Abstract
Lipoprotein apheresis (LA) is a therapeutic option for patients with severe hypercholesterolemia who have persistently elevated LDL-C levels despite attempts at drug therapy. MicroRNAs (miRNAs), important posttranscriptional gene regulators, are involved in the pathogenesis of atherosclerosis. Our study aimed to monitor the dynamics of twenty preselected circulating miRNAs in patients under long-term apheresis treatment. Plasma samples from 12 FH patients (men = 50%, age = 55.3 ± 12.2 years; mean LA overall treatment time = 13.1 ± 7.8 years) were collected before each apheresis therapy every sixth month over the course of four years of treatment. Eight complete follow-up (FU) samples were measured in each patient. Dynamic changes in the relative quantity of 6 miRNAs (miR-92a, miR-21, miR-126, miR-122, miR-26a, and miR-185; all p < 0.04) during FU were identified. Overall apheresis treatment time influenced circulating miR-146a levels (p < 0.04). In LDLR mutation homozygotes (N = 5), compared to heterozygotes (N = 7), we found higher plasma levels of miR-181, miR-126, miR-155, and miR-92a (all p < 0.03). Treatment with PCSK9 inhibitors (N = 6) affected the plasma levels of 7 miRNAs (miR-126, miR-122, miR-26a, miR-155, miR-125a, miR-92a, and miR-27a; all p < 0.04). Long-term monitoring has shown that LA in patients with severe familial hypercholesterolemia influences plasma circulating miRNAs involved in endothelial dysfunction, cholesterol homeostasis, inflammation, and plaque development. The longer the treatment using LA, the better the miRNA milieu depicting the potential cardiovascular risk.
1. Introduction
Familial hypercholesterolemia (FH) is a frequent, severe, and mostly autosomal dominant genetic disorder associated with elevated plasma low-density lipoprotein cholesterol (LDL-C) levels that predisposes patients towards premature atherosclerotic cardiovascular disease, especially if they remain undiagnosed or inadequately treated [1]. The disorder most commonly results from loss-of-function mutations in the low-density lipoprotein cholesterol receptor (LDLR) gene encoding LDL receptor protein and genes encoding proteins that interact with the receptor, including apolipoprotein B (APOB), proprotein convertase subtilisin/kexin type 9 (PCSK9), or LDLR adaptor protein 1 (LDLRAP1) [2,3].
Lipoprotein apheresis (LA) is a therapeutic option for patients with severe hypercholesterolemia who have persistently elevated LDL-C levels despite attempts at drug therapy [4]. LA is an extracorporeal elimination technique that removes LDL particles and other pathogenic lipoproteins, such as lipoprotein (a) or triglyceride-rich lipoproteins, from the circulation. The main indications for lipoprotein apheresis are (i) the diagnosis of homozygous FH, (ii) heterozygous FH that is refractory to the standard care and intolerant to routine care, and (iii) patients with lipoprotein (a) and increased resistance to pharmacotherapy [5]. LA is also a potent therapy that impacts inflammation and related mediators [6].
MicroRNAs (miRNAs) are small endogenous noncoding RNAs that regulate the mRNA translation of target genes via the RNA interference pathway, strongly influencing a wide range of cellular processes and biological pathways [7,8]. MiRNAs are released into human plasma and serum to serve as biomarkers for clinical diagnosis, prognosis, and follow-up monitoring of the consequences of treatments [9]. Extracellular miRNAs retain their stability and avoid degradation via incorporation into membrane-derived vesicles or bound to circulating proteins to mediate cell communication with neighbouring or remote cells [10]. Atherosclerosis is characterised by endothelial dysfunction, which promotes inflammatory responses to cholesterol and lipid accumulation within the arterial wall. Endothelial dysfunction encompasses a spectrum of biological processes, and miRNAs have emerged as critical regulators of endothelial gene regulatory networks [11].
Based on our previous study [12], we hypothesised that long-term LA could influence the dynamics of circulating miRNAs involved in atherosclerosis pathophysiology. We have already reported that the long-term elimination of pathogenic lipoproteins from plasma related to endothelial dysfunction and inflammation suggests improving cardiovascular prognosis in most FH patients [13]. Twenty candidate miRNAs were selected based on screening of miRNAs involved in atherosclerosis development (Supplementary Material Table S1). Predominantly, plasma-stable miRNAs targeting genes involved in the regulation of endothelial dysfunction, cholesterol homeostasis, inflammation, and plaque development were measured (Supplementary Material Table S2).
2. Materials and Methods
2.1. Design and Study Population
We studied a group of 12 patients with familial hypercholesterolemia under long-term apheresis treatment (men = 50%, age = 55.3 ± 12.2 years). The characteristics of the patients have been previously described [14]. DNA-based evidence of a mutation in the LDLR gene was the criterion for the diagnosis of homozygous FH (N = 5). None of the patients had a mutation in the APOB gene. All patients were treated daily with statins (rosuvastatin 40 mg or 20 mg) combined with ezetimibe (10 mg) or in one subject with fenofibrate (267 mg). At the time of sampling, 6 patients were treated with PCSK9 inhibitors (Table 1).

Table 1.
Baseline characteristics of patients.
The patients had been regularly treated with LDL apheresis (immunoadsorption) or rheohemapheresis (cascade filtration) for an average of 13.1 ± 7.8 years. Several events had occurred in some patients during this period: one patient with homozygous FH, who had aortic valve stenosis, underwent a Bentall procedure and triple aortocoronary bypass at the age of 42 years. The second patient with homozygous FH had an ischemic stroke at the age of 64 years. A third homozygous FH patient had a myocardial infarction and aortocoronary bypass surgery at the age of 27 years (five years after starting LA). Plasma collected during the period from 2016 to 2020 was measured in our study. During this time, any cardiovascular events did not occur in any person.
