Advanced Skeletal Ossification Is Associated with Genetic Variants in Chronologically Young Beef Heifers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Cattle Record Collection
2.3. DNA Isolation and Quantification
2.4. Allelic Discrimination and Sequencing
2.5. Sequence Mapping and Variant Calling
2.6. Genetic Association Analyses
3. Results
3.1. Association Analyses
3.2. Regression Analyses
4. Discussion
4.1. SNP Validation
4.2. Targeted Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasser, C.L.; Grum, D.E.; Mussard, M.L.; Fluharty, F.L.; Kinder, J.E.; Day, M.L. Induction of Precocious Puberty in Heifers I: Enhanced Secretion of Luteinizing Hormone. J. Anim. Sci. 2006, 84, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Funston, R.N.; Deutscher, G.H. Comparison of Target Breeding Weight and Breeding Date for Replacement Beef Heifers and Effects on Subsequent Reproduction and Calf Performance. J. Anim. Sci. 2004, 82, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.J.; Perry, R.C.; Kiracofe, G.H.; Bellows, R.A.; Staigmiller, R.B.; Corah, L.R. Management Considerations in Heifer Development and Puberty. J. Anim. Sci. 1992, 70, 4018–4035. [Google Scholar] [CrossRef] [PubMed]
- Lesmeister, J.L.; Burfening, P.J.; Blackwell, R.L. Date of First Calvin in Beef Cows and Subsequent Calf Productions. J. Anim. Sci. 1973, 36, 1–6. [Google Scholar]
- Duckett, S.K.; Andrae, J.G. Implant Strategies in an Integrated Beef Production System. J. Anim. Sci. 2001, 79, E110. [Google Scholar] [CrossRef]
- Herrington, M.A.; Tonsor, G.T. Beef Premiums and Discounts; Kansas State University: Manhattan, KS, USA, 2012; pp. 1–12. [Google Scholar]
- Tatum, D. Beef Grading; National Cattlemen’s Beef Association: Centennial, CO, USA, 2007; pp. 1–4. [Google Scholar]
- Lawrence, T.E.; Whatley, J.D.; Montgomery, T.H.; Perino, L.J. A Comparison of the USDA Ossification-Based Maturity System to a System Based on Dentition. J. Anim. Sci. 2001, 79, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- USDA. Institutional Meat Purchase Specifications: Fresh Beef Series 100; USDA: Washington, DC, USA, 2014; pp. 1–46.
- Webb, N.B.; Kahlenberg, O.J.N.H.D. Lowing Sequence Beginning at the Anterior. Eckert Pack. CO Defiance Ohio 1955, 2722, 1027–1031. [Google Scholar]
- Tuma, H.J.; Henrickson, R.L.; Stephens, D.F.; Moore, R. Influence If Marbling and Animal Age on Factors Associated with Beef Quality. J. Anim. Sci. 1962, 21, 848–851. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Monteils, V. Associations among Animal, Carcass, Muscle Characteristics, and Fresh Meat Color Traits in Charolais Cattle. Meat Sci. 2018, 140, 145–156. [Google Scholar] [CrossRef]
- Hoffman, K.C.; Colle, M.J.; Nasados, J.A.; Gray, S.J.; Rogers, J.; Van Buren, J.B.; Puga, K.J.; Murdoch, G.K.; Richard, R.P.; Doumit, M.E. Relationship between Heifer Carcass Maturity and Beef Quality Characteristics. Transl. Anim. Sci. 2019, 4, 1206–1215. [Google Scholar] [CrossRef]
- Acheson, R.J.; Woerner, D.R.; Tatum, J.D. Effects of USDA Carcass Maturity on Sensory Attributes of Beef Produced by Grain- Finished Steers and Heifers Classified as Less than 30 Months Old Using Dentition. J. Anim. Sci. 2014, 92, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.D.; Ellis, K.W.I.; Lang, M.M.; Noyes, E.V. Influence of Carcass Maturity on the Palatability of Beef. J. Anim. Sci. 1976, 43, 35. [Google Scholar] [CrossRef]
- USDA. United States Department of Agriculture Agricultural Marketing Service Livestock, Poultry, and Seed Program United States Standards for Grades of Carcass Beef; USDA: Washington, DC, USA, 2017.
