Genome-Wide Characterization and Gene Expression Analysis of TRP Channel Superfamily Genes in the Migratory Locust, Locusta migratoria
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Gene Identification and Sequence Analysis
2.3. Sample Preparation
2.4. Total RNA Extraction and cDNA Synthesis
2.5. RNA-Seq and Differential Expression Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. The Identification of TRP Superfamily Genes of L. migratoria
3.2. The Number of TRP Superfamily Genes in L. migratoria and Other Insects
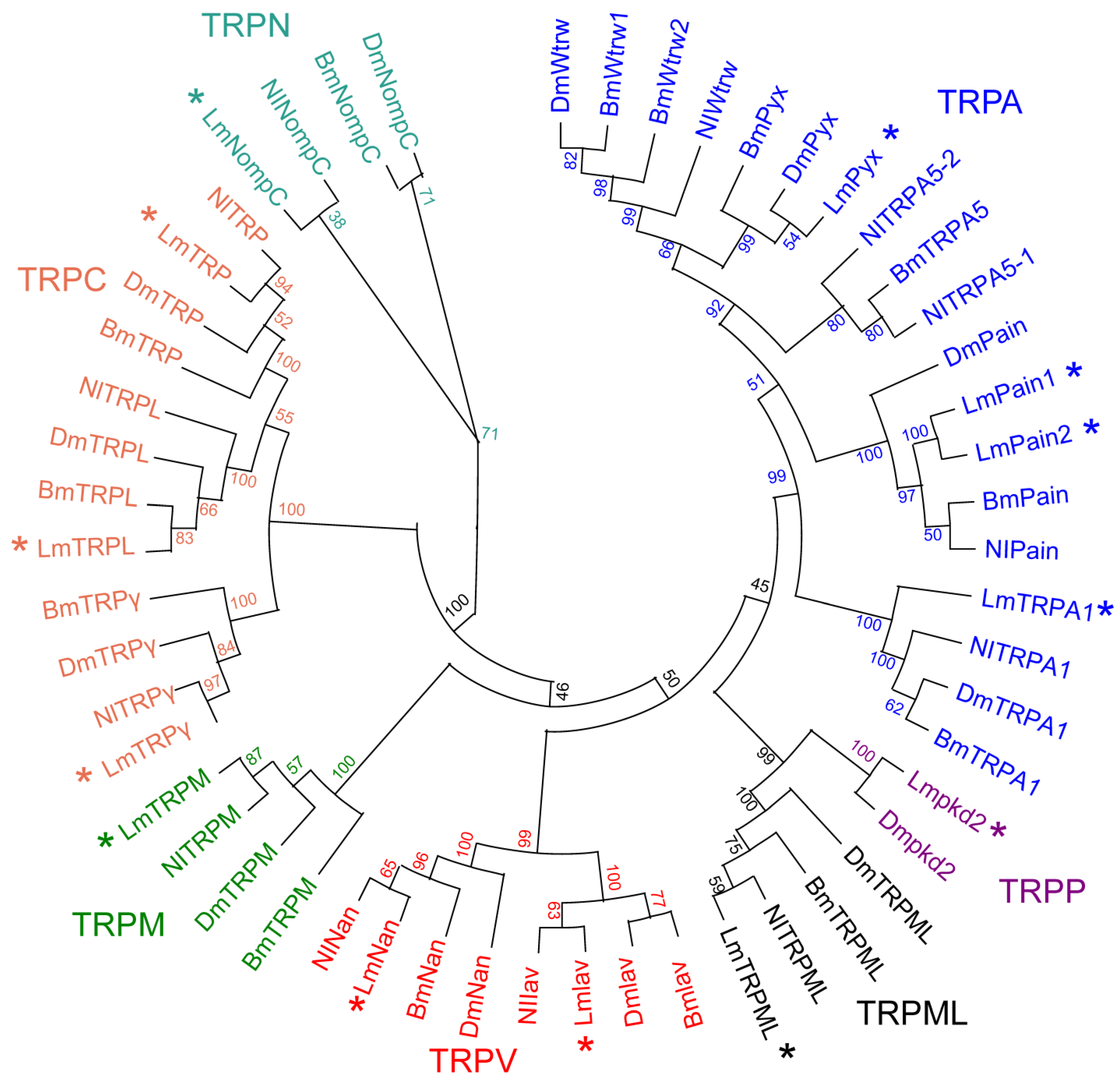
3.3. Phylogenetic Analysis of TRP Superfamily Genes
3.4. Phylogenetic, Conserved Motifs, and Domains in L. migratoria
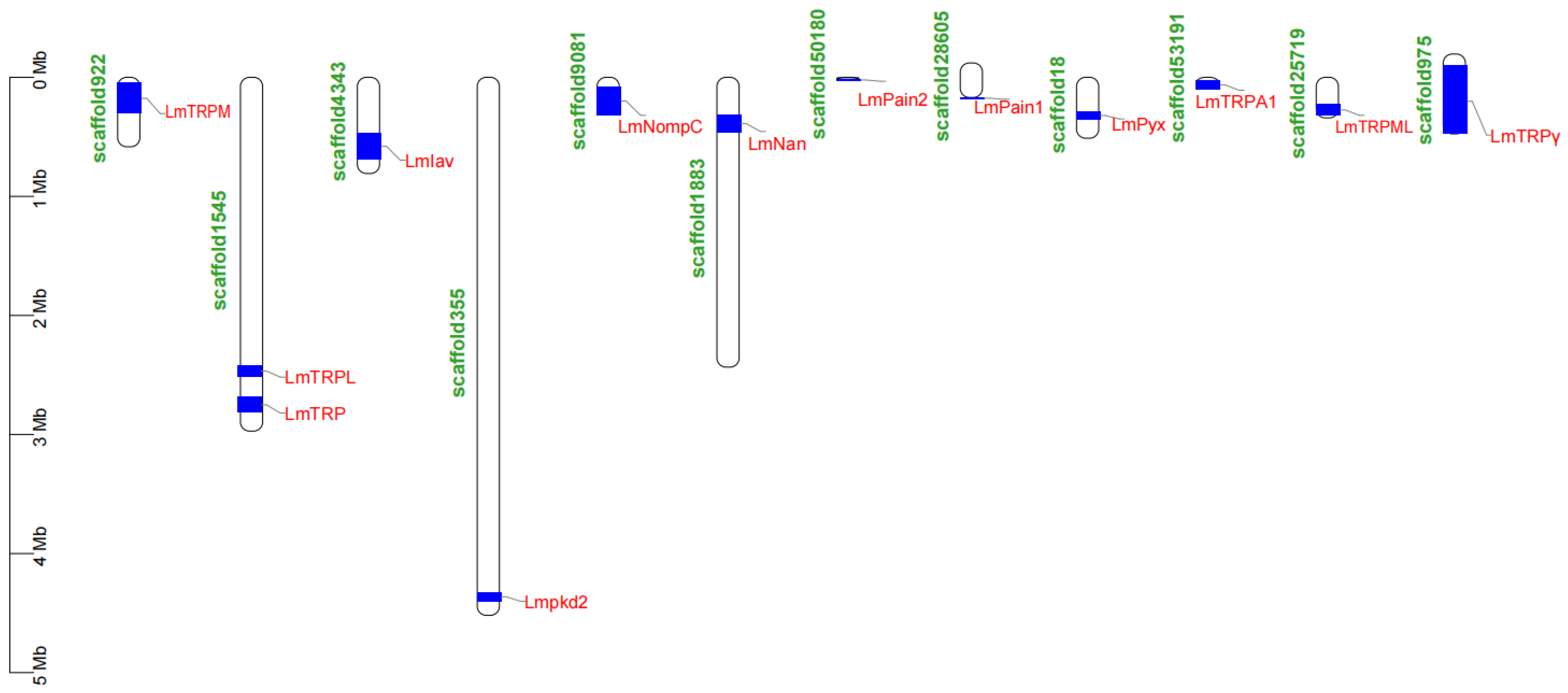
3.5. Location of TRP Superfamily Genes in Genome of L. migratoria
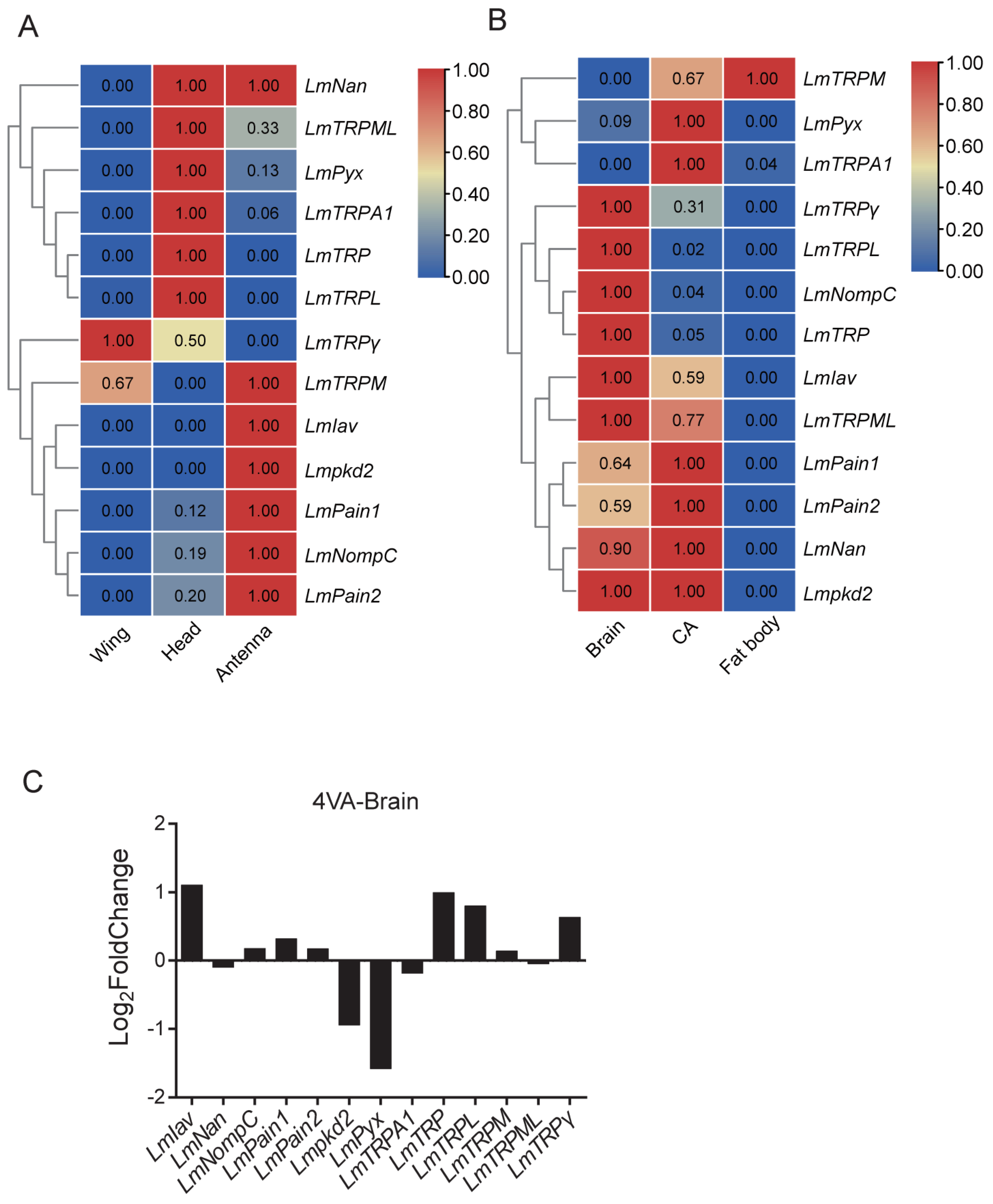
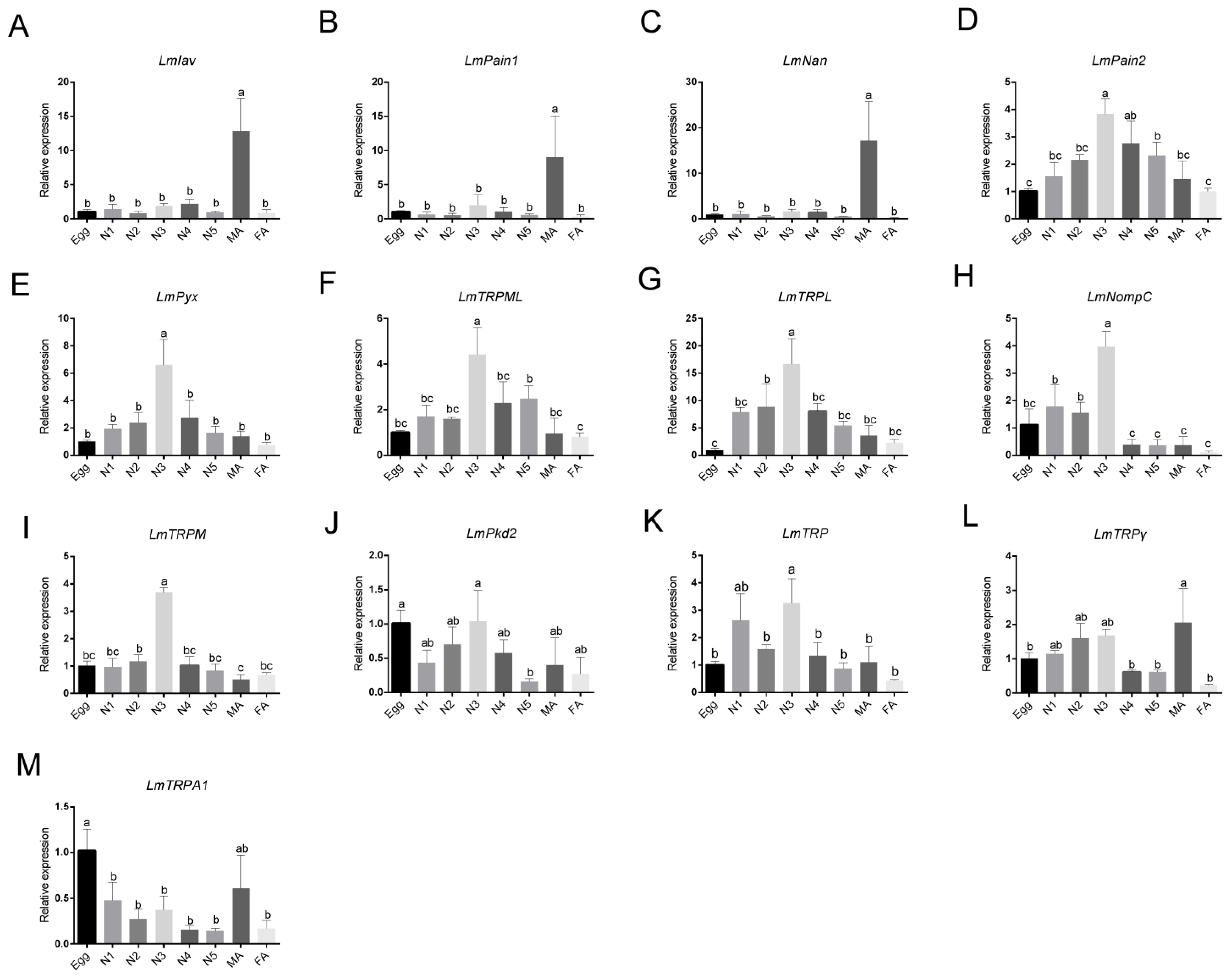
3.6. Expression Patterns Analysis of TRP Superfamily Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, M.A.; Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 2013, 92, 394–403. [Google Scholar] [CrossRef]
- Shimomura, K.; Oikawa, H.; Hasobe, M.; Suzuki, N.; Yajima, S.; Tomizawa, M. Contact repellency by l-menthol is mediated by TRPM channels in the red flour beetle Tribolium castaneum. Pest. Manag. Sci. 2021, 77, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Tribbensee, S.M.; Karna, M.; Khalil, M.; Neurath, M.F.; Reeh, P.W.; Engel, M.A. Differential contribution of TRPA1, TRPV4 and TRPM8 to colonic nociception in mice. PLoS ONE 2015, 10, e0128242. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, M.; Fiorio Pla, A.; Prevarskaya, N.; Gkika, D. Human transient receptor potential (TRP) channel expression profiling in carcinogenesis. Int. J. Dev. Biol. 2015, 59, 399–406. [Google Scholar] [CrossRef]
