High Expression of CDCA7 in the Prognosis of Glioma and Its Relationship with Ferroptosis and Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Bioinformatics Analysis
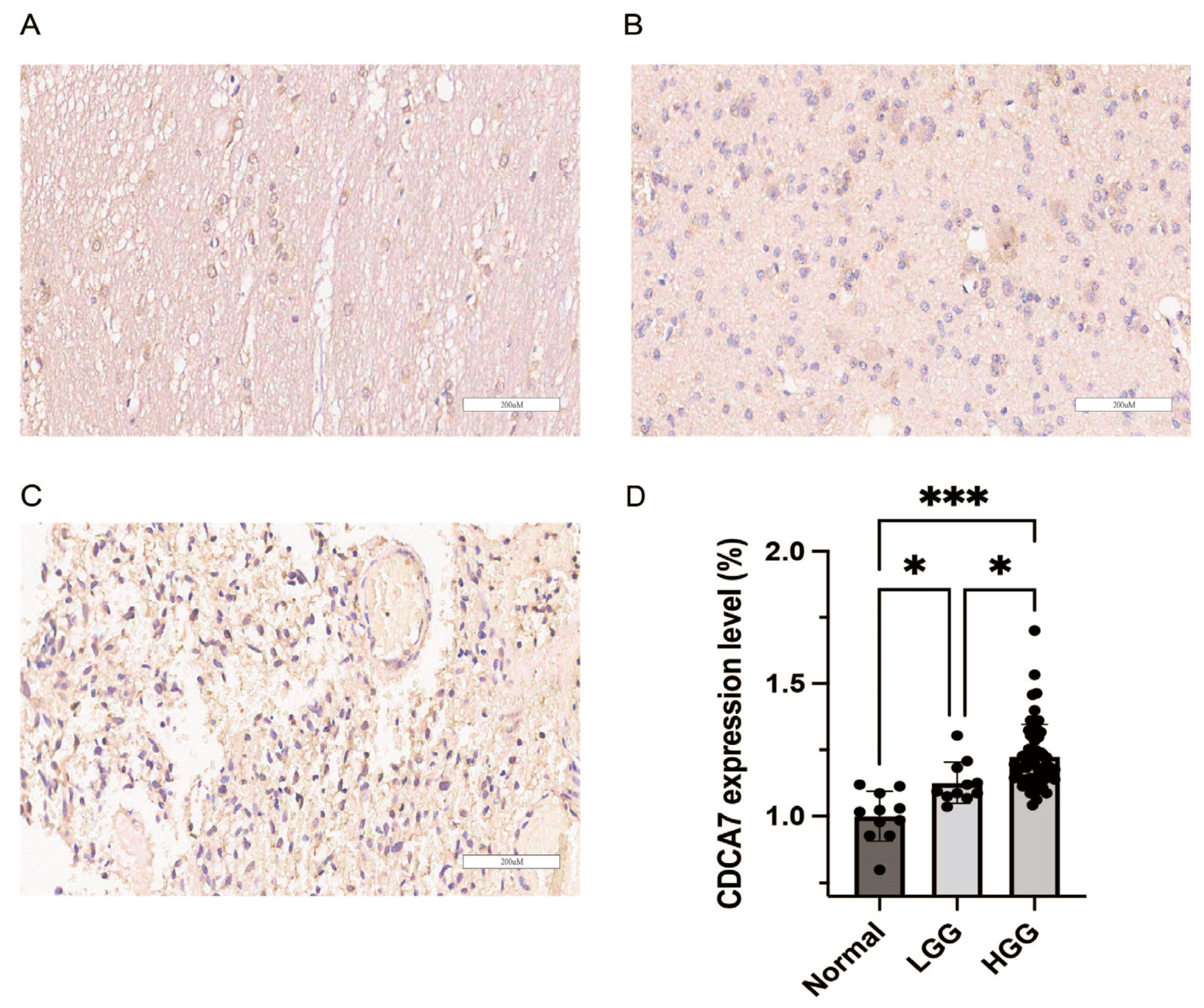
2.2. Glioma Tissue Microarray, Specimen, and Immunohistochemistry
2.3. Enrichment Analysis