- Berendsen, A.D.; Olsen, B.R. Bone Development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- de Crombrugghe, B.; Lefebvre, V.; Nakashima, K. Regulatory Mechanisms in the Pathways of Cartilage and Bone Formation. Curr. Opin. Cell Biol. 2001, 13, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), 131–139. [Google Scholar] [CrossRef] [PubMed]
- Colacchio, A.M. Association Between Carcass Maturity Grade and Genes Involved in Bone Growth, in Young Heifers; University of Idaho: Moscow, ID, USA, 2019; Volume 8, p. 55. [Google Scholar]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Cloning: A Laboratory Manual. Cold Spring Harb. Lab. 1982. [Google Scholar] [CrossRef]
- Davenport, K.M.; Bret Taylor, J.; Henslee, D.; Southerland, C.; Yelich, J.; Ellison, M.J.; Murdoch, B.M. Variation in Type Two Taste Receptor Genes Is Associated with Bitter Tasting Phenylthiocarbamide Consumption in Mature Targhee and Rambouillet Rams. Transl. Anim. Sci. 2021, 5, txab142. [Google Scholar] [CrossRef] [PubMed]
- Minina, E.; Kreschel, C.; Naski, M.C.; Ornitz, D.M.; Vortkamp, A. Interaction of FGF, Ihh/Pthlh, and BMP Signaling Integrates Chondrocyte Proliferation and Hypertrophic Differentiation. Dev. Cell 2002, 3, 439–449. [Google Scholar] [CrossRef]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; Von Der Mark, H.; et al. SOX9 Is a Major Negative Regulator of Cartilage Vascularization, Bone Marrow Formation and Endochondral Ossification. Development 2010, 137, 901–911. [Google Scholar] [CrossRef]
- Liu, Z.; Kennedy, O.D.; Cardoso, L.; Basta-Pljakic, J.; Partridge, N.C.; Schaffler, M.B.; Rosen, C.J.; Yakar, S. DMP-1-Mediated Ghr Gene Recombination Compromises Skeletal Development and Impairs Skeletal Response to Intermittent PTH. FASEB J. 2016, 30, 635–652. [Google Scholar] [CrossRef]
- Andrew, T.W.; Koepke, L.S.; Wang, Y.; Lopez, M.; Steininger, H.; Struck, D.; Boyko, T.; Ambrosi, T.H.; Tong, X.; Sun, Y.; et al. Sexually Dimorphic Estrogen Sensing in Skeletal Stem Cells Controls Skeletal Regeneration. Nat. Commun. 2022, 13, 6491. [Google Scholar] [CrossRef] [PubMed]
- Kaback, L.A.; Soung, D.Y.; Naik, A.; Smith, N.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Osterix/Sp7 Regulates Mesenchymal Stem Cell Mediated Endochondral Ossification. J. Cell. Physiol. 2008, 214, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qiao, L.; Lei, G.; Mira, R.R.; Gu, J.; Zheng, Q. Col10a1 Gene Expression and Chondrocyte Hypertrophy during Skeletal Development and Disease. Front. Biol. 2014, 9, 195–204. [Google Scholar] [CrossRef]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De Novo Assembly of the Cattle Reference Genome with Single-Molecule Sequencing. Gigascience 2020, 9, giaa021. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genomee Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Quinlan, A.R. BEDTools: The Swiss-Army Tool for Genome Feature Analysis. Curr. Protoc. Bioinform. 2014, 47, 11–12. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2014; Available online: http://lib.stat.cmu.edu/R/CRAN/doc/manuals/r-devel/fullrefman.pdf (accessed on 15 March 2023).