- Chouquet, B.; Debernard, S.; Bozzolan, F.; Solvar, M.; Maibeche-Coisne, M.; Lucas, P. A TRP channel is expressed in Spodoptera littoralis antennae and is potentially involved in insect olfactory transduction. Insect Mol. Biol. 2009, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.J.; Brent, C.S.; Fu, T.; Wang, G.; Christie, A.E. Mining Lygus hesperus (western tarnished plant bug) transcriptomic data for transient receptor potential channels: Expression profiling and functional characterization of a Painless homolog. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2022, 44, 101027. [Google Scholar] [CrossRef]
- Wang, L.X.; Niu, C.D.; Wu, S.F.; Gao, C.F. Molecular characterizations and expression profiles of transient receptor potential channels in the brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2021, 173, 104780. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, re3. [Google Scholar] [CrossRef]
- Cattaneo, A.M.; Bengtsson, J.M.; Montagne, N.; Jacquin-Joly, E.; Rota-Stabelli, O.; Salvagnin, U.; Bassoli, A.; Witzgall, P.; Anfora, G. TRPA5, an ankyrin subfamily insect TRP channel, is expressed in antennae of Cydia pomonella (Lepidoptera: Tortricidae) in multiple splice variants. J. Insect Sci. 2016, 16, 83. [Google Scholar] [CrossRef]
- Craig Montell, L.B.; Flockerzi, A.V. The TRP Channels, a Remarkably Functional Family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Sidi, S.; Friedrich, R.W.; Nicolson, T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 2003, 301, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Son, W.; Chung, Y.D.; Kim, J.; Shin, D.W.; McClung, C.A.; Lee, Y.; Lee, H.W.; Chang, D.J.; Kaang, B.K.; et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 2004, 24, 9059–9066. [Google Scholar] [CrossRef] [PubMed]