Samples before each apheresis/rheopheresis therapy at 6-month intervals for 4 years of treatment were analysed. Eight complete follow-up (FU) samples were measured in 6 patients, and 7 FU samples were measured in 3 patients. In the remaining three patients, 4 to 6 blood samples were collected during the FU. Characteristics, medical history, laboratory assessments, and medications were noted over the course of the study at the defined FU times.
2.2. LDL-Apheresis
Plasma separation was performed using a Cobe-Spectra or Optia continuous centrifugal separator (Terumo, Likewood, CO, USA) in 9 patients. An adsorption–desorption automatic device (Adasorb, Medicap, Germany) controlled repeated fillings and washings of Lipopak adsorbers (Pocard, Moscow, Russia). In 2 patients, Lipocollect adsorbers (Medicollect, Germany) were used. Briefly, patients’ blood was taken via peripheral venous access to a Cobe-Spectra or Optia blood cell separator (Terumo, Likewood, CO, USA) that, acting as a centrifuge, separated plasma and cellular components of the blood. In accordance with the immunoadsorption technique, plasma was pumped through Lipopak affinity columns (Pocard, Moscow, Russia) containing antibodies against the main lipoprotein of LDL-cholesterol—apolipoprotein B100 [15].
2.3. Rheohemapheresis
Three patients simultaneously received long-term therapy due to hypercholesterolemia and increased levels of fibrinogen. Rheohemapheresis therapy was used in accordance with Borberg et al. with our own modification [16]. In rheopheresis, plasma is pumped through a filter that separates out lipoproteins and other large molecules. Purified plasma is mixed with blood cells separated earlier and returned back to the patient via another peripheral vein. The adsorption is fully automated; the plasma flow through the adsorption columns is directed by a secondary device, adsorption desorption automat Adasorb (Medicap, Ulrichstein, Germany). To obtain plasma, we used continuous separators (Cobe Spectra or Spectra Optia, Terumo BCT, Lakewood, CO, USA) and Evaflux 4A filters (Kawasumi, Tokyo, Japan) to wash the obtained plasma. The flow through the filter was controlled using a CF100 automatic machine (Infomed, Geneva, Switzerland). Anticoagulation was performed using a combination of heparin and ACD-A (Baxter, Munich, Germany). Of the circulating plasma volume, 1 to 1.5% was washed and was calculated via the on-board computer of the blood cell separator. The procedures were performed from blood taken from the peripheral vein in the elbow pit or in the forearm.
2.4. Plasma Samples
Blood samples (10 mL) were collected in EDTA-containing tubes and centrifuged at 1500× g for 15 min at room temperature. Plasma samples were processed within 30 min of blood collection, aliquoted into RNase-free tubes, and stored at −80 °C before RNA extraction. The median storage time (ST) of plasma samples was 966 days (range: 10 to 1882 days). Free haemoglobin levels (f-Hb), one of the pre-analytical factors that significantly influence circulating plasma miRNA levels, were measured as an indicator of haemolysis. The concentration of f-Hb was calculated as we reported previously [12]. Samples with f-Hb above 25 mg/dL (N = 8) were considered to be affected by haemolysis and were excluded from the analysis.
Total serum cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HD-CL), and triglycerides (TAG) were determined using a commercial kit on a Modular Roche analyser according to the manufacturer’s instructions [17].
2.5. MiRNA Measurement
Total RNA was extracted from 200 μL plasma using the miRCURYTM RNA isolation kit for biofluids (Exiqon, Vedbaek, Denmark) as we reported previously [12,18]. SYBR green-based real-time quantitative PCR was performed using a QuantStudio6 instrument (Thermo Scientific, Waltham, MA, USA). Passive reference dye (ROXTM 50 nm) was included for all PCRs. Interplate calibrators and spike-in controls were included in each analysis to ensure the quality of RNA isolation, cDNA synthesis, and PCR. Twenty-three individual miRCURY miRNA assays (Qiagen, Hilden, Germany) were used for quantitative PCR (Supplementary Material Table S2). As endogenous miRNA controls, hsa-miR-103-3p, hsa-miR-191-5p and hsa-let-7a-5p were selected. GenEx SW (Multid Analysis AB, Göteborg, Sweden) was used for miRNA expression analysis. Four miRNAs, namely, miR-758, miR-370, miR-33a and miR-34a, with a call rate <50% (i.e., more than 50% of the data were invalid for that miRNA) were excluded from the analysis. Ct values higher than 35, which indicated a low concentration of miRNAs in plasma, were replaced by the value 35. The Genorm and NormFinder algorithms selected let-7a-5p for normalization. The missing data, very low miRNA levels, were replaced by deltaCt+2 (which represents at least 1/4 of the detectable miRNA amount). Data were converted to relative quantities and to the log transformations of the values.
2.6. Statistical Analysis
Data were tested for normality using the Shapiro–Wilk test and are presented as the mean ± SD or as median (IQR). In the graphs, the mean and standard error are shown. The nonparametric Kruskal–Wallis test was used for biochemical parameter comparisons. Partial correlation was used for comparison of biochemical parameters with miRNA quantity, and then Bonferroni correction was applied to significance levels. GenEx SW (Multid Analysis AB, Göteborg, Sweden) was used for miRNA expression analysis. Data were converted to relative quantities and to the log transformations of the values. Calculations were performed using JMP software 16.2.0 2020–2021 SAS Institute Inc. (Cary, NC, USA). A p value <0.05 was considered statistically significant. MicroRNA gene targets were predicted using the miRWalk program released January 2022.