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer Fertility and Carry over Consequences for Life Time Production in Dairy and Beef Cattle. Animal 2014, 8, 91–104. [Google Scholar] [CrossRef]
- Sarko, J. Bone and Mineral Metabolism. Emerg. Med. Clin. N. Am. 2005, 23, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Wilhelm, D.; Rodgers, R.J. Development of Mammalian Ovary. J. Endocrinol. 2014, 221, R145–R161. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Elia, G.; O’Boyle, P.; Dunn, M.; Morris, D. Composition of the Bovine Uterine Proteome Is Associated with Stage of Cycle and Concentration of Systemic Progesterone. Proteomics 2013, 13, 3333–3353. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; An, B.; Du, L.; Chang, T.; Liang, M.; Yang, B.G.; Xu, L.; Zhang, L.; Li, J.; Guangxin, E.; et al. Genome-Wide Association Analysis of Growth Curve Parameters in Chinese Simmental Beef Cattle. Animals 2021, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.K.; Alatalo, S.L.; Halleen, J.M.; Mohan, S.; Gustafsson, J.Å.; Ohlsson, C. Estrogen Receptor Specificity in the Regulation of the Skeleton in Female Mice. J. Endocrinol. 2001, 171, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Lindberg, M.; Sävendahl, L.; Lubahn, D.B.; Ritzen, E.M.; Gustafsson, J.Å.; Ohlsson, C. Disproportional Body Growth in Female Estrogen Receptor-α-Inactivated Mice. Biochem. Biophys. Res. Commun. 1999, 265, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Windahl, S.H.; Vidal, O.; Andersson, G.; Gustafsson, J.A.; Ohlsson, C. Increased Cortical Bone Mineral Content but Unchanged Trabecular Bone Mineral Density in Female Mice. J. Clin. Investig. 1999, 104, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sanyal, S.; Chattopadhyay, N. The Role of Estrogen in Bone Growth and Formation: Changes at Puberty. Cell Health Cytoskelet. 2011, 3, 2–12. [Google Scholar]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and Bone Metabolism; Maturitas: Tampere, Finland, 1996; Volume 23. [Google Scholar]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, Sex-Nonspecific Signaling through the Estrogen or Androgen Receptors: Dissociation from Transcriptional Activity. Cell 2001, 104, 719–730. [Google Scholar]
- Velders, M.; Schleipen, B.; Fritzemeier, K.H.; Zierau, O.; Diel, P. Selective Estrogen Receptor-β Activation Stimulates Skeletal Muscle Growth and Regeneration. FASEB J. 2012, 26, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Rastelli, C.; Bulzomi, P.; Acconcia, F.; Pallottini, V.; Marino, M.; Marino, M. 7-Estradiol Regulates the First Steps of Skeletal Muscle Cell Differentiation via ER-Mediated Signals. Am. J. Physiol. Cell. Physiol. 2009, 297, 1249–1262. [Google Scholar] [CrossRef]