- Bellemer, A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature 2015, 2, 227–243. [Google Scholar] [CrossRef]
- Mao, F.; Lu, W.J.; Yang, Y.; Qiao, X.; Ye, G.Y.; Huang, J. Identification, characterization and expression analysis of TRP channel genes in the vegetable pest, Pieris rapae. Insects 2020, 11, 192. [Google Scholar] [CrossRef]
- Peng, G.; Shi, X.; Kadowaki, T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet Evol. 2015, 84, 145–157. [Google Scholar] [CrossRef]
- Matsuura, H.; Sokabe, T.; Kohno, K.; Tominaga, M.; Kadowaki, T. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 2009, 9, 228. [Google Scholar] [CrossRef]
- Su, H.A.; Bai, X.; Zeng, T.; Lu, Y.Y.; Qi, Y.X. Identification, characterization and expression analysis of transient receptor potential channel genes in the oriental fruit fly, Bactrocera dorsalis. BMC Genom. 2018, 19, 674. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, X.Z. Function and regulation of TRP family channels in C. elegans. Pflugers Arch. 2009, 458, 851–860. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.J.; Guo, D.; Wang, L.X.; Niu, C.D.; Wu, S.F.; Zhang, Y.V.; Gao, C.F. Function of transient receptor potential-like channel in insect egg laying. Front. Mol. Neurosci. 2022, 15, 823563. [Google Scholar] [CrossRef] [PubMed]
- Zermoglio, P.F.; Latorre-Estivalis, J.M.; Crespo, J.E.; Lorenzo, M.G.; Lazzari, C.R. Thermosensation and the TRPV channel in Rhodnius prolixus. J. Insect Physiol. 2015, 81, 145–156. [Google Scholar] [CrossRef]
- Nesterov, A.; Spalthoff, C.; Kandasamy, R.; Katana, R.; Rankl, N.B.; Andres, M.; Jahde, P.; Dorsch, J.A.; Stam, L.F.; Braun, F.J.; et al. TRP channels in insect stretch receptors as insecticide targets. Neuron 2015, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Costea, P.I.; Stam, L.; Nesterov, A. TRPV channel nanchung and TRPA channel water witch form insecticide-activated complexes. Insect Biochem. Mol. Biol. 2022, 149, 103835. