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 1597. [Google Scholar] [CrossRef]
- Liloglou, T.; Walters, M.; Maloney, P.; Youngson, J.; Field, J.K. A T2517C polymorphism in the GSTM4 gene is associated with risk of developing lung cancer. Lung Cancer 2002, 37, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, S.E.; Timpson, N.; Relton, C.; Davey Smith, G.; Nordestgaard, B.G. AHRR (cg05575921) hypomethylation marks smoking behaviour, morbidity and mortality. Thorax 2017, 72, 646–653. [Google Scholar] [CrossRef]
- Cosin-Tomas, M.; Cilleros-Portet, A.; Aguilar-Lacasaña, S.; Fernandez-Jimenez, N.; Bustamante, M. Prenatal Maternal Smoke, DNA Methylation, and Multi-omics of Tissues and Child Health. Curr. Environ. Health Rep. 2022, 9, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Maravic, V.; Ćorić, V.; Mitkovic-Voncina, M.; Djordjevic, M.; Savić-Radojević, A.; Ercegovac, M.; Matić, M.; Simić, T.; Lečić-Toševski, D.; Tošković, O.; et al. Interaction of glutathione S-transferase polymorphisms and tobacco smoking during pregnancy in susceptibility to autism spectrum disorders. Sci. Rep. 2019, 9, 3206. [Google Scholar] [CrossRef]
- de Jong, K.; Boezen, H.M.; Hacken, N.H.; Postma, D.S.; Vonk, J.M. GST-omega genes interact with environmental tobacco smoke on adult level of lung function. Respir. Res. 2013, 14, 83. [Google Scholar] [CrossRef]
- Grazuleviciene, R.; Danileviciute, A.; Nadisauskiene, R.; Vencloviene, J. Maternal smoking, GSTM1 and GSTT1 polymorphism and susceptibility to adverse pregnancy outcomes. Int. J. Environ. Res. Public Health 2009, 6, 1282–1297. [Google Scholar] [CrossRef]
- Witschi, H.; Espiritu, I.; Maronpot, R.R.; Pinkerton, K.E.; Jones, A.D. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis 1997, 18, 2035–2042. [Google Scholar] [CrossRef]
- Låg, M.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir. Res. 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M.T.M.; Steegers, E.A.P.; Peters, W.H.M. Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues. Hum. Reprod. 2001, 16, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).