3. Results
3.1. Dynamics of miRNA Quantity during FU
The overall apheresis treatment time ranged between 0 and 22.2 years in our study group. There was no emergence within the monitored period of new coronary events or deaths in any patient treated with LDL-apheresis or rheopheresis.
Only one patient (LDLR homozygote, female, 54 years) initiated her first-ever apheresis in the study. The patient was treated daily with rosuvastatin (40 mg) in combination with ezetimibe (10 mg) and anopyrine (100 mg). The sample collected before the first LA ever we used as a baseline for comparison with other measured plasma samples. Relative quantification was performed to this sample. Since patients were treated with LA for a long time, the sample from an untreated subject was ideal for comparing of treatment effect.
Sixteen miRNAs were successfully measured, namely: miR-126-3p; miR-155-5p; miR-122-5p; miR-125a-5p; miR-146a-5p; miR-181a-5p; miR-17-5p; miR-144-5p; miR-26a-5p; miR-29c-3p; miR-143-5p; miR-92a-3p; miR-27a-3p; miR-365a-3p; miR-185-5p; and miR-21-5p. As we reported previously [18], there exists a correlation between the quantity of miRNAs in plasma and ST. After ST adjustment, we found changes in the quantity of miR-92a (p < 0.0001), miR-185 (p < 0.01), miR-21 (p < 0.02), miR-126 and miR-122 (all p < 0.03), and miR-26a (p < 0.04) at the FU time points. Most miRNAs oscillated during FU; only miR-92a showed an increasing trend (Figure 1).
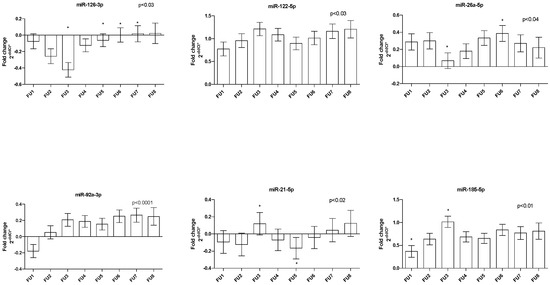
Figure 1.
Dynamics of circulating miRNAs in patients under long-term LA treatment during FU. Bar graphs demonstrate the mean ± standard error. * depicts differences between FUs.
We divided patients into 2 groups, with mean LA overall treatment times of 17.9 ± 4.3 years (N = 6) and 5.5 ± 3.3 years (N = 6), and compare effect of treatment duration. In patients with shorter treatment times, we found a higher relative quantity of plasma miR-146, briefly 0.52 ± 0.04 vs. 0.39 ± 0.03; p < 0.04 (Supplementary Material Figure S1). We didn’t find differences in separation methods on plasma miRNA levels (Supplementary Material Figure S2).
Four patients had type 2 diabetes mellitus (T2DM). Further statistical analyses revealed lower levels of miR-155 in patients with T2DM during FU (p = 0.01; Supplementary Material Figure S3).
3.2. MiRNA Quantity and LDLR Mutation Genotype
In LDLR mutation homozygotes (N = 5; 42% of patients), we detected higher plasma levels of miR-155 (p < 0.0006), miR-126 (p < 0.01) and miR-181 and miR-92a (both p < 0.03) compared to LDLR heterozygotes (N = 7; 58%) (Figure 2A). Moreover, we identified significant differences in miR-155 quantity between LDLR homo vs. heterozygous patients during FU (p < 0.05) (Figure 2B).
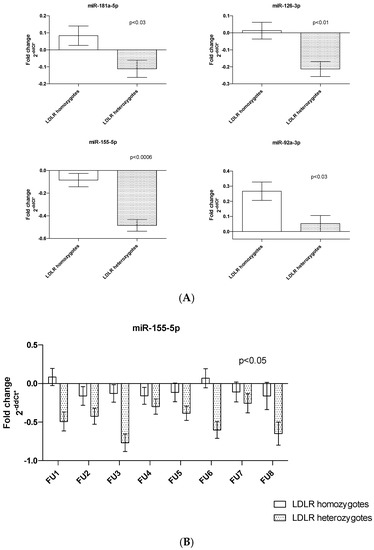
Figure 2.
Differences in plasma miRNA quantity (A) in LDLR mutation carriers of one or two risk alleles; (B) changes in the quantity of miR-155 levels according to LDLR genotype during FU. Bar graphs demonstrate the mean ± standard error.
3.3. PCSK9 Inhibitor Treatment and miRNA Levels
Six patients (4 LDLR homozygotes/2 LDLR heterozygotes) were under combined LA treatment with PCSK9 inhibitors. Two patients were treated with PCSK9 inhibitors throughout the FU. Three patients started treatment with PCSK9 inhibitors during the FU, and one patient had interrupted treatment at 2 FU points. Treatment with PCSK9 inhibitors affected the plasma levels of seven miRNAs. Briefly, increased quantities of miR-126, miR-27a, miR-26a (p < 0.002), miR-122 and miR-155 (p < 0.02) and miR-92a (p < 0.04) were determined in patients treated with PCSK9 inhibitors (Figure 3).
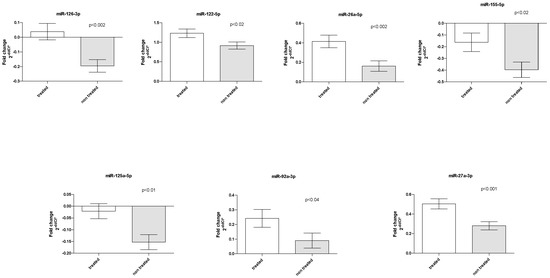
Figure 3.
PCSK9 inhibitor treatment effect on miRNA levels. Bar graphs demonstrate the mean ± standard error.
