Proteomic Analysis of Rat Duodenum Reveals the Modulatory Effect of Boron Supplementation on Immune Activity
Abstract
1. Introduction
2. Methods
2.1. Experimental Animals and Design
2.2. Sample Collection and Preparation
2.3. Protein Extraction and Quantification
2.4. Trypsin Digestion and TMT Peptide Labeling
2.5. Reversed-Phase High-Performance Liquid Chromatography (HPLC) Analysis
2.6. LC-MS/MS Analysis
2.7. Proteins Identification and Quantification
2.8. Bioinformatics Analysis
3. Results
3.1. Global Profiling of Proteins in Duodenum Tissue
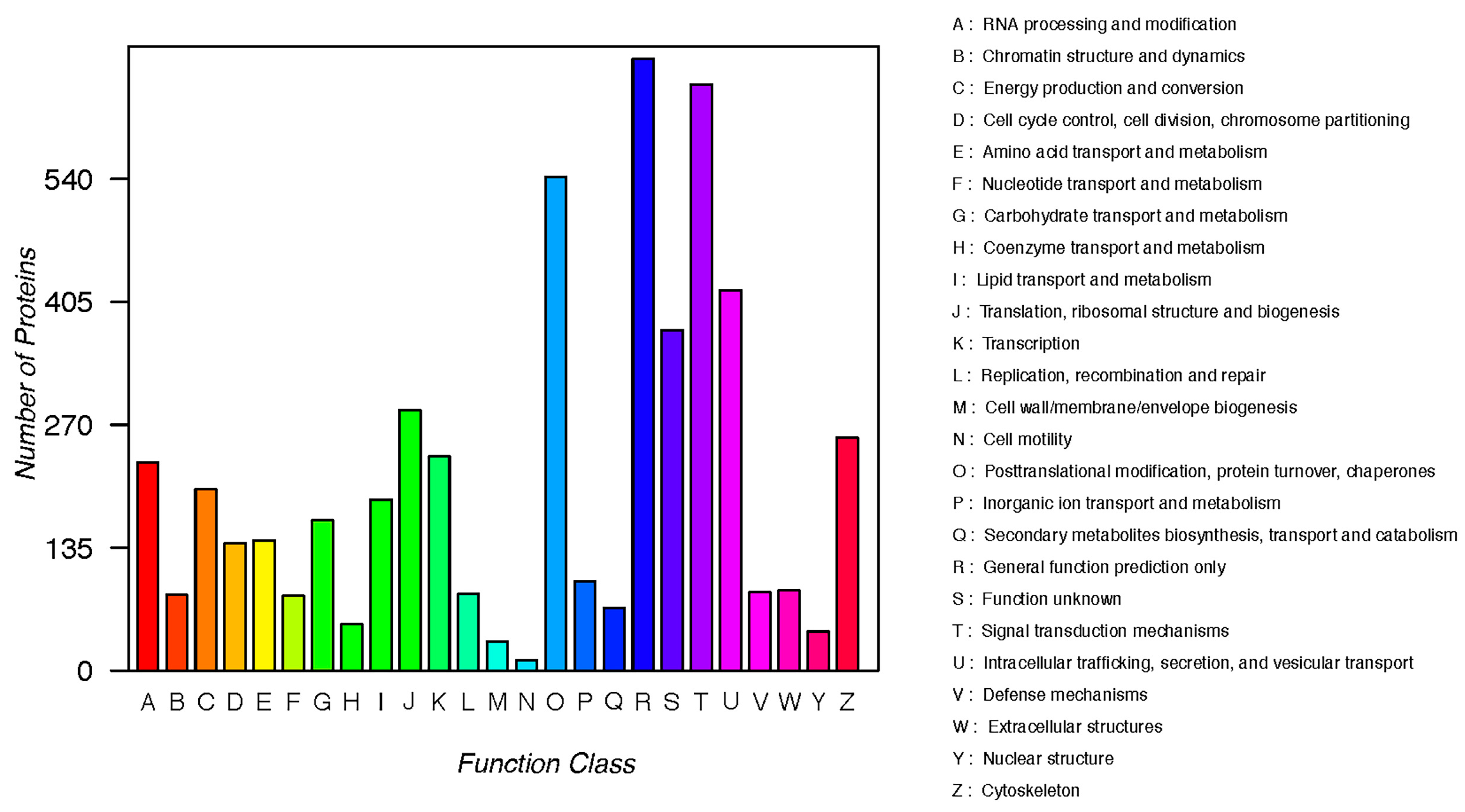
3.2. KOG Annotation
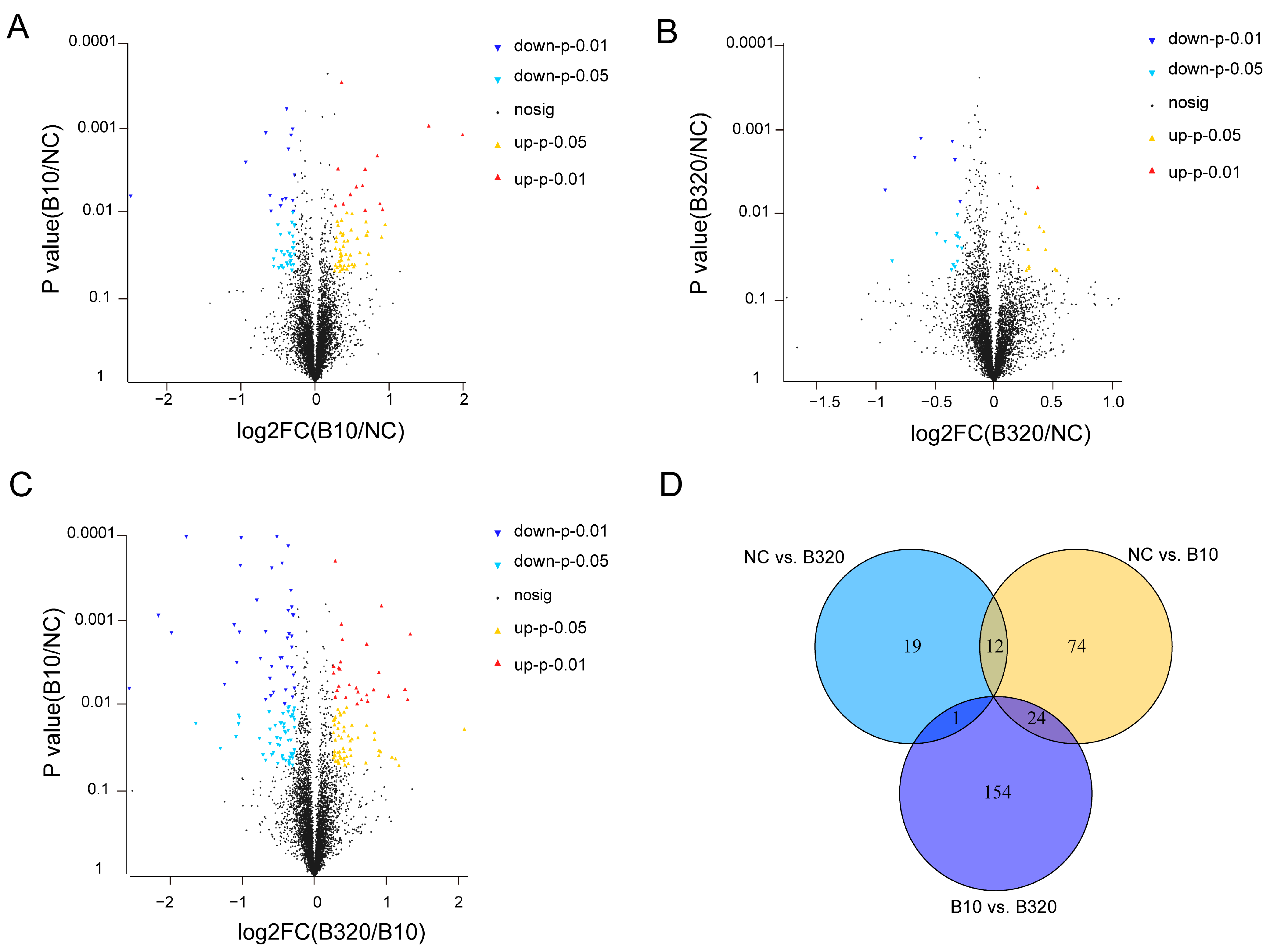
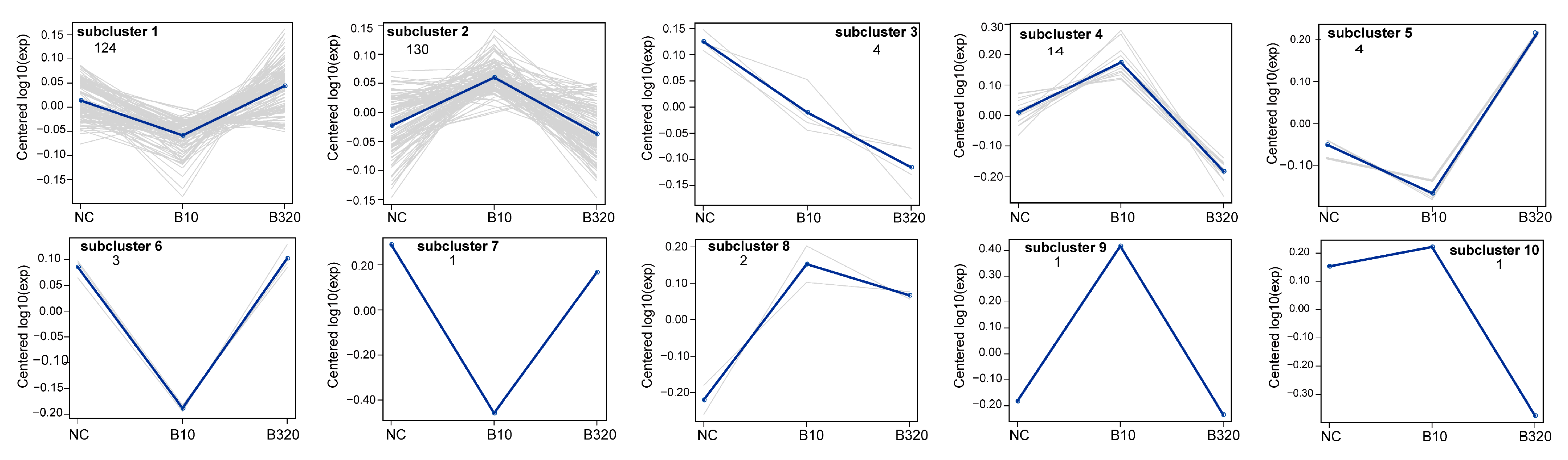
3.3. Differential Expressed Proteins among Three Treatment Groups
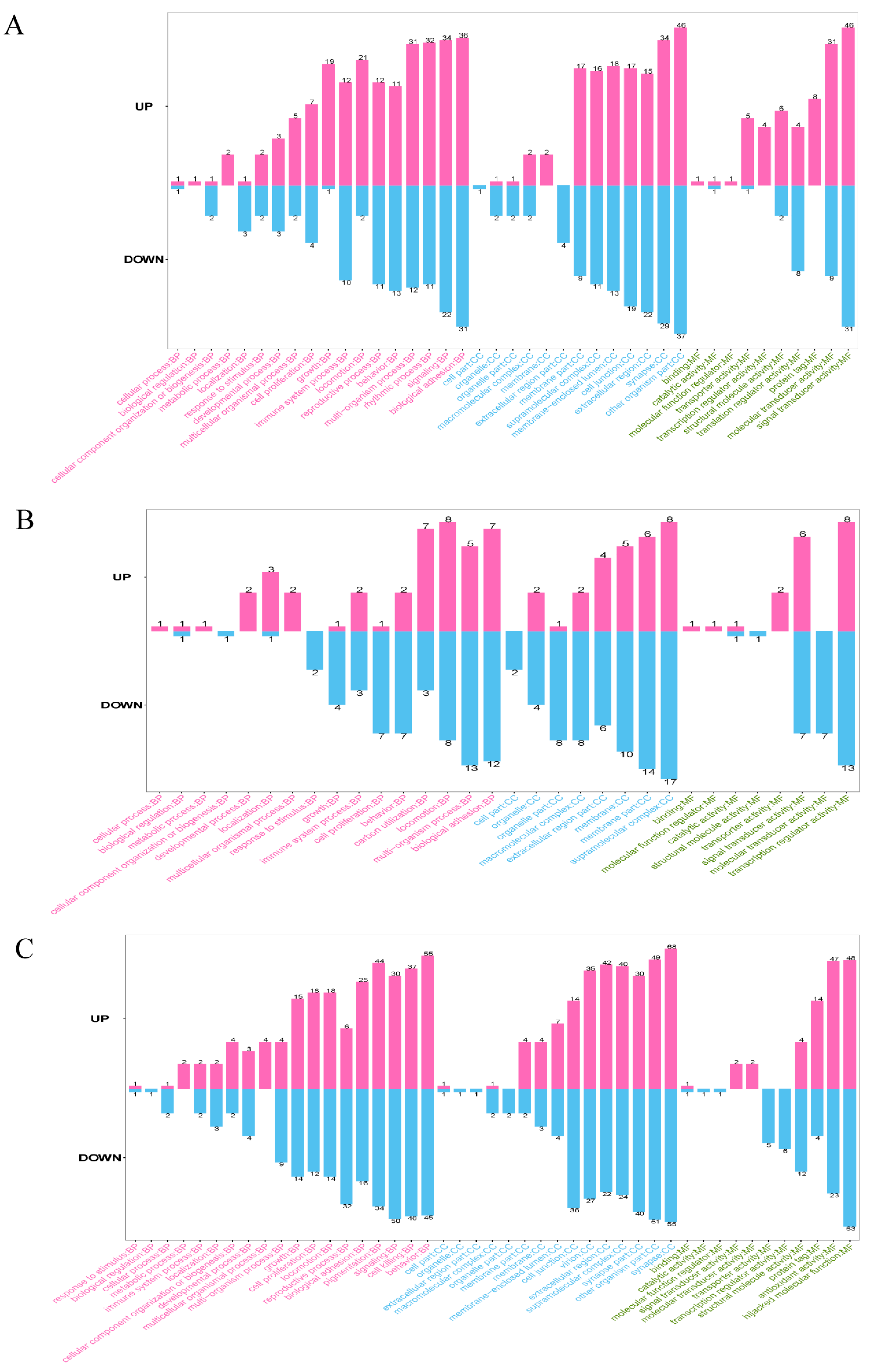
3.4. GO Annotation of DEPs
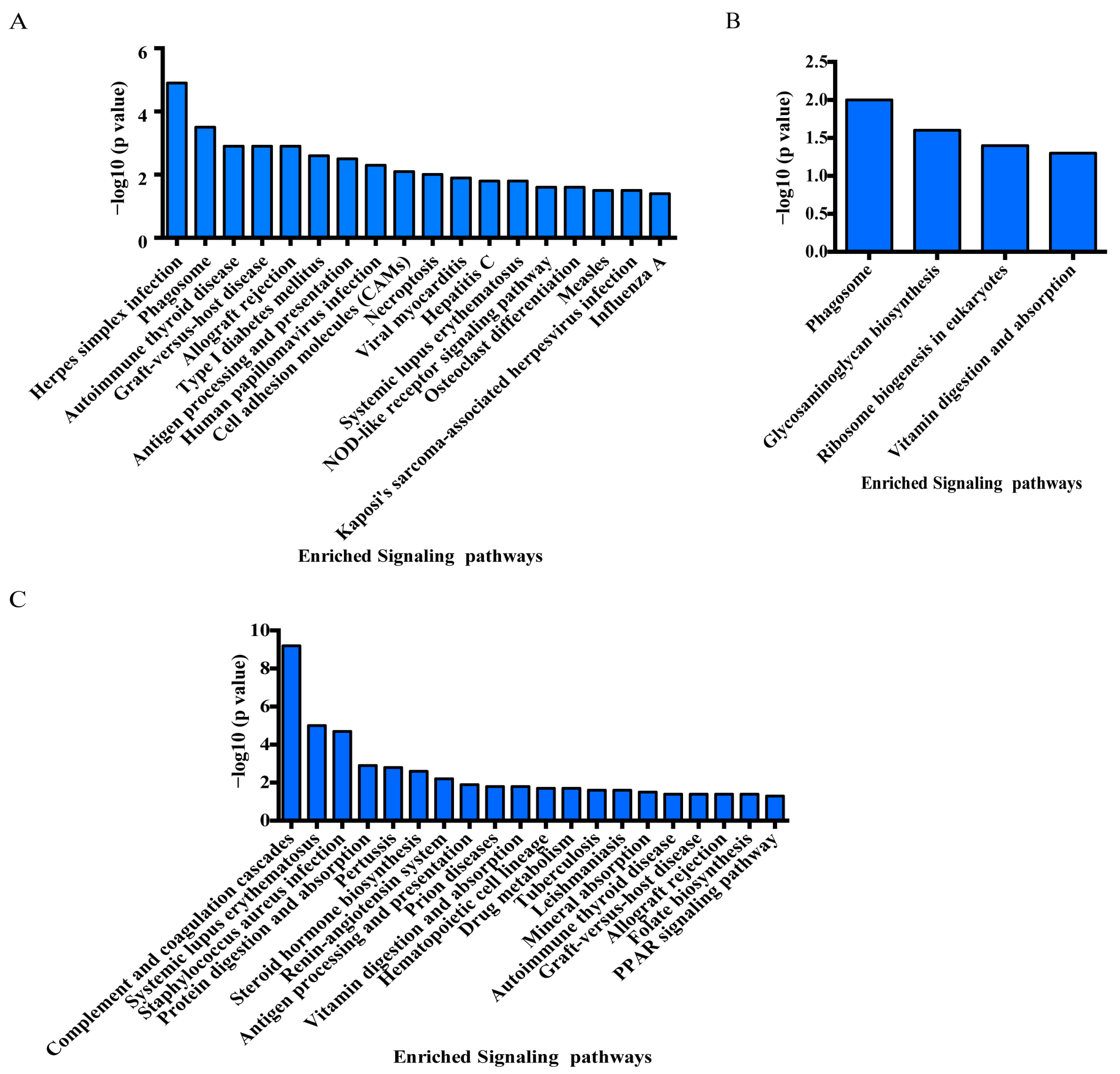
3.5. KEGG Enrichment Analysis of DEPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Cakir, S.; Eren, M.; Senturk, M.; Sarica, Z.S. The effect of boron on some biochemical parameters in experimental diabetic rats. Biol. Trace Elem. Res. 2018, 184, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, E.; Deng, J.; Pei, Y.; Ren, M.; Hu, Q.; Gu, Y.; Li, S. GPR30 mediated effects of boron on rat spleen lymphocyte proliferation, apoptosis, and immune function. Food Chem. Toxicol. 2020, 146, 111838. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Matiullah; Iqbal, A.; Husaini, S.N.; Malik, F. Determination of boron contents in water samples collected from the Neelum valley, Azad Kashmir, Pakistan. Biol. Trace Elem. Res. 2011, 139, 287–295. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing boring about boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Hunt, C.D. Dietary boron: An overview of the evidence for its role in immune function. J. Trace Elem. Exp. Med. 2003, 16, 291–306. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Nielsen, F.H. Effects of nutritional deficiency of boron on the bones of the appendicular skeleton of mice. Biol. Trace Elem. Res. 2019, 188, 221–229. [Google Scholar] [CrossRef]
- Akbari, N.; Ostadrahimi, A.; Tutunchi, H.; Pourmoradian, S.; Farrin, N.; Najafipour, F.; Soleimanzadeh, H.; Kafil, B.; Mobasseri, M. Possible therapeutic effects of boron citrate and oleoylethanolamide supplementation in patients with COVID-19: A pilot randomized, double-blind, clinical trial. J. Trace Elem. Med. Biol. 2022, 71, 126945. [Google Scholar] [CrossRef]
- Bouchareb, R.; Katz, M.; Saadallah, N.; Sassi, Y.; Ali, S.; Lebeche, D. Boron improves cardiac contractility and fibrotic remodeling following myocardial infarction injury. Sci. Rep. 2020, 10, 17138. [Google Scholar] [CrossRef]
- Mohammed, E.E.; Turkel, N.; Yigit, U.M.; Dalan, A.B.; Sahin, F. Boron Derivatives Inhibit the Proliferation of Breast Cancer Cells and Affect Tumor-Specific T Cell Activity In Vitro by Distinct Mechanisms. Biol. Trace Elem. Res. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, A.; Zhuang, L.; Wang, X.; Song, Z.; Liang, R.; Ren, M.; Long, M.; Jia, X.; Li, Z.; et al. Enrichment of boron element in follicular fluid and its potential effect on the immune function. Environ. Pollut. 2022, 304, 119147. [Google Scholar] [CrossRef]
- Wang, C.; Kong, Z.; Duan, L.; Deng, F.; Chen, Y.; Quan, S.; Liu, X.; Cha, Y.E.; Gong, Y.; Wang, C.; et al. Reproductive toxicity and metabolic perturbations in male rats exposed to boron. Sci. Total Environ. 2021, 785, 147370. [Google Scholar] [CrossRef]