2.4. Screening for Ferroptosis-Associated Genes
2.5. Protein–Protein Interaction (PPI) Network Analysis
2.6. Statistical Analysis
3. Results
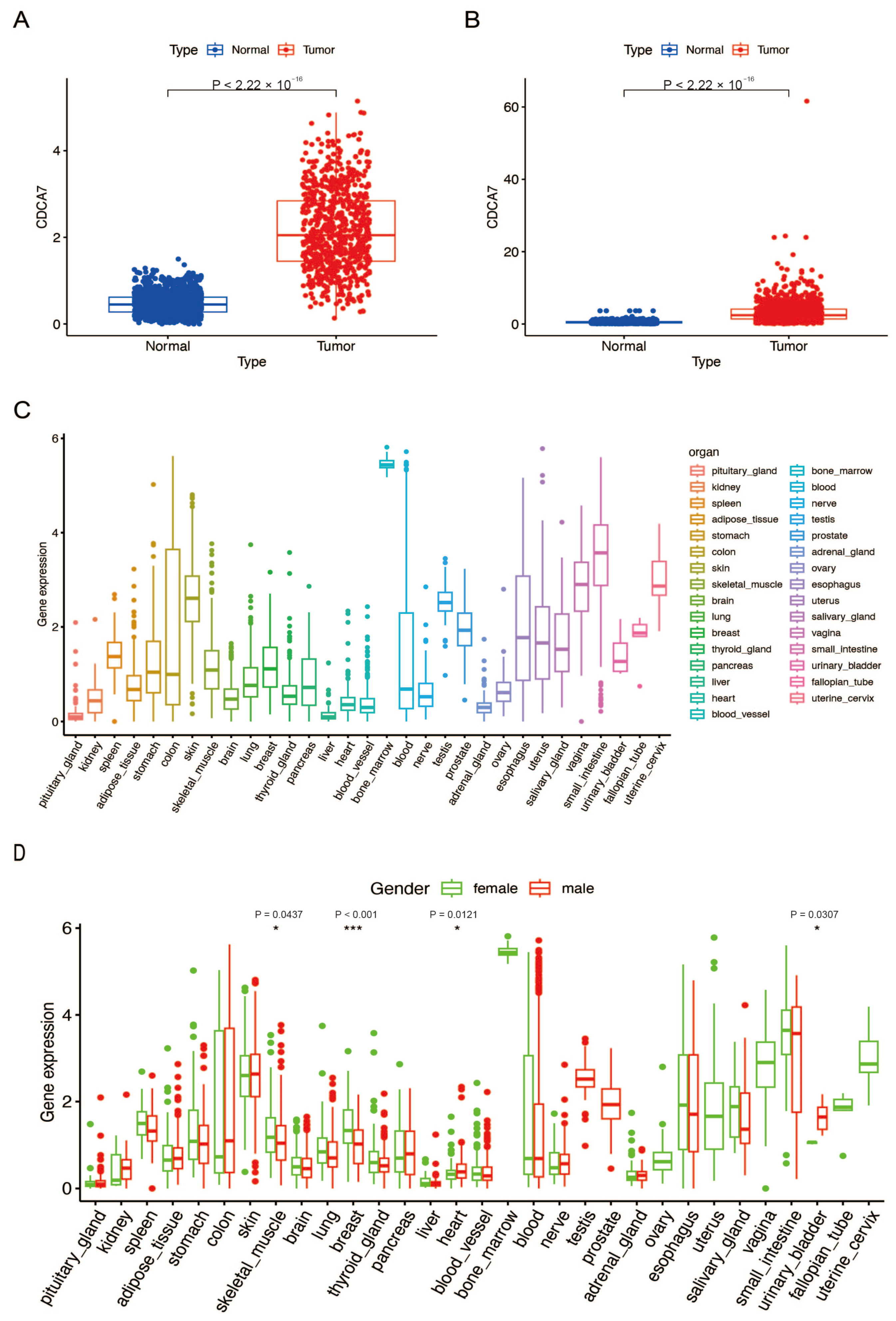
3.1. CDCA7 Shows High Expression in Gliomas and Low Expression in Normal Human Brain Tissues