3.4. Correlation between miRNAs and Biochemical Parameters
Levels of measured biochemical and lipidemic parameters, including TC, LDL-C, HDL-C, and TAG, were stable during FU (Supplementary Material Table S3). We detected variability only between LDLR genotypes, with slightly higher levels of plasma LDL-C in LDLR homozygotes (p < 0.03). In contrast, the levels of TAG and glycaemia were lower in LDLR homozygotes than in heterozygotes (p < 0.02 and p < 0.05, Supplementary Material Figure S4). PCSK9 inhibitor treatment did not affect any parameters.
Partial correlation adjusted for ST revealed all miRNAs were associated with measured biochemical parameters (p < 0.05). After Bonferroni correction, we identified significance (p < 0.0004) in eight of sixteen miRNAs. A negative relationship between the relative quantity of miR-92a, miR-181a, and miR-185 and plasma levels of ApoB (all p < 0.0004) was detected. Furthermore, an inverse correlation was found between miR-27a and Lp(a) (p < 0.0003). In contrast, a positive correlation was detected between miR-155 and TAG (p < 0.0001; Table 2). MiR-122, miR-21 and miR-365a were inversely correlated with ALT (p < 0.0001), and miR-122 also with AST (p < 0.0001; Table 2).

Table 2.
Nonparametric Spearman’s correlation of miRNAs and biochemical parameters. A p value <0.0004 was considered statistically significant after Bonferroni correction.
4. Discussion
Our centre is the first one in the Czech Republic to start the treatment of FH patients using LDL apheresis/rheopheresis. Over 38 years of performing LDL apheresis, we encountered 19 patients who were either homozygous or more severely heterozygous and required lipid apheresis. The aim of the current study was to monitor the dynamics of twenty preselected circulating miRNAs in patients under long-term apheresis treatment. To the best of our knowledge, no work has been published addressing the issue of miRNAs in a larger cohort of patients followed for such a long period of time for familial hypercholesterolemia.
4.1. Long-Term LA and Changes in miRNA Quantity
Our retrospective study detected quantitative changes in miRNAs predominantly associated with endothelial dysfunction (miR-92a and miR-126), cholesterol metabolism (miR-122, mir-26, and miR-185), and vascular remodelling (miR-21) in FH patients under long-term apheresis treatment. The majority of miRNAs oscillated during FU points, and only miR-92a showed an increasing trend. MiR-92a, a member of the miR-17-92 cluster, is important for cell angiogenesis, apoptosis, migration, and inflammation [19,20,21]. Endothelial miR-92a is responsive to atheroprone micro-environmental cues, and its expression level is increased by oscillatory low shear stress and oxidised LDL [10,22]. Among the targets of miR-92a are sirtuin 1 (SIRT1), Krüppel-like factor 2 (KLF2), and KLF4, all of which positively regulate eNOS-derived NO. By inhibiting these endothelial-protective molecules, miR-92a can promote the endothelial innate immune response and the ensuing vascular inflammation [22]. Recently, it has been demonstrated that extracellular miR-92a mediates endothelial cell-macrophage communication. MiR-92a can be transported to macrophages via extracellular vesicles to regulate KLF4 levels, thus leading to atheroprone phenotypes of macrophages and hence atherosclerotic lesion formation [10]. A strong association between circulating miR-92a levels and coronary artery disease in humans was previously reported [23]. The increased quantity of circulating miR-92a during FU probably reflects worsened endothelial dysfunction in FH patients despite long-term LA treatment combined with hypolipidemic drugs of the new generation. Despite an intensive hypolipidemic intervention, patients with severe hypercholesterolemia are not reaching the desired LDL-C targets (this is evident in Table S3, which shows biomarkers of FH patients during aphaeresis treatment). Long-term monitoring of miRNAs in patients with FH could elucidate the progression of atherosclerosis.
Detection of higher levels of miR-146 in patients with shorter LA treatment times could support the finding that FH patients benefit from long-term apheresis procedures that reduce inflammation, which might be important for the prevention of cardiovascular events [5].
The plasma concentration of miR-155 was lower in subjects with diabetes during FUs. Decreased levels of miR-155 probably reflect declined pancreatic function and insulin resistance as reported previously [24].
4.2. LDLR Mutation
In LDLR mutation homozygotes compared to heterozygotes, we identified higher levels of miRNAs predominantly involved in vascular integrity, angiogenesis and inflammation (miR-126, miR-92a, miR-155 and miR-181a). MiR-155 was the only miRNA that increased during whole FUs. MiRNA-155 is a typical multifunctional miRNA that has been associated with the occurrence and development of atherosclerosis by regulating the functions of CD4+ T lymphocytes, monocytes/macrophages, endothelial cells (ECs), and vascular smooth muscle cells [25,26]. MiR-155 is upregulated in human cardiac disease [27] and is significantly increased in plasma and plaques in atherosclerotic patients [28]. Higher levels of miR-155 may reflect worse vascular integrity in LDLR homozygous patients.
4.3. PCSK9 Inhibitor Treatment
Finally, add-on treatment with PCSK9 inhibitors showed increased plasma levels of miRNAs participating in cholesterol metabolism (miR-122 and miR-27a), endothelial dysfunction (miR-126 and mir-26a), and inflammation (miR-92a, miR-155, and miR-125a).