- Moustafa, A.; Sugiyama, T.; Saxon, L.K.; Zaman, G.; Sunters, A.; Armstrong, V.J.; Javaheri, B.; Lanyon, L.E.; Price, J.S. The Mouse Fibula as a Suitable Bone for the Study of Functional Adaptation to Mechanical Loading. Bone 2009, 44, 930–935. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline Phosphatase: Structure, Expression and Its Function in Bone Mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Talwar, R.M.; Wong, B.S.; Svoboda, K.; Harper, R.P. Effects of Estrogen on Chondrocyte Proliferation and Collagen Synthesis in Skeletally Mature Articular Cartilage. J. Oral Maxillofac. Surg. 2006, 64, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Widlund, H.R.; Puigserver, P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer 2016, 2, 619–631. [Google Scholar] [CrossRef]
- Ishii, K.A.; Fumoto, T.; Iwai, K.; Takeshita, S.; Ito, M.; Shimohata, N.; Aburatani, H.; Taketani, S.; Lelliott, C.J.; Vidal-Puig, A.; et al. Coordination of PGC-1β and Iron Uptake in Mitochondrial Biogenesis and Osteoclast Activation. Nat. Med. 2009, 15, 259–266. [Google Scholar] [CrossRef]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The Skeleton: A Multi-Functional Complex Organ. The Growth Plate Chondrocyte and Endochondral Ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Ross, F.P. Genetic Regulation of Osteoclast Development and Function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Kressler, D.; Schreiber, S.N.; Knutti, D.; Kralli, A. The PGC-1-Related Protein PERC Is a Selective Coactivator of Estrogen Receptor α. J. Biol. Chem. 2002, 277, 13918–13925. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Wu, J.; Chen, W.; Fan, H.; Liu, H. KLF14 Inhibits Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells by Downregulating WNT3A. Am. J. Transl. Res. 2020, 12, 4445–4455. [Google Scholar] [PubMed]
- Small, K.S.; Todorčević, M.; Civelek, M.; El-Sayed Moustafa, J.S.; Wang, X.; Simon, M.M.; Fernandez-Tajes, J.; Mahajan, A.; Horikoshi, M.; Hugill, A.; et al. Regulatory Variants at KLF14 Influence Type 2 Diabetes Risk via a Female-Specific Effect on Adipocyte Size and Body Composition. Nat. Genet. 2018, 50, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Pant, S.D.; Wani, A.K.; Mohamed, H.H.; Khalifa, N.E.; Almohaimeed, H.M.; Alshanwani, A.R.; Assiri, R.; Aggad, W.S.; Noreldin, A.E.; et al. Krüppel-like Factors Family Regulation of Adipogenic Markers Genes in Bovine Cattle Adipogenesis. Mol. Cell. Probes 2022, 65, 101850. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, C.; Kitajima, H.; Yamamoto, K.; Maeda, Y.; Sonoda, N.; Shibata, H.; Inoguchi, T. DNA Methylation of the Klf14 Gene Region in Whole Blood Cells Provides Prediction for the Chronic Inflammation in the Adipose Tissue. Biochem. Biophys. Res. Commun. 2018, 497, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.D.; Cheng, P.; Liu, T.; Wang, Z. BMSC-Derived Exosomal MiR-29a Promotes Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2020, 8, 608521. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, T.; Kessler, C.B.; Lee, S.K.; Delany, A.M. MiR-29 Promotes Murine Osteoclastogenesis by Regulating Osteoclast Commitment and Migration. J. Biol. Chem. 2013, 288, 33347–33360. [Google Scholar] [CrossRef] [PubMed]
- Roberto, V.P.; Tiago, D.M.; Silva, I.A.L.; Cancela, M.L. MiR-29a Is an Enhancer of Mineral Deposition in Bone-Derived Systems. Arch. Biochem. Biophys. 2014, 564, 173–183. [Google Scholar] [CrossRef]