- Ergul, A.B.; Kara, M.; Karakukcu, C.; Tasdemir, A.; Aslaner, H.; Ergul, M.A.; Muhtaroglu, S.; Zararsiz, G.E.; Torun, Y.A. High doses of boron have no protective effect against nephrolithiasis or oxidative stress in a rat model. Biol. Trace Elem. Res. 2018, 186, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Macelline, S.P.; Wickramasuriya, S.S.; Shin, T.K.; Kim, E.; Son, H.C.; Heo, J.M. Moderate dietary boron supplementation improved growth performance, crude protein digestibility and diarrhea index in weaner pigs regardless of the sanitary condition. Anim. Biosci. 2022, 35, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Sizmaz, O.; Koksal, B.H.; Yildiz, G. Rumen microbial fermentation, protozoan abundance and boron availability in yearling rams fed diets with different boron concentrations. J. Anim. Feed Sci. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Sharma, A.; Mani, V.; Pal, R.P.; Sarkar, S.; Datt, C. Boron supplementation in peripartum Murrah buffaloes: The effect on calcium homeostasis, bone metabolism, endocrine and antioxidant status. J. Trace Elem. Med. Biol. 2020, 62, 126623. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, K.; Wang, J.; Sun, P.; Huang, H.; Khaliq, H.; Naeem, M.A.; Zhong, J.; Peng, K. Transcriptional study revealed that boron supplementation may alter the immune-related genes through MAPK signaling in ostrich chick thymus. Biol. Trace Elem. Res. 2019, 189, 209–223. [Google Scholar] [CrossRef]
- Agace, W.W.; McCoy, K.D. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef]
- Mucida, D.; Esterhazy, D. SnapShot: Gut immune niches. Cell 2018, 174, 1600–1600.e1. [Google Scholar] [CrossRef]
- Abo-Shaban, T.; Sharna, S.S.; Hosie, S.; Lee, C.Y.Q.; Balasuriya, G.K.; McKeown, S.J.; Franks, A.E.; Hill-Yardin, E.L. Issues for patchy tissues: Defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J. Neural Transm. 2023, 130, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Ihara, E.; Minoda, Y.; Ogino, H.; Sasaki, T.; Fujiwara, M.; Oda, Y.; Ogawa, Y. The Altered Mucosal Barrier Function in the Duodenum Plays a Role in the Pathogenesis of Functional Dyspepsia. Dig. Dis. Sci. 2019, 64, 3228–3239. [Google Scholar] [CrossRef] [PubMed]