MiR-27 was the most increased miRNA in PCSK9 inhibitor-treated patients. Several studies have identified important roles for miR-27 in lipid metabolism, inflammation, angiogenesis, adipogenesis, oxidative stress, the renin-angiotensin system, insulin resistance, and type 2 diabetes, which have important roles in the onset and outcome of atherosclerosis [29,30]. Interestingly, miR-27a also directly decreases LRP6 and LDLRAP1, two other key players in the LDLR pathway that are required for efficient endocytosis of the LDLR-LDL-C complex in the liver [31]. Moreover, serum levels of miR-27a were found to be inversely related to two cholesterol transporters: ABCA1 and ABCG1 gene expression in coronary heart disease patients [32]. It should be noted that four patients under combined LA treatment with PCSK9 inhibitors were LDLR homozygotes. Three up-regulated miRNAs: miR-92a, miR-155, and miR-126 were also increased when subjects with different LDLR genotypes were compared. In such a small number of analysed individuals, a strong effect of LDLR homozygotes on the result cannot be excluded. In addition, we cannot exclude the distortion of the results due to differences in the PCSK9 inhibitors usage protocol.
4.4. Biochemical Parameters
Unlike in our previous studies [12,14], we only analysed samples before each LA in approximately 6-month intervals in the present study. Stable levels of lipidemic parameters, including TC, LDL-C, HDL-C, and TAG, during FU probably reflect the persistent effect of LA treatment. The lower LDL-C at the last FU interval may reflect the effect of combined therapy using PCSK9 inhibitors that were used from that time point. Not surprisingly, LDL-C concentration was higher in LDLR homozygotes, but interestingly, lower levels of glycemia and TAG were detected in these patients.
Furthermore, we identified an inverse correlation between the levels of ApoB and miR-92a, miR-185, and miR-181a. As we mentioned above, miR-92a targets SIRT1, which associates with many modulators regulating lipid metabolism and results in increased expression of sterol regulatory element-binding proteins (SREBPs), which acts as a key modulator in lipid synthesis [33]. Recently it has been demonstrated that miR-92a promoted HUVEC apoptosis and suppressed proliferation of ox-LDL-induced HUVECs by targeting SIRT6 expression and activating MAPK signalling pathway [34]. MiR-185, a liver-specific miRNA, targets sterol regulatory element-binding transcription factor (SREBF1), which encodes SREBP1. SREBP1 plays a crucial role in regulating cholesterol, and fatty acid metabolism induces the expression of genes involved in de novo cholesterol biosynthesis and LDL uptake [35]. Moreover, SREBF1 targets, i.e., the genes LDLR, FASN, and INSIG, are involved in cholesterol and fatty acid metabolism (https://rgd.mcw.edu/wg/home/pathway2/molecular-pathways2/srebf-targets/ (accessed on 22 January 2022)). An in vitro study on human hepatoma cells showed that miR-185 controls cholesterol uptake by directly targeting LDLR and the LDLR-destabilizing RNA-binding protein KH-type splicing regulatory protein (KSRP), adding another layer of complexity to the mechanism by which miR-185 regulates cholesterol homeostasis [36]. MiR-181a is another predominant atheroprotective miRNA. MiR-181a targets phosphatase and tensin homolog (PTEN). Loss of PTEN activates PI3K/AKT/mTOR pathway, which in turn upregulates SREBP and LDLR. LDL is then hydrolysed to free fatty acids and free cholesterol in lysosome [37]. Generally, both miR-181-5p and miR-181-3p reduce the expression of genes involved in inflammation, such as adhesion molecules and inhibitors of the inflammatory signalling pathway. Both miRNAs also suppress the recruitment of macrophages into lesions [38]. Recently, another group showed that miR-181-5p and miR-181-3p cooperatively inhibit NF-κB signalling by binding to TGF-β-activated kinase 1-binding protein (TAB2) and NF-κB essential modulator (NEMO), respectively [39].
Furthermore, we detected an inverse correlation between Lp(a) and miR-27a levels. It was reported miR-27a decreases low-density lipoprotein receptor-related protein 6 (LRP6) and low-density lipoprotein receptor adapter protein 1 (LDLRAP1), two other key players in the LDLR pathway [30]. MiR-27a/b affects the efflux, influx, esterification and hydrolysis of cellular cholesterol by regulating the expression of ABCA1, APOA1, LPL, CD36 and ACAT1 [40].
Not surprisingly, expression of miR-155, a regulator of cholesterol and fatty acid metabolism, was positively associated with TAG concentration in our study Wang et al. [41] reported that liver X receptor (LXR)α is the target gene of miR-155, and silencing miR-155 reduced the expression of SREBP1 and FAS. An in vivo study showed that upregulation of miR-155 decreased hepatic lipid accumulation mainly by suppressing the LXRα-dependent lipogenic signalling pathway. All mentioned miRNA targets act in regulating pathways of cholesterol and fatty acid metabolism. The inverse correlation between ApoB and miR-92a, miR-181a, and miR-185a, as well as between Lp(a) and miR-27a, suggests an indirect atheroprotective role of analysed miRNAs. Positive correlation between plasma miR-155 and TAG may provide a new biomarker for clinical usage.
The changes in circulating miRNA levels relate to the pathophysiological background of dyslipidaemia and/or the specific type of LDL receptor mutation (homozygote or heterozygote patients) in familial hypercholesterolemia. Our results could help to elucidate the pathways critical for modulation of lipid metabolism, enhancement of endothelial function, inhibition of inflammation, improvement of plaque stability, and immune regulation and thus provide new avenues for tailored therapies.
The first limitation of this single-centre retrospective study is that include the relatively small number of patients, which may affect the accuracy of our results. Moreover, thanks to the advancement of science and the development of new effective hypolipidemics, especially PCSK9 inhibitors, some patients are achieving target LDL cholesterol levels without the need for lipid apheresis support. Thus, our findings need to be verified by multicentre prospective studies. Furthermore, since the lipoprotein apheresis treatment technique is carried out only in a few large medical centres, many FH patients are unable to receive apheresis treatment, resulting in a particular bias in patient selection
Second, our study group is not completely homogeneous, which may have influenced the occurrence or dynamics of miRNAs. There exist differences in overall treatment time between patients. The cohort is not completely identical in some other parameters either. However, we have performed basic clinical tests at the same time, such as mineral levels, indicators of proper liver and kidney function, endothelial activity, coagulation parameters, and others. Changes in these parameters were correlated with changes in miRNA levels and dynamics to be as close as possible to the clinical condition and also to assess whether changes in some clinical parameters are related to miRNA levels.