- Martins, R.; Brito, L.F.; Machado, P.C.; Pinto, L.F.B.; Silva, M.R.; Schenkel, F.S.; Pedrosa, V.B. Genome-Wide Association Study and Pathway Analysis for Carcass Fatness in Nellore Cattle Measured by Ultrasound. Anim. Genet. 2021, 52, 730–733. [Google Scholar] [CrossRef]
- Purfield, D.C.; Evans, R.D.; Berry, D.P. Reaffirmation of Known Major Genes and the Identification of Novel Candidate Genes Associated with Carcass-Related Metrics Based on Whole Genome Sequence within a Large Multi-Breed Cattle Population. BMC Genom. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Reinhardt, C.D.; Busby, W.D.; Corah, L.R. Relationship of Various Incoming Cattle Traits with Feedlot Performance and Carcass Traits. J. Anim. Sci. 2009, 87, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.B.; Da Silva, M.C.; Magnabosco, C.U.; Narciso, M.G.; Sainz, R.D. Selection Indices and Multivariate Analysis Show Similar Results in the Evaluation of Growth and Carcass Traits in Beef Cattle. PLoS ONE 2016, 11, e0147180. [Google Scholar] [CrossRef]
- Hay, E.H.; Roberts, A. Genome-Wide Association Study for Carcass Traits in a Composite Beef Cattle Breed. Livest. Sci. 2018, 213, 35–43. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Orr, T.E.; Fyhrie, D.P.; Schurman, D.J. Influences of Mechanical Stress on Prenatal and Postnatal Skeletal Development. Clin. Orthop. Relat. Res. 1987, 219, 237–250. [Google Scholar] [CrossRef]
- Gross, N.; Strillacci, M.G.; Peñagaricano, F.; Khatib, H. Characterization and Functional Roles of Paternal RNAs in 2–4 Cell Bovine Embryos. Sci. Rep. 2019, 9, 20347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, M.; Zhang, Y.; Song, Y.; Cheng, H.; Zhou, R. The Germline-Enriched Ppp1r36 Promotes Autophagy. Sci. Rep. 2016, 6, 24609. [Google Scholar] [CrossRef]
- Shen, J.; Lin, X.; Dai, F.; Chen, G.; Lin, H.; Fang, B.; Liu, H. Ubiquitin-specific Peptidases: Players in Bone Metabolism. Cell Prolif. 2023, 56, e13444. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Xie, J.; Lee, O.; Kim, J.H.; Hong, J.; Boldyreff, B.; Filhol, O.; Chun, H.; Greenblatt, M.B.; et al. Regulation of Sclerostin by the SIRT1 Stabilization Pathway in Osteocytes. Cell Death Differ. 2022, 29, 1625–1638. [Google Scholar] [CrossRef]
- Shirakawa, J.; Harada, H.; Noda, M.; Ezura, Y. PTH-Induced Osteoblast Proliferation Requires Upregulation of the Ubiquitin-Specific Peptidase 2 (Usp2) Expression. Calcif. Tissue Int. 2016, 98, 306–315. [Google Scholar] [CrossRef]
- Williams, S.A.; Maecker, H.L.; French, D.M.; Liu, J.; Gregg, A.; Silverstein, L.B.; Cao, T.C.; Carano, R.A.D.; Dixit, V.M. USP1 Deubiquitinates ID Proteins to Preserve a Mesenchymal Stem Cell Program in Osteosarcoma. Cell 2011, 146, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, F.; Fang, P.; Dai, T.; Yang, B.; van Dam, H.; Jia, J.; Zheng, M.; Zhang, L. Ubiquitin-Specific Protease 4 Antagonizes Osteoblast Differentiation Through Dishevelled. J. Bone Miner. Res. 2016, 31, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, K.H.; Lee, K.M.; Chun, Y.M.; Lee, J.W. Deubiquitinating Enzyme USP7 Is Required for Self-Renewal and Multipotency of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 8674. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.W.; Pringle, L.M.; Quick, L.; Riquelme, D.N.; Ye, Y.; Oliveira, A.M.; Chou, M.M. TRE17/Ubiquitin-Specific Protease 6 (USP6) Oncogene Translocated in Aneurysmal Bone Cyst Blocks Osteoblastic Maturation via an Autocrine Mechanism Involving Bone Morphogenetic Protein Dysregulation. J. Biol. Chem. 2010, 285, 37111–37120. [Google Scholar] [CrossRef] [PubMed]
- Hock, A.K.; Vigneron, A.M.; Vousden, K.H. Ubiquitin-Specific Peptidase 42 (USP42) Functions to Deubiquitylate Histones and Regulate Transcriptional Activity. J. Biol. Chem. 2014, 289, 34862–34870. [Google Scholar] [CrossRef] [PubMed]
- Markova, E.N.; Petrova, N.V.; Razin, S.V.; Kantidze, O.L. Transcription Factor RUNX1. Mol. Biol. 2012, 46, 755–767. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Yoo, K.J.; Lee, H.J.; Lee, D.R.; Yeo, C.Y.; Baek, K.H. The Expression of Usp42 during Embryogenesis and Spermatogenesis in Mouse. Gene Expr. Patterns 2007, 7, 143–148. [Google Scholar] [CrossRef]
- Paulsson, K.; Békássy, A.N.; Olofsson, T.; Mitelman, F.; Johansson, B.; Panagopoulos, I. A Novel and Cytogenetically Cryptic t(7;21)(P22;Q22) in Acute Myeloid Leukemia Results in Fusion of RUNX1 with the Ubiquitin-Specific Protease Gene USP42. Leukemia 2006, 20, 224–229. [Google Scholar] [CrossRef]
- Kane, E.I.; Spratt, D.E. Structural Insights into Ankyrin Repeat-Containing Proteins and Their Influence in Ubiquitylation. Int. J. Mol. Sci. 2021, 22, 609. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; De Crombrugghe, B. The Transcription Factor Sox9 Has Essential Roles in Successive Steps of the Chondrocyte Differentiation Pathway and Is Required for Expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic Analysis of the Roles of BMP2, BMP4, and BMP7 in Limb Patterning and Skeletogenesis. PLoS Genet. 2006, 2, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Van Rechem, C.; Rood, B.R.; Touka, M.; Pinte, S.; Jenal, M.; Guérardel, C.; Ramsey, K.; Monté, D.; Bégue, A.; Tschan, M.P.; et al. Scavenger Chemokine (CXC Motif) Receptor 7 (CXCR7) Is a Direct Target Gene of HIC1 (Hypermethylated in Cancer 1). J. Biol. Chem. 2009, 284, 20927–20935. [Google Scholar] [CrossRef] [PubMed]
- Sobh, A.; Loguinov, A.; Stornetta, A.; Balbo, S.; Tagmount, A.; Zhang, L.; Vulpe, C.D. Genome-Wide CRISPR Screening Identifies the Tumor Suppressor Candidate OVCA2 as a Determinant of Tolerance to Acetaldehyde. Toxicol. Sci. 2019, 169, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Prowse, A.H.; Vanderveer, L.; Milling, S.W.F.; Pan, Z.Z.; Dunbrack, R.L.; Xu, X.X.; Godwin, A.K. OVCA2 Is Downregulated and Degraded during Retinoid-Induced Apoptosis. Int. J. Cancer 2002, 99, 185–192. [Google Scholar] [CrossRef]