- Abtisam, J.; Al-Awadi, A.; Ali, B. Comparative study of histopathological changes between nano-boron and boron in small intestine of layer chickens. Iraqi J. Agric. Sci. 2023, 54, 724–729. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Wu, X.; Ren, M.; Hu, Q.; Jin, E.; Gu, Y. Effect of boron on microstructure, immune function, expression of tight junction protein, cell proliferation and apoptosis of duodenum in rats. Biol. Trace Elem. Res. 2021, 199, 205–215. [Google Scholar] [CrossRef]
- Zhao, C.; Han, Y.; Wang, C.; Ren, M.; Hu, Q.; Gu, Y.; Ye, P.; Li, S.; Jin, E. Transcriptome Profiling of Duodenum Reveals the Importance of Boron Supplementation in Modulating Immune Activities in Rats. Biol. Trace Elem. Res. 2021, 200, 3762–3773. [Google Scholar] [CrossRef]
- Khaliq, H.; Zhong, J.; Peng, K.-M. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Liu, B.; Yin, X.; Wei, H.; Wang, Z.; Tang, H.; Qiu, Y.; Hao, Y.; Zhang, X.; Bi, H.; Guo, D. Quantitative proteomic analysis of rat retina with experimental autoimmune uveitis based on tandem mass tag (TMT) peptide labeling coupled with LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1153, 122293. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Gao, C.; Chen, W.; Chen, G.; Zhang, M.; Luo, C.; Wang, T.; Chen, X.; Tao, L. TMT-based proteomics analysis to screen potential biomarkers of acute-phase TBI in rats. Life Sci. 2021, 264, 118631. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.H.; Kim, H.; Kim, S.J.; Bae, J.; Kim, H.K.; Lee, S.W. A fully automated dual-online multifunctional ultrahigh pressure liquid chromatography system for high-throughput proteomics analysis. J. Chromatogr. A 2014, 1329, 83–89. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006, 34, D322–D326. [Google Scholar] [CrossRef]