Third, two separation methods (immunoadsorption vs. cascade filtration) were used in our study. MiRNAs are released into circulation bound to different types of vesicles, such as apoptotic bodies, microvesicles, exosomes, and lipoproteins. We did not expect to find differences between apheresis and rheopheresis because both methods remove lipoproteins from plasma.
5. Conclusions
Long-term monitoring has shown that LDL apheresis in patients with severe familial hypercholesterolemia has an impact on plasma-circulating miRNAs involved in endothelial dysfunction, cholesterol homeostasis, inflammation, and plaque development. The longer the treatment using LDL apheresis, the better the miRNA milieu depicting the potential cardiovascular risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081571/s1, Table S1: MiRNAs involved in atherosclerosis development. References [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] are cited in the supplementary materials; Table S2: Identification of measured miRNA assays and their mature miRNA sequences; Table S3: Biomarkers of FH patients during time points of apheresis treatment; Figure S1: Overall treatment time effect on variances in levels of pro-inflammatory miR-146a. Group 1: patients with mean overall treatment times of 17.9 ± 4.3 years (N = 6) and Group 2: patients with mean overall treatment times 5.5 ± 3.3 years (N = 6). Bar graphs demonstrate the mean ± standard error; Figure S2: Comparison of miRNAs plasma concentration during FUs in respect to absorption technique: rheopheresis (red line) vs. LDL apheresis (blue line) in FU time points (1–8). Data are expressed as means ± standard error. All p = n.s.; Figure S3: Differences in miRNAs levels between patients with type 2 diabetes mellitus (T2DM) vs. non diabetes. diabetes (blue line) vs. non-diabetes (red line) in FU time points (1–8). Data are expressed as means ± standard error. For miR-155 is p ≤ 0.01; for others all p = n.s.; Figure S4: Differences in plasma concentrations of LDL-C, TAG, and glycemia parameters between LDLR mutations.
Author Contributions
The original draft was written by D.D.; V.B. and M.B. designed and directed the study, performed the clinical aspects of the project, contributed to the interpretation of the results, and wrote and edited the article. D.D. and P.H. designed and performed the experiments, analysed the data, and wrote the article. Data analysis was conducted by V.L. Data review was performed by J.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Health of the Czech Republic (Grant no. NU22-01-00151). All rights reserved.
Institutional Review Board Statement
Institutional ethics committee approval of the protocol was obtained. The study was approved on the 31 May 2016 with approval number 201606 S14P by the Ethics Committee, University Hospital Hradec Králové, Czech Republic and was performed under the guidelines within the Declaration of Helsinki (2000) of the World Medical Association.
Informed Consent Statement
All patients provided written informed consent before study enrolment. The protocol of this study was carried out according to the principles of the Declaration of Helsinki. The study was a single-centre, retrospective observational study.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- Rader, D.J.; Kathiresan, S. Disorders of lipoprotein metabolism. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Kasper, D.L., Longo, D.L., Fauci, A.S., Hauser, S.L., Loscalzo, J., Eds.; McGraw Hill Ed.: New York, NY, USA, 2018; pp. 2889–2902. [Google Scholar]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Tichý, L.; Freiberger, T.; Blaha, V.; Satny, M.; Hubacek, J.A. Genetics of Familial Hypercholesterolemia: New Insights. Front. Genet. 2020, 11, 574474. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, R.; Bambauer, C.; Lehmann, B.; Latza, R.; Schiel, R. LDL-apheresis: Technical and clinical aspects. Sci. World J. 2012, 2012, 314283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaha, V.; Blaha, M.; Lanska, M.; Solichova, D.; Kujovska Krcmová, L.; Havel, E.; Vyroubal, P.; Zadak, Z.; Žák, P.; Sobotka, L. Lipoprotein apheresis in the treatment of dyslipidaemia—The Czech Republic experience. Physiol. Res. 2017, 66, S91–S100. [Google Scholar] [CrossRef]
- Stefanutti, C.; Zenti, M.G. Lipoprotein Apheresis and PCSK9-Inhibitors. Impact on Atherogenic Lipoproteins and Anti-Inflammatory Mediators in Familial Hypercholesterolaemia. Curr. Pharm. Des. 2018, 24, 3634–3637. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Dlouha, D.; Hubacek, J.A. Regulatory RNAs and cardiovascular disease—With a special focus on circulating microRNAs. Physiol. Res. 2017, 66 (Suppl. 1), S21–S38. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; Zeiher, A.M.; Dimmeler, S. Circulating microRNAs: Biomarkers or mediators of cardiovascular diseases? Arterioscler. Thromb. Vasc. Biol. 2011, 11, 2383–2390. [Google Scholar] [CrossRef]
- Chang, Y.J.; Li, Y.S.; Wu, C.C.; Wang, K.C.; Huang, T.C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 12, 2492–2504. [Google Scholar] [CrossRef]
- Wiese, C.B.; Zhong, J.; Xu, Z.Q.; Zhang, Y.; Ramirez Solano, M.A.; Zhu, W.; Linton, M.F.; Sheng, Q.; Kon, V.; Vickers, K.C. Dual inhibition of endothelial miR-92a-3p and miR-489-3p reduces renal injury-associated atherosclerosis. Atherosclerosis 2019, 282, 121–131. [Google Scholar] [CrossRef]