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, W.; He, Y.; Gorczyca, D.; Xiang, Y.; Cheng, L.E.; Meltzer, S.; Jan, L.Y.; Jan, Y.N. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 2013, 493, 221–225. [Google Scholar] [CrossRef]
- Hehlert, P.; Zhang, W.; Gopfert, M.C. Drosophila Mechanosensory Transduction. Trends Neurosci. 2021, 44, 323–335. [Google Scholar] [CrossRef]
- Göpfert, M.C.; Albert, J.T.; Nadrowski, B.; Kamikouchi, A. Specification of auditory sensitivity by Drosophila TRP channels. Nat. Neurosci. 2006, 9, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Niu, C.D.; Zhang, Y.; Jia, Y.L.; Zhang, Y.J.; Zhang, Y.; Zhang, Y.Q.; Gao, C.F.; Wu, S.F. The NompC channel regulates Nilaparvata lugens proprioception and gentle-touch response. Insect Biochem. Mol. Biol. 2019, 106, 55–63. [Google Scholar] [CrossRef]
- Effertz, T.; Wiek, R.; Gopfert, M.C. NompC TRP channel is essential for Drosophila sound receptor function. Curr. Biol. 2011, 21, 592–597. [Google Scholar] [CrossRef]
- Kim, H.G.; Margolies, D.; Park, Y. The roles of thermal transient receptor potential channels in thermotactic behavior and in thermal acclimation in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2015, 76, 47–55. [Google Scholar] [CrossRef]
- Sato, A.; Sokabe, T.; Kashio, M.; Yasukochi, Y.; Tominaga, M.; Shiomi, K. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2014, 111, E1249–E1255. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Hull, J.J.; Yang, T.; Wang, G. Identification and functional characterization of four transient receptor potential ankyrin 1 variants in Apolygus lucorum (Meyer-Dur). Insect Mol. Biol. 2016, 25, 370–384. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, R.; Hu, K.; Qiu, L.; Ding, W.; Li, Y. A novel negative thermotaxis behavior in rice planthoppers is regulated by TRPA1 channel. Pest. Manag. Sci. 2020, 76, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Heather, A.; Colbert, T.L.S.; Bargmann, C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997, 17, 8259–8269. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, Y.T.; Lu, T.; Kwon, H.W.; Pitts, R.J.; Van Loon, J.J.; Takken, W.; Zwiebel, L.J. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 2009, 30, 967–974. [Google Scholar] [CrossRef]
- Yin, Y.L.; Wang, H.H.; Gui, Z.C.; Mi, S.; Guo, S.; Wang, Y.; Wang, Q.Q.; Yue, R.Z.; Lin, L.B.; Fan, J.X.; et al. Citronellal Attenuates Oxidative Stress-Induced Mitochondrial Damage through TRPM2/NHE1 Pathway and Effectively Inhibits Endothelial Dysfunction in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 2241. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tong, X.; Zhang, X.; Gui, W.; Ai, G.; Huang, L.; Ding, D.; Zhang, J.; Kang, L. Sulfation modification of dopamine in brain regulates aggregative behavior of animals. Natl. Sci. Rev. 2022, 9, nwab163. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, X.; Yang, P.; Jiang, X.; Jiang, F.; Zhao, D.; Li, B.; Cui, F.; Wei, J.; Ma, C.; et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014, 5, 2957. [Google Scholar] [CrossRef]
- Tanaka, S.; Harano, K.I.; Nishide, Y.; Sugahara, R. The mechanism controlling phenotypic plasticity of body color in the desert locust: Some recent progress. Curr. Opin. Insect Sci. 2016, 17, 10–15. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhang, L.; Xu, X.; Cao, Z.; Zhang, L. Expressions of olfactory proteins in locust olfactory organs and a palp odorant receptor involved in plant aldehydes detection. Front. Physiol. 2018, 9, 663. [Google Scholar] [CrossRef]