3.2. The Relationship between CDCA7 Expression and Clinicopathological Features
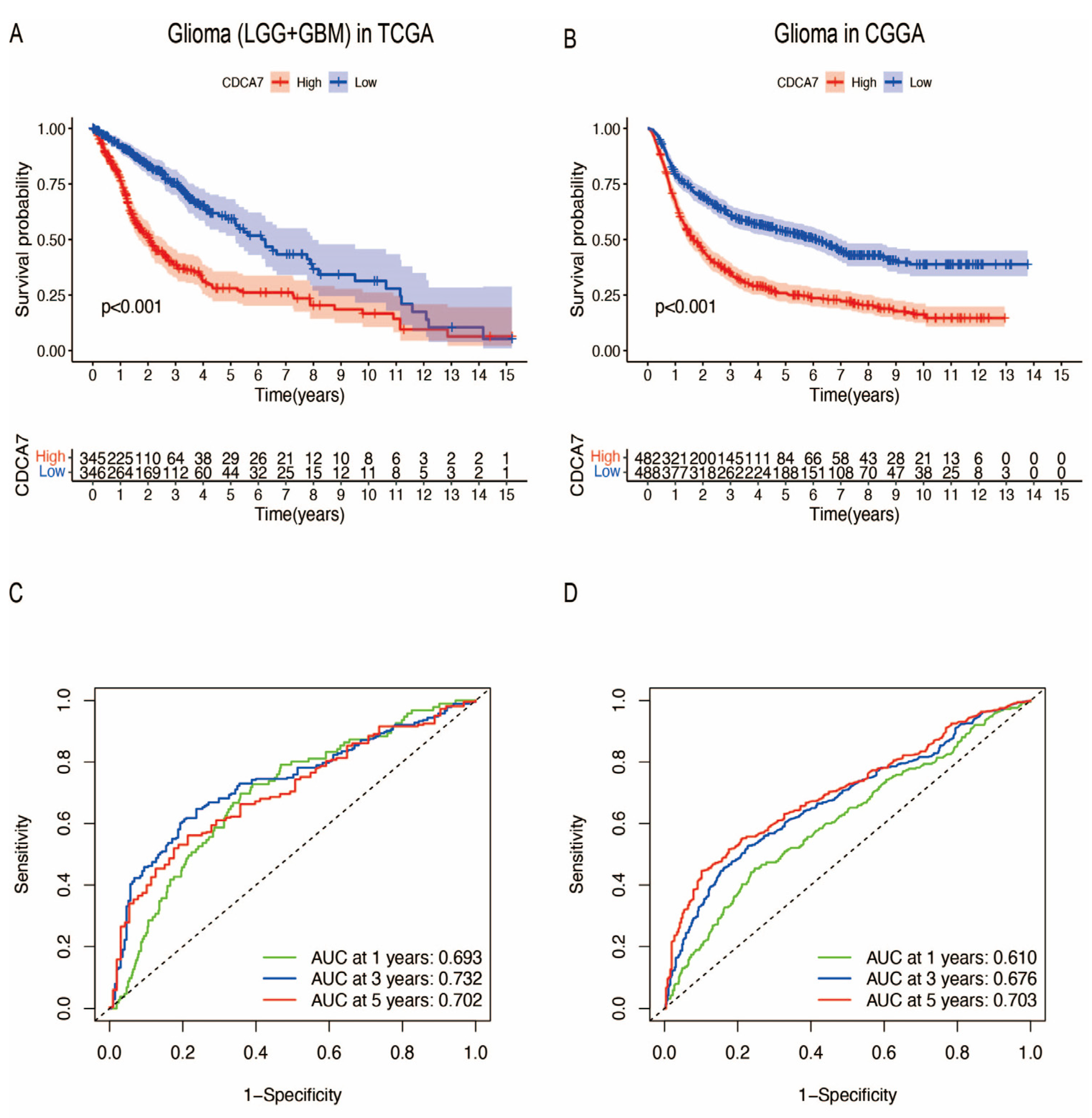
3.3. The Relationship between CDCA7 Expression and the Prognosis of Glioma Patients
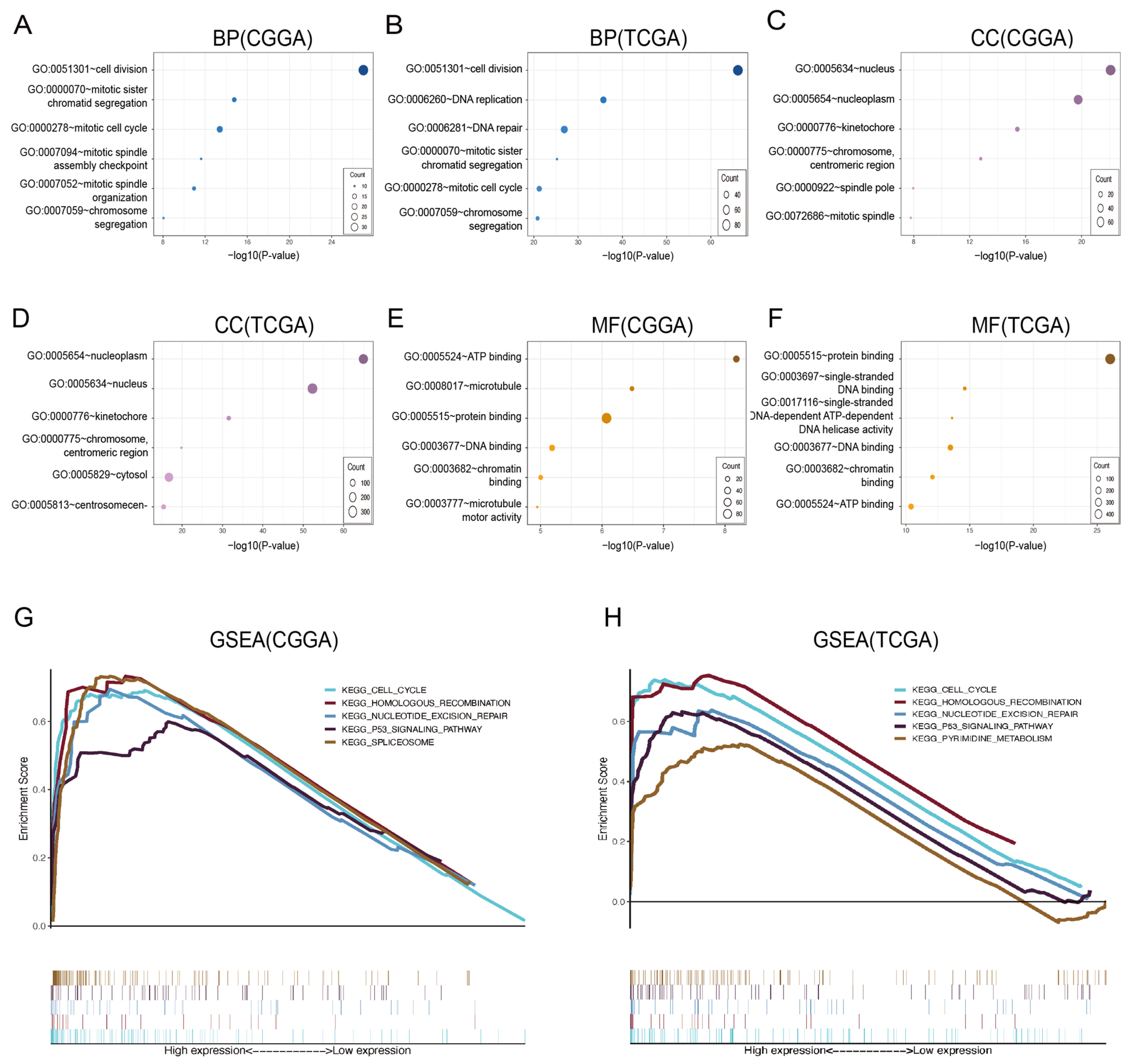
3.4. Enrichment Analysis of CDCA7
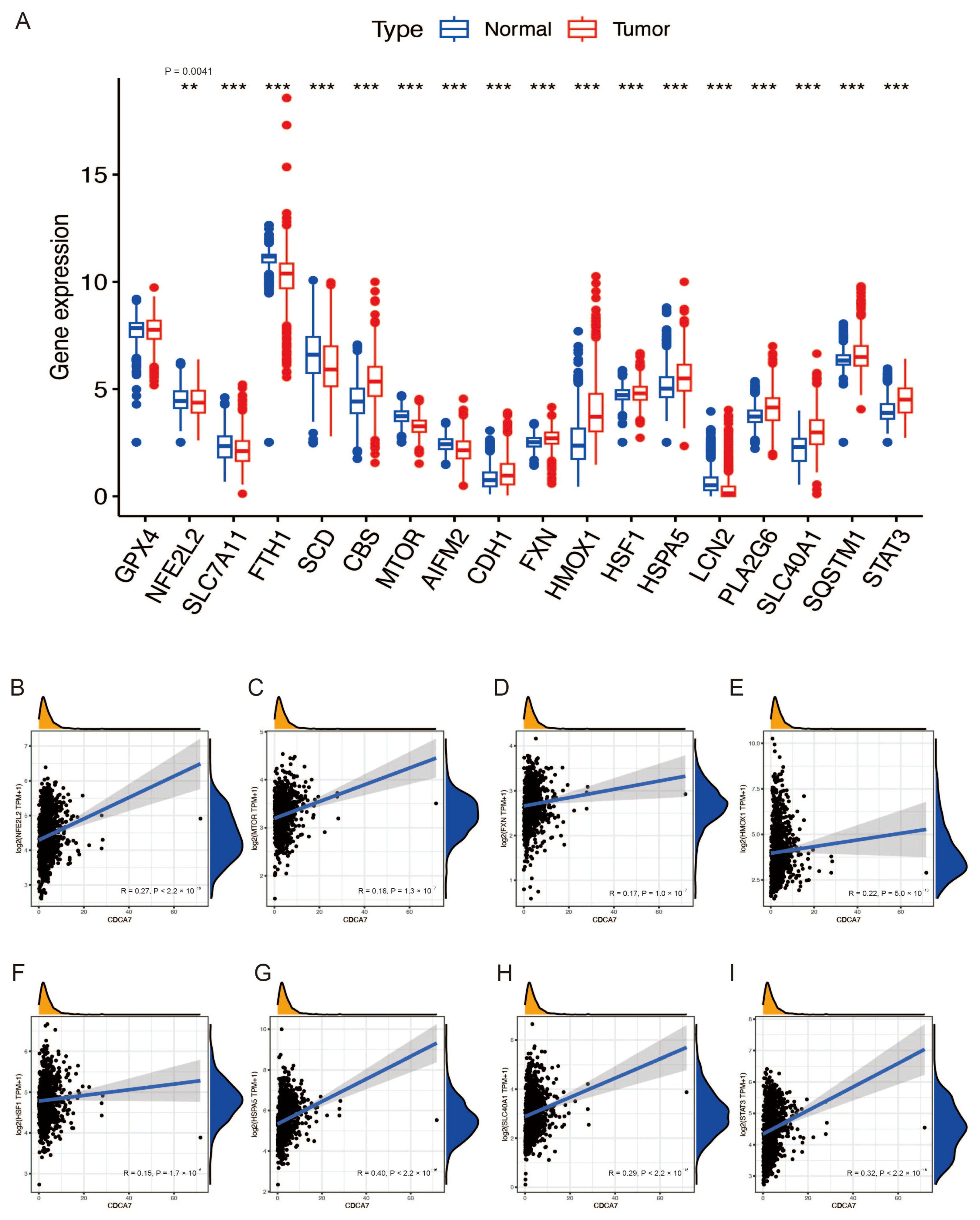