- Dlouha, D.; Blaha, M.; Blaha, V.; Fatorova, I.; Hubacek, J.A.; Stavek, P.; Lanska, V.; Parikova, A.; Pitha, J. Analysis of circulating miRNAs in patients with familial hypercholesterolaemia treated by LDL/Lp(a) apheresis. Atheroscler. Suppl. 2017, 30, 128–134. [Google Scholar] [CrossRef]
- Visek, J.; Blaha, M.; Blaha, V.; Lasicova, M.; Lanska, M.; Andrýs, C.; Tebbens, J.D.; e Sá, I.C.I.; Tripská, K.; Vicen, M.; et al. Monitoring of up to 15 years effects of lipoprotein apheresis on lipids, biomarkers of inflammation, and soluble endoglin in familial hypercholesterolemia patients. Orphanet J. Rare Dis. 2021, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Dlouha, D.; Blaha, M.; Rohlova, E.; Hubacek, J.A.; Lanska, V.; Visek, J.; Blaha, V. Multiplex Protein Biomarker Profiling in Patients with Familial Hypercholesterolemia. Genes 2021, 12, 1599. [Google Scholar] [CrossRef] [PubMed]
- Blaha, V.; Blaha, M.; Solichová, D.; Krčmová, L.K.; Lánská, M.; Havel, E.; Vyroubal, P.; Zadák, Z.; Žák, P.; Sobotka, L. Antioxidant defense system in familial hypercholesterolemia and the effects of lipoprotein apheresis. Atheroscler. Suppl. 2017, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Borberg, H.; Tauchert, M. Rheohaemapheresis of ophthalmological diseases and diseases of the microcirculation. Transfus. Apher. Sci. 2006, 34, 41–49. [Google Scholar] [CrossRef]
- Solichova, D.; Melichar, B.; Blaha, V.; Klejna, M.; Vavrova, J.; Palicka, V.; Zadak, Z. Biochemical profile and survival in nonagenarians. Clin. Biochem. 2001, 34, 563–569. [Google Scholar] [CrossRef]
- Dlouha, D.; Ivak, P.; Netuka, I.; Novakova, S.; Konarik, M.; Tucanova, Z.; Lanska, V.; Hlavacek, D.; Wohlfahrt, P.; Hubacek, J.A.; et al. The effect of long-term left ventricular assist device support on flow-sensitive plasma microRNA levels. Int. J. Cardiol. 2021, 339, 138–143. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, Y.; Huang, W.; Qin, G.; Weintraub, N.L.; Tang, Y. miR-92a inhibits vascular smooth muscle cell apoptosis: Role of the MKK4-JNK pathway. Apoptosis 2014, 19, 975–983. [Google Scholar] [CrossRef]
- Daniel, J.M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef]
- Shang, F.; Wang, S.C.; Hsu, C.Y.; Miao, Y.; Martin, M.; Yin, Y.; Wu, C.-C.; Wang, Y.-T.; Wu, G.; Chien, S.; et al. MicroRNA-92a Mediates Endothelial Dysfunction in CKD. J. Am. Soc. Nephrol. 2017, 28, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, J.; Xu, N.; Han, G.; Geng, Q.; Song, J.; Li, S.; Zhao, J.; Chen, H. Signature of Circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS ONE 2013, 8, e80738. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Gambardella, J.; Sardu, C.; Lombardi, A.; Santulli, G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Noncoding RNA 2021, 7, 39. [Google Scholar] [CrossRef]
- Weber, M.; Kim, S.; Patterson, N.; Rooney, K.; Searles, C.D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1192–H1203. [Google Scholar] [CrossRef]
- Virtue, A.; Johnson, C.; Lopez-Pastraña, J.; Shao, Y.; Fu, H.; Li, X.; Li, Y.-F.; Yin, Y.; Mai, J.; Rizzo, V.; et al. MicroRNA-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease a novel mouse model of obesity paradox. J. Biol. Chem. 2017, 292, 1267–1287. [Google Scholar] [CrossRef]
- Krishnan, R.; Nair, A.S.; Dhar, P.K. Computational study of ‘HUB’ microRNA in human cardiac diseases. Bioinformation 2017, 13, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, D.; Chen, H.; Liu, S.; Hu, H.; Wu, T.; Wang, J.; Chen, W.; Ning, Y.; Li, Y.; et al. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef]
- Jennewein, C.; von Knethen, A.; Schmid, T.; Brüne, B. MicroRNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor γ (PPARgamma) mRNA destabilization. J. Biol. Chem. 2010, 285, 11846–11853. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chimica Acta 2011, 412, 66–70. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Done, S.C. MicroRNA-27a decreases the level and efficiency of the LDL receptor and contributes to the dysregulation of cholesterol homeostasis. Atherosclerosis 2015, 242, 595–604. [Google Scholar] [CrossRef]
- Rafiei, A.; Ferns, G.A.; Ahmadi, R.; Khaledifar, A.; Rahimzadeh-Fallah, T.; Mohmmad-Rezaei, M.; Emami, S.; Bagheri, N. Expression levels of miR-27a, miR-329, ABCA1, and ABCG1 genes in peripheral blood mononuclear cells and their correlation with serum levels of oxidative stress and hs-CRP in the patients with coronary artery disease. IUBMB Life 2021, 73, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X. The decreased SIRT1 level may account for the lipid profile in chronic kidney disease. J. Biol. Res. 2019, 26, 9. [Google Scholar] [CrossRef]