- Chen, D.; Hou, L.; Wei, J.; Guo, S.; Cui, W.; Yang, P.; Kang, L.; Wang, X. Aggregation pheromone 4-vinylanisole promotes the synchrony of sexual maturation in female locusts. eLife 2022, 11, 74581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory Mechanisms of Vitellogenesis in Insects. Front. Cell Dev. Biol. 2020, 8, 593613. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Q.; Chen, D.; Wei, J.; Yang, P.; Yu, J.; Wang, X.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef]
- Turner, H.N.; Armengol, K.; Patel, A.A.; Himmel, N.J.; Sullivan, L.; Iyer, S.C.; Bhattacharya, S.; Iyer, E.P.R.; Landry, C.; Galko, M.J.; et al. The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 2016, 26, 3116–3128. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zheng, H.; Wen, X.; Chen, S.; Ye, J.; Tang, S.; Yao, F.; Li, Y.; Yan, Z. Brv1 is required for Drosophila larvae to sense gentle touch. Cell Rep. 2018, 23, 23–31. [Google Scholar] [CrossRef]
- Voets, T.; Janssens, A.; Droogmans, G.; Nilius, B. Outer pore architecture of a Ca2+-selective TRP channel. J. Biol. Chem. 2004, 279, 15223–15230. [Google Scholar] [CrossRef]
- Wang, L.X.; Niu, C.D.; Salgado, V.L.; Lelito, K.; Stam, L.; Jia, Y.L.; Zhang, Y.; Gao, C.F.; Wu, S.F. Pymetrozine activates TRPV channels of brown planthopper Nilaparvata lugens. Pestic. Biochem. Physiol. 2019, 153, 77–86. [Google Scholar] [CrossRef]
- Sun, L.; Pan, X.; Li, H.; Zhang, X.; Zhao, X.; Zhang, L.; Zhang, L. Odor-induced vomiting is combinatorially triggered by palp olfactory receptor neurons that project to the lobus glomerulatus in locust brain. Front. Physiol. 2022, 13, 855522. [Google Scholar] [CrossRef] [PubMed]