- Routray, I.; Ali, S. Boron Induces Lymphocyte Proliferation and Modulates the Priming Effects of Lipopolysaccharide on Macrophages. PLoS ONE 2016, 11, e0150607. [Google Scholar] [CrossRef]
- Arciniega-Martinez, I.M.; Romero-Aguilar, K.S.; Farfan-Garcia, E.D.; Garcia-Machorro, J.; Resendiz-Albor, A.A.; Soriano-Ursua, M.A. Diversity of effects induced by boron-containing compounds on immune response cells and on antibodies in basal state. J. Trace Elem. Med. Biol. 2022, 69, 126901. [Google Scholar] [CrossRef]
- Ji, S.G.; Juran, B.D.; Mucha, S.; Folseraas, T.; Jostins, L.; Melum, E.; Kumasaka, N.; Atkinson, E.J.; Schlicht, E.M.; Liu, J.Z.; et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 2017, 49, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.M.; Fodil, N.; Torre, S.; Bongfen, S.E.; Olivier, J.F.; Leung, V.; Langlais, D.; Meunier, C.; Berghout, J.; Langat, P.; et al. CCDC88B is a novel regulator of maturation and effector functions of T cells during pathological inflammation. J. Exp. Med. 2014, 211, 2519–2535. [Google Scholar] [CrossRef] [PubMed]
- Fodil, N.; Moradin, N.; Leung, V.; Olivier, J.F.; Radovanovic, I.; Jeyakumar, T.; Flores Molina, M.; McFarquhar, A.; Cayrol, R.; Bozec, D.; et al. CCDC88B is required for pathogenesis of inflammatory bowel disease. Nat. Commun. 2017, 8, 932. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Luo, Y.; Wu, X.T.; Ansari, A.R.; Wang, J.; Yang, K.; Xiao, K.; Peng, K. Effects of Supplemental Boron on Intestinal Proliferation and Apoptosis in African Ostrich Chicks. Int. J. Morphol. 2016, 34, 830–835. [Google Scholar] [CrossRef]