- Schillo, K.K.; Hall, J.B.; Hielman, S.M. Effects of Nutrition and Season on the Onset of Puberty in the Beef Heifer. J. Anim. Sci. 1992, 70, 3994–4005. [Google Scholar] [CrossRef]
- Moorey, S.E.; Biase, F.H. Beef Heifer Fertility: Importance of Management Practices and Technological Advancements. J. Anim. Sci. Biotechnol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
| Position Chr:BP | Ref./Alt. | Test | p-Value | Effect Size | Alt Allele Frequency |
|---|---|---|---|---|---|
| 4:94,584,161 | A/G | C vs. AB | 0.032 | 0.099 | 0.026|0.008 |
| 7:61,142,080 | T/C | A vs. B vs. C | 0.006 | 0.107 | 0.750|0.805|0.847 |
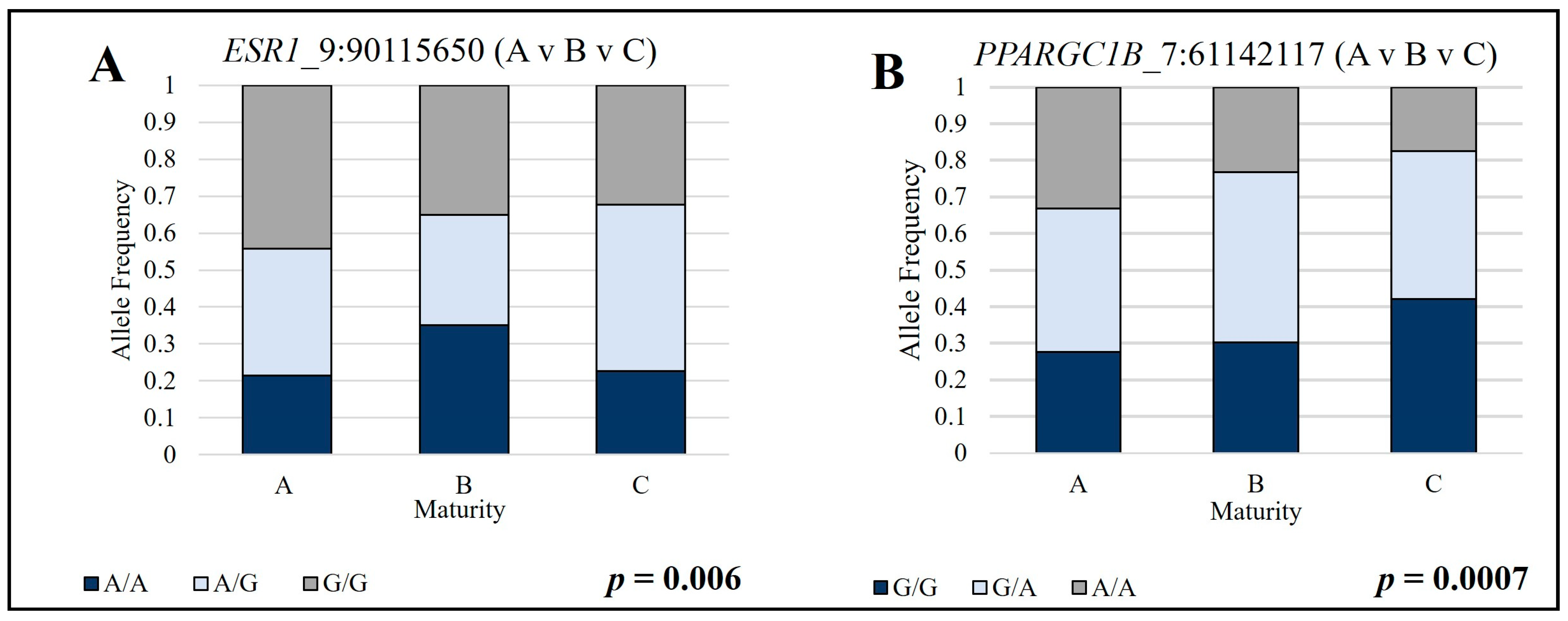
| 7:61,142,117 | A/G | A vs. B vs. C | 0.0007 | 0.128 | 0.472|0.535|0.623 |
| 10:76,769,055 | G/A | A vs. BC | 0.008 | 0.489 | 0.094|0.155 |
| 10:76,769,110 | G/A | A vs. B vs. C | 0.030 | 0.086 | 0.472|0.400|0.390 |
| 19:23,066,541 | C/T | A vs. BC | 0.045 | 0.101 | 0.017|0.041 |
| 25:38,081,982 | G/C | A vs. B vs. C | 0.002 | 0.104 | 0.000|0.005|0.025 |
| 26:28,004,085 | T/C | A vs. BC | 0.012 | 0.974 | 0.040|0.013 |
| * 9:90115650 | A/G | A vs. B vs. C | 0.006 | 0.087 | 0.740|0.683|0.705 |
| * 9:90015095 | C/T | A vs. B vs. C | 0.220 | 0.090 | 0.665|0.670|0.722 |
| * 2:131837201 | G/A | A vs. B vs. C | 0.074 | 0.092 | 0.769|0.783|0.577 |
| Position Chr:BP | Ref./Alt. | Test | Model | Inheritance | p-Value | Genomic Context (±100 kb) |
|---|---|---|---|---|---|---|
| 4:15,158,192 | T/G | A vs. BC | Logistic Regression | Dominant | 0.0634 | Intronic ASNS |
| 5:38,540,781 | A/G | A vs. BC | Association | Recessive | 0.0745 | Within YAF2 3′ UTR, upstream of GXYLT1; downstream of LOC785294 |
| 9:84,791,505 | C/T | A vs. BC | Association | Recessive | 0.0997 | Upstream of SAMD5, LOC100850276; downstream of LOC512881, LOC101907062, LOC101906979 |
| 9:94,978,531 | T/C | A vs. BC | Association | Dominant | 0.0920 | Upstream of TMEM181, LOC101903438, SYTL3; downstream of TULP4, LOC104969639, DYNLT1 |
| 11:102,238,700 | G/C | A vs. BC | Allelic Association | 0.0838 | Upstream of NTNG2, MED27; downstream of SETX | |
| 11:102,238,757 | C/T | C vs. AB | Logistic Regression | Dominant | 0.0618 | Upstream of NTNG2, MED27; downstream of SETX |
| 12:15,818,603 | C/T | A vs. BC | Logistic Regression | Dominant | 0.0967 | Upstream of ERICH6B, LOC101902486; downstream of SIAH3, CBY2 |
| 14:23,564,180 | A/G | A vs. B vs. C | Logistic Regression | Dominant | 0.0563 | Upstream of PENK, SDR16C6 |
| 19:23,346,922 | G/C | C vs. AB | Association Test | Recessive | 0.0908 | Upstream of LOC112442622, TSR1, LOC112442829, LOC112442828, SMG6; downstream of SGSM2, MNT, METTL16, SRR |
| 20:63,542,232 | A/G | A vs. BC | Logistic Regression | Recessive | 0.0837 | Upstream of TAS2R1; downstream of LOC104975290 |
| 20:63,542,246 | T/C | A vs. B vs. C | Logistic Regression | Recessive | 0.0557 | Upstream of TAS2R1; downstream of LOC104975290 |
| 22:41,302,660 | C/T | C vs. AB | Genotypic Association | 0.0987 | Intronic FHIT |
| Position Chr:BP | Genomic Context (±100 kb) |
|---|---|