- Störtkuhl, K.F.; Hovemann, B.T.; Carlson, J.R. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J. Neurosci. 1999, 19, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, Y.; Wang, F.; Wang, Z. Drosophila TRPA channel painless inhibits male-male courtship behavior through modulating olfactory sensation. PLoS ONE 2011, 6, e25890. [Google Scholar] [CrossRef]
- Shimomura, K.; Ino, S.; Tamura, K.; Terajima, T.; Tomizawa, M. TRPA1-mediated repellency behavior in the red flour beetle Tribolium castaneum. Sci. Rep. 2022, 12, 15270. [Google Scholar] [CrossRef]
- Dhakal, S.; Ren, Q.; Liu, J.; Akitake, B.; Tekin, I.; Montell, C.; Lee, Y. Drosophila TRPgamma is required in neuroendocrine cells for post-ingestive food selection. eLife 2022, 11, 56726. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Saito, S.; Shimoda, M.; Kobayashi, M.; Takasu, Y.; Sezutsu, H.; Kato, Y.; Tominaga, M.; Mizoguchi, A.; Shiomi, K. Comparisons in temperature and photoperiodic-dependent diapause induction between domestic and wild mulberry silkworms. Sci. Rep. 2021, 11, 8052. [Google Scholar] [CrossRef]

| Gene Name | Genomic ID | CDS Length (bp) | Amino Acid (aa) | Molecular Weight (Da) | Isoelectric Point |
|---|---|---|---|---|---|
| LmIav | LOCMI10002 | 3081 | 1026 | 114,537.08 | 8.81 |
| LmNan | LOCMI14722 | 2757 | 918 | 102,814.22 | 5.9 |
| LmNompC | LOCMI07947 | 3990 | 1329 | 146,348.41 | 9.22 |
| LmPain1 | LOCMI06538 | 2976 | 991 | 112,666.78 | 5.41 |
| LmPain2 | LOCMI03163 | 2775 | 924 | 105,285.22 | 5.34 |
| Lmpkd2 | LOCMI15283 | 1473 | 490 | 56,483.04 | 4.98 |
| LmPyx | LOCMI07175 | 1395 | 465 | 51,742.73 | 9.1 |
| LmTRPA1 | LOCMI03266 | 1929 | 643 | 72,038.88 | 8.89 |
| LmTRP | LOCMI14741 | 2697 | 899 | 103,911.28 | 8.42 |
| LmTRPL | LOCMI14740 | 2754 | 918 | 105,869.56 | 6.32 |
| LmTRPM | LOCMI11192 | 4587 | 1529 | 174,403.82 | 5.97 |