- Xu, L.-Z.; Deng, J.; Liu, T.; Ren, M.; Hu, Q.-Q.; Li, S.-H.; Gu, Y.-F.; Wang, C.-F.; Jin, E.-H. Boron Modulates the Barrier Function, Antioxidant Activity, and Epithelial Cell Proliferation in Rat Jejunum. Curr. Topics Nutr. Res. 2022, 20, 97. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Adhikari, B.; Pontin, K.P.; Latorre, J.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Merino-Guzman, R.; Mendez-Albores, A.; Kwon, Y.M.; et al. Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella enteritidis: Proof of concept. Res. Vet. Sci. 2019, 123, 7–13. [Google Scholar] [CrossRef]
- Bourgeois, A.C.; Scott, M.E.; Sabally, K.; Koski, K.G. Low dietary boron reduces parasite (nematoda) survival and alters cytokine profiles but the infection modifies liver minerals in mice. J. Nutr. 2007, 137, 2080–2086. [Google Scholar] [CrossRef]
- Donoiu, I.; Militaru, C.; Obleaga, O.; Hunter, J.M.; Neamtu, J.; Bita, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester Chlorogenoborate Complex: A New Naturally Occurring Boron-Containing Compound. Inorganics 2023, 11, 112. [Google Scholar] [CrossRef]
- Chisari, E.; Wouthuyzen-Bakker, M.; Friedrich, A.W.; Parvizi, J. The relation between the gut microbiome and osteoarthritis: A systematic review of literature. PLoS ONE 2021, 16, e0261353. [Google Scholar] [CrossRef] [PubMed]
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.A.; Schott, E.M.; Gill, S.R.; Zuscik, M.J. The gut microbiome-joint connection: Implications in osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101. [Google Scholar] [CrossRef]
- Scorei, I.; Bita, A.; Dinca, L.; Mogosanu, G.; Rangavajla, N. Borate Complexes of Chlorogenic Acid and Uses Thereof. U.S. Patent Application No. PCT/US22/78488, 21 October 2022. Available online: https://www.uspto.gov/patents (accessed on 25 October 2022).