| 4:94,584,161 | Upstream of KLF14, LOC112446482, LOC107132431; downstream of LOC107131356, SNORA13, mir-29a, mir-29b-1 |
| 7:61,142,080 | Intronic PPARGC1B; downstream of PDE6A, mir-378-1 |
| 7:61,142,117 | Intronic PPARGC1B; downstream of PDE6A, mir-378-1 |
| 10:76,769,055 | Upstream of PLEKHG3; downstream of LOC104973208, PPP1R36, HSPA2, ZBTB1 |
| 10:76,769,110 | Upstream of PLEKHG3; downstream of LOC104973208, PPP1R36, HSPA2, ZBTB1 |
| 19:23,066,541 | Upstream of HIC1, RTN4RL1, LOC112442776, LOC112442621; downstream of DPH1, OVCA2, mir-132, mir-212, SMG6 |
| 25:38,081,982 | Upstream of USP42, EIF2AK1, PMS2, RSPH10B; downstream of ANKRD61, AIMP2, CYTH3 |
| 26:28,004,085 | Intronic SORCS1 |
| Brand | Estrogen | Testosterone | N Receiving 20 or 28 mg Estrogen |
|---|---|---|---|
| Component TE-200 with Tylan and Revlor XH | 20 mg estradiol | 200 mg trenbolone acetate | n = 802 |
| Synovex Plus | 28 mg estradiol benzoate | 200 mg trenbolone acetate | n = 18 |
| Position Chr:BP | Ref./Alt. | Test | Inheritance | p-Value | Alt Allele Frequency |
|---|---|---|---|---|---|
| 7:61,142,117 | A/G | A vs. B vs. C | Dominant | 0.045 | 0.472|0.529|0.571 |
| 10:76,769,055 | G/A | A vs. BC | Additive | 0.004 | 0.094|0.160 |
| 10:76,769,110 | G/A | A vs. B vs. C | Additive | 0.009 | 0.472|0.404|0.370 |
| 19:23,066,541 | C/T | A vs. BC | Additive | 0.036 | 0.017|0.045 |
| 25:38,081,982 | G/C | A vs. B vs. C | Dominant | 0.012 | 0.000|0.005|0.038 |
| 26:28,004,085 | T/C | A vs. BC | Additive | 0.009 | 0.040|0.010 |
| * 9:90115650 | A/G | A vs. B vs. C | Genotypic | 7.54 × 10−19 | 0.740|0.683|0.705 |
| * 9:90015095 | C/T | A vs. B vs. C | Genotypic | 4.07 × 10−15 | 0.665|0.670|0.722 |
| * 2:131837201 | G/A | A vs. B vs. C | Genotypic | 0.002 | 0.769|0.783|0.577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shira, K.A.; Murdoch, B.M.; Davenport, K.M.; Becker, G.M.; Xie, S.; Colacchio, A.M.; Bass, P.D.; Colle, M.J.; Murdoch, G.K. Advanced Skeletal Ossification Is Associated with Genetic Variants in Chronologically Young Beef Heifers. Genes 2023, 14, 1629. https://doi.org/10.3390/genes14081629
Shira KA, Murdoch BM, Davenport KM, Becker GM, Xie S, Colacchio AM, Bass PD, Colle MJ, Murdoch GK. Advanced Skeletal Ossification Is Associated with Genetic Variants in Chronologically Young Beef Heifers. Genes. 2023; 14(8):1629. https://doi.org/10.3390/genes14081629
Chicago/Turabian StyleShira, Katie A., Brenda M. Murdoch, Kimberly M. Davenport, Gabrielle M. Becker, Shangqian Xie, Antonetta M. Colacchio, Phillip D. Bass, Michael J. Colle, and Gordon K. Murdoch. 2023. "Advanced Skeletal Ossification Is Associated with Genetic Variants in Chronologically Young Beef Heifers" Genes 14, no. 8: 1629. https://doi.org/10.3390/genes14081629
APA StyleShira, K. A., Murdoch, B. M., Davenport, K. M., Becker, G. M., Xie, S., Colacchio, A. M., Bass, P. D., Colle, M. J., & Murdoch, G. K. (2023). Advanced Skeletal Ossification Is Associated with Genetic Variants in Chronologically Young Beef Heifers. Genes, 14(8), 1629. https://doi.org/10.3390/genes14081629