| LmTRPML | LOCMI07586 | 1779 | 592 | 67,777.94 | 6.42 |
| LmTRPγ | LOCMI11247 | 2463 | 821 | 94,101.82 | 7.34 |
| Species Name | TRPV | TRPA | TRPC | TRPM | TRPML | TRPN | TRPP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iav | Nan | Pain | Wtrw | TRPA5 | Pyx | TRPA1 | TRP | TRPL | TRPγ | TRPM | TRPML | NompC | pkd2 | Brv | Total | |
| L. migratoria | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 13 |
| B. dorsalis | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 15 |
| B. mori | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 14 |
| A. mellifera | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 |
| D. melanogaster | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 16 |
| N. lugens | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Guo, W.; Wang, M.; Zhang, D. Genome-Wide Characterization and Gene Expression Analysis of TRP Channel Superfamily Genes in the Migratory Locust, Locusta migratoria. Genes 2023, 14, 1427. https://doi.org/10.3390/genes14071427
Yang Y, Guo W, Wang M, Zhang D. Genome-Wide Characterization and Gene Expression Analysis of TRP Channel Superfamily Genes in the Migratory Locust, Locusta migratoria. Genes. 2023; 14(7):1427. https://doi.org/10.3390/genes14071427
Chicago/Turabian StyleYang, Yong, Wenhui Guo, Mingjun Wang, and Daochuan Zhang. 2023. "Genome-Wide Characterization and Gene Expression Analysis of TRP Channel Superfamily Genes in the Migratory Locust, Locusta migratoria" Genes 14, no. 7: 1427. https://doi.org/10.3390/genes14071427
APA StyleYang, Y., Guo, W., Wang, M., & Zhang, D. (2023). Genome-Wide Characterization and Gene Expression Analysis of TRP Channel Superfamily Genes in the Migratory Locust, Locusta migratoria. Genes, 14(7), 1427. https://doi.org/10.3390/genes14071427