- Li, L.; Fu, F.; Xue, M.; Chen, W.; Liu, J.; Shi, H.; Chen, J.; Bu, Z.; Feng, L.; Liu, P. IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-α. Antivir. Res. 2017, 140, 76–82. [Google Scholar] [CrossRef]
- Sanchez-Tacuba, L.; Rojas, M.; Arias, C.F.; Lopez, S. Rotavirus Controls Activation of the 2′-5′-Oligoadenylate Synthetase/RNase L Pathway Using at Least Two Distinct Mechanisms. J. Virol. 2015, 89, 12145–12153. [Google Scholar] [CrossRef]
- Di Fiore, I.J.; Holloway, G.; Coulson, B.S. Innate immune responses to rotavirus infection in macrophages depend on MAVS but involve neither the NLRP3 inflammasome nor JNK and p38 signaling pathways. Virus Res. 2015, 208, 89–97. [Google Scholar] [CrossRef]
- Calmon, M.F.; Rodrigues, R.V.; Kaneto, C.M.; Moura, R.P.; Silva, S.D.; Mota, L.D.; Pinheiro, D.G.; Torres, C.; de Carvalho, A.F.; Cury, P.M.; et al. Epigenetic silencing of CRABP2 and MX1 in head and neck tumors. Neoplasia 2009, 11, 1329–1339. [Google Scholar] [CrossRef]
- Neil, J.A.; Matsuzawa-Ishimoto, Y.; Kernbauer-Holzl, E.; Schuster, S.L.; Sota, S.; Venzon, M.; Dallari, S.; Galvao Neto, A.; Hine, A.; Hudesman, D.; et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat. Microbiol. 2019, 4, 1737–1749. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Wang, F.; Wang, H.; Wu, Z.; Wu, S.; Bao, W. Expression Pattern Analysis of Antiviral Genes and Inflammatory Cytokines in PEDV-Infected Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2020, 7, 75. [Google Scholar] [CrossRef]
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic Analysis of the Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells: Influence of Immunobiotic Lactobacilli. Front. Immunol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Liu, Q.; Jiang, Y.L.; Yang, W.T.; Huang, H.B.; Shi, C.W.; Yang, G.L.; Wang, C.F. Surface-Displayed Porcine IFN-lambda3 in Lactobacillus plantarum Inhibits Porcine Enteric Coronavirus Infection of Porcine Intestinal Epithelial Cells. J. Microbiol. Biotechnol. 2020, 30, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.K.; Kaczmarek, M.E.; Dallari, S.; Chen, Y.H.; Tada, T.; Axelrad, J.; Landau, N.R.; Stapleford, K.A.; Cadwell, K. Variable susceptibility of intestinal organoid-derived monolayers to SARS-CoV-2 infection. PLoS Biol. 2022, 20, e3001592. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Qiu, W.; Wang, J.; Li, P.; Peng, K. The Immune Regulatory Effect of Boron on Ostrich Chick Splenic Lymphocytes. Biol. Trace Elem. Res. 2021, 199, 2695–2706. [Google Scholar] [CrossRef] [PubMed]
- Gunther, E.; Walter, L. The major histocompatibility complex of the rat (Rattus norvegicus). Immunogenetics 2001, 53, 520–542. [Google Scholar] [CrossRef] [PubMed]
- Hurt, P.; Walter, L.; Sudbrak, R.; Klages, S.; Müller, I.; Shiina, T.; Inoko, H.; Lehrach, H.; Günther, E.; Reinhardt, R. The genomic sequence and comparative analysis of the rat major histocompatibility complex. Genome Res. 2004, 14, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Dressel, R.; Walter, L.; Günther, E. Genomic and funtional aspects of the rat MHC, the RT1 complex. Immunol. Rev. 2001, 184, 82–95. [Google Scholar] [CrossRef]
- Saxena, T.; Lyon, J.G.; Pai, S.B.; Pare, D.; Amero, J.; Karumbaiah, L.; Carroll, S.L.; Gaupp, E.; Bellamkonda, R.V. Engineering Controlled Peritumoral Inflammation to Constrain Brain Tumor Growth. Adv. Healthc. Mater. 2019, 8, e1801076. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Du, K.; Liu, X.; Li, C.; Wang, D.; Zhang, Y.; Wang, C.; Li, J. Integrative Pan-Cancer Analysis Confirmed that FCGR3A is a Candidate Biomarker Associated with Tumor Immunity. Front. Pharmacol. 2022, 13, 900699. [Google Scholar] [CrossRef]