- Xu, Y.; Miao, C.; Cui, J.; Bian, X. miR-92a-3p promotes ox-LDL induced-apoptosis in HUVECs via targeting SIRT6 and activating MAPK signaling pathway. Braz. J. Med. Biol. Res. 2021, 54, e9386. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, W.; Pellicane, C.; Sahyoun, C.; Joseph, B.K.; Gallo-Ebert, C.; Donigan, M.; Pandya, D.; Giordano, C.; Bata, A.; et al. Identification of miR-185 as a regulator of de novo cholesterol biosynthesis and low density lipoprotein uptake. J. Lipid Res. 2014, 55, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, J.; Du, Y.; Jia, X.; Yang, F.; Si, S.; Wang, L.; Hong, B. microRNA-185 modulates low density lipoprotein receptor expression as a key posttranscriptional regulator. Atherosclerosis 2015, 243, 523–532. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Y.; Wu, Q.; Huang, L.; Xu, J.; Zeng, Q. Pathogenic role of microRNAs in atherosclerotic ischemic stroke: Implications for diagnosis and therapy. Genes Dis. 2022, 9, 682–696. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.F.; Chen, W.J.; Tang, S.L.; Mo, Z.C.; Tang, Y.-Y.; Li, Y.; Wang, J.-L.; Liu, X.-Y.; Peng, J.; et al. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 2014, 234, 54–64. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, N.; Wang, Z.; Ai, D.M.; Cao, Z.Y.; Pan, H.-P. Decreased MiR-155 Level in the Peripheral Blood of Non-Alcoholic Fatty Liver Disease Patients May Serve as a Biomarker and may Influence LXR Activity. Cell Physiol. Biochem. 2016, 39, 2239–2248. [Google Scholar] [CrossRef]
- Asgeirsdottir, S.A.; Van Solingen, C.; Murniati, N.F.; Zwiers, P.J.; Heeringa, P.; Van Meurs, M.; Satchell, S.C.; Saleem, M.A.; Mathieson, P.W.; Banas, B.; et al. Microrna-126 Contributes To Renal Macrovascular Heterogeneity Of Vcam-1 Protein Expression In Acute Inflammation. Am. J. Physiol. Renal. Physiol. 2012, 302, F1630–F1639. [Google Scholar] [CrossRef]
- Chen, T.; Huang, Z.; Wang, L.; Wang, Y.; Wu, F.; Meng, S.; Wang, C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc. Res. 2009, 83, 131–139. [Google Scholar] [CrossRef]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef]
- Fang, Y.; Davies, P.F. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Moore, K.J. Microrna Regulation Of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Guo, M.; Mao, X.; Ji, Q.; Lang, M.; Li, S.; Peng, Y.; Zhou, W.; Xiong, B.; Zeng, Q. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol. Cell Biol. 2010, 88, 555–564. [Google Scholar] [CrossRef]
- Huang, R.S.; Hu, G.Q.; Lin, B.; Lin, Z.Y.; Sun, C.C. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J. Investig. Med. 2010, 58, 961–967. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yagi, S.; Yamakuchi, M. Microrna-34a Regulation Of Endothelial Senescence. Biochem. Biophys. Res. Commun. 2010, 398, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Raiesdana, A.; Kojima, Y.; Chun, H.J.; Azuma, J.; Maegdefessel, L.; Kundu, R.K.; Quertermous, T.; Tsao, P.S.; Spin, J.M. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell Physiol. 2011, 226, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, X.; Cheng, Y.; Yang, J.; Huo, Y.; Zhang, C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 7903–7913. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012, 126, S81–S90. [Google Scholar] [CrossRef]
- Qin, B.; Xiao, B.; Liang, D.; Xia, J.; Li, Y.; Yang, H. MicroRNAs expression in ox-LDL treated HUVECs: MiR-365 modulates apoptosis and Bcl-2 expression. Biochem. Biophys. Res. Commun. 2011, 410, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.M.; Dávalos, A.; Goedeke, L.; Salerno, A.G.; Warrier, N.; Cirera-Salinas, D.; Suárez, Y.; Fernández-Hernando, C. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.M.; Rotllan, N.; Vlassov, A.V.; Davalos, A.; Li, M.; Goedeke, L.; Aranda, J.F.; Cirera-Salinas, D.; Araldi, E.; Salerno, A.; et al. Control of cholesterol metabolism and plasma high density lipoprotein levels by microRNA-144. Circ. Res. 2013, 112, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011, 478, 404–407. [Google Scholar] [CrossRef]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; Van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism Of Mir-33 In Mice Promotes Reverse Cholesterol Transport And Regression Of Atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef]
- Shirasaki, T.; Honda, M.; Shimakami, T.; Horii, R.; Yamashita, T.; Sakai, Y.; Sakai, A.; Okada, H.; Watanabe, R.; Murakami, S.; et al. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J. Virol. 2013, 87, 5270–5286. [Google Scholar] [CrossRef]
- Suarez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Suárez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. J. Immunol. 2010, 184, 21–25. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, J.; Xie, J.; Wei, W.; Chen, M.; Zhao, X. MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett. 2012, 586, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Zeng, D.X.; Li, R.T.; Pang, R.P.; Yang, H.; Hu, Y.L.; Zhang, Q.; Jiang, Y.; Huang, L.Y.; Tang, Y.B.; et al. Essential role of microRNA-155 in regulating endothelium dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, V.; Rotllan, N.; Araldi, E.; Luciano, A.; Skroblin, P.; Abonnenc, M.; Perrotta, P.; Yin, X.; Bauer, A.; Leslie, K.L.; et al. Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol. Med. 2016, 8, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kaluza, D.; Frömel, T.; Knau, A.; Bennewitz, K.; Boon, R.A.; Bonauer, A.; Doebele, C.; Boeckel, J.N.; Hergenreider, E.; et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood 2012, 119, 1607–1616. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.J.; Jiang, H.J.; Du, Y.; Yang, F.; Si, S.Y.; Hong, B. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Mol. Cell Biol. 2013, 33, 1956–1964. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).