- Sun, K.; Fei, X.; Xu, M.; Xu, R.; Xu, M. FCGR3A Is a Prognostic Biomarker and Correlated with Immune Infiltrates in Lower-Grade Glioma. J. Oncol. 2022, 2022, 9499317. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Okada, S. Inborn errors of STAT1 immunity. Curr. Opin. Immunol. 2021, 72, 59–64. [Google Scholar] [CrossRef]
- Lee, C.J.; An, H.J.; Cho, E.S.; Kang, H.C.; Lee, J.Y.; Lee, H.S.; Cho, Y.Y. Stat2 stability regulation: An intersection between immunity and carcinogenesis. Exp. Mol. Med. 2020, 52, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Favis, R.; Sun, Y.; van de Velde, H.; Broderick, E.; Levey, L.; Meyers, M.; Mulligan, G.; Harousseau, J.L.; Richardson, P.G.; Ricci, D.S. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharm. Genom. 2011, 21, 121–129. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, H.; Liang, J.; Yan, H.; Chen, Y.; Shen, Y.; Kong, Y.; Wang, S.; Zhao, G.; Jin, W. Digital karyotyping reveals probable target genes at 7q21.3 locus in hepatocellular carcinoma. BMC Med. Genom. 2011, 4, 60. [Google Scholar] [CrossRef]
- Liu, Z.D.; Zhang, S.; Hao, J.J.; Xie, T.R.; Kang, J.S. Cellular model of neuronal atrophy induced by DYNC1I1 deficiency reveals protective roles of RAS-RAF-MEK signaling. Protein Cell 2016, 7, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.B.; Wen, T.; Li, Z.; Xin, X.; Che, X.F.; Wang, J.; Liu, Y.P.; Qu, X.J. DYNC1I1 Promotes the Proliferation and Migration of Gastric Cancer by Up-Regulating IL-6 Expression. Front. Oncol. 2019, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, J.; Wang, Z.; Feng, Y.; Wei, Z.; Hou, X.; Xu, J.; He, Y.; Yang, D. Transcriptome hallmarks in Helicobacter pylori infection influence gastric cancer and MALT lymphoma. Epigenomics 2020, 12, 661–671. [Google Scholar] [CrossRef]
- Somasundaram, R.; Deuring, J.J.; van der Woude, C.J.; Peppelenbosch, M.P.; Fuhler, G.M. Linking risk conferring mutations in NCF4 to functional consequences in Crohn’s disease. Gut 2012, 61, 1097–1098. [Google Scholar] [CrossRef]
- Jiang, C.; Feng, D.; Zhang, Y.; Yang, K.; Hu, X.; Xie, Q. SCAT8/miR-125b-5p axis triggers malignant progression of nasopharyngeal carcinoma through SCARB1. BMC Mol. Cell. Biol. 2023, 24, 15. [Google Scholar] [CrossRef]
- Rodriguez, A. High HDL-Cholesterol Paradox: SCARB1-LAG3-HDL Axis. Curr. Atheroscler. Rep. 2021, 23, 5. [Google Scholar] [CrossRef]
- Uluisik, I.; Kaya, A.; Fomenko, D.E.; Karakaya, H.C.; Carlson, B.A.; Gladyshev, V.N.; Koc, A. Boron stress activates the general amino acid control mechanism and inhibits protein synthesis. PLoS ONE 2011, 6, e27772. [Google Scholar] [CrossRef] [PubMed]
- Abdik, E.A.; Abdik, H.; Tasli, P.N.; Deniz, A.A.H.; Sahin, F. Suppressive role of boron on adipogenic differentiation and fat deposition in human mesenchymal stem cells. Biol. Trace Elem. Res. 2019, 188, 384–392. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Chen, S.; Han, Y.; Zhang, F.; Ren, M.; Hu, Q.; Ye, P.; Li, X.; Jin, E.; Li, S. Proteomic Analysis of Rat Duodenum Reveals the Modulatory Effect of Boron Supplementation on Immune Activity. Genes 2023, 14, 1560. https://doi.org/10.3390/genes14081560
Zhao C, Chen S, Han Y, Zhang F, Ren M, Hu Q, Ye P, Li X, Jin E, Li S. Proteomic Analysis of Rat Duodenum Reveals the Modulatory Effect of Boron Supplementation on Immune Activity. Genes. 2023; 14(8):1560. https://doi.org/10.3390/genes14081560
Chicago/Turabian StyleZhao, Chunfang, Shuqin Chen, Yujiao Han, Feng Zhang, Man Ren, Qianqian Hu, Pengfei Ye, Xiaojin Li, Erhui Jin, and Shenghe Li. 2023. "Proteomic Analysis of Rat Duodenum Reveals the Modulatory Effect of Boron Supplementation on Immune Activity" Genes 14, no. 8: 1560. https://doi.org/10.3390/genes14081560
APA StyleZhao, C., Chen, S., Han, Y., Zhang, F., Ren, M., Hu, Q., Ye, P., Li, X., Jin, E., & Li, S. (2023). Proteomic Analysis of Rat Duodenum Reveals the Modulatory Effect of Boron Supplementation on Immune Activity. Genes, 14(8), 1560. https://doi.org/10.3390/genes14081560