3.5. The Relationship between CDCA7 and Ferroptosis Suppressor Genes in Glioma
3.6. Analysis of Potential Functional Links between CDCA7 and Ferroptosis through PPI
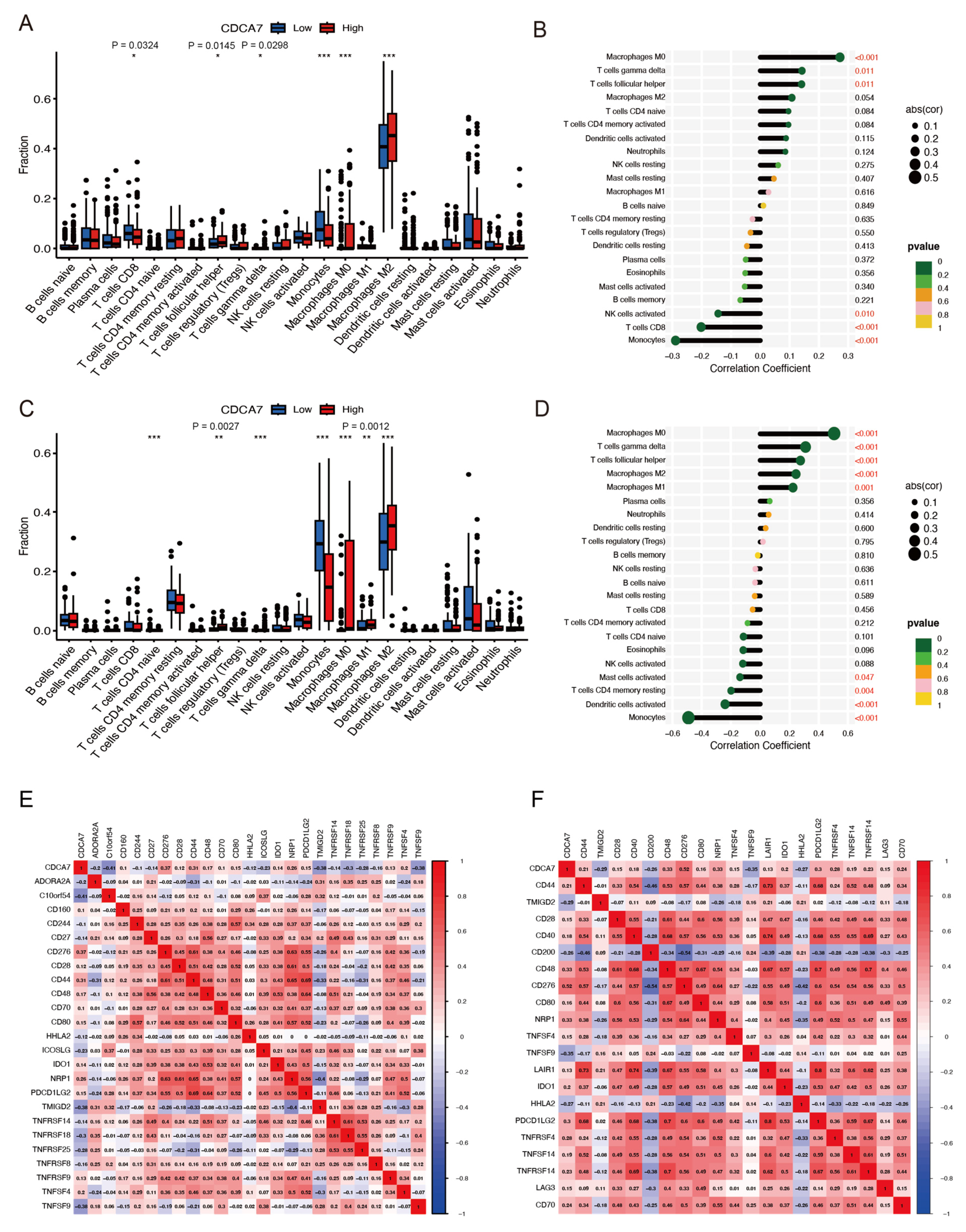
3.7. Correlation between CDCA7 Expression Level and Tumor Immune Cell Infiltration
3.8. Association of CDCA7 with Immune Checkpoint Molecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Schneider, E.; Westhoff, M.-A.; Wirtz, C.R.; Karpel-Massler, G. Current state and future perspective of drug repurposing in malignant glioma. Semin. Cancer Biol. 2021, 68, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Hoshide, R.; Jandial, R. 2016 World Health Organization Classification of Central Nervous System Tumors: An Era of Molecular Biology. World Neurosurg. 2016, 94, 561–562. [Google Scholar] [CrossRef]
- Hu, W.; Yang, Y.; Xi, S.; Sai, K.; Su, D.; Zhang, X.; Lin, S.; Zeng, J. Expression of CPEB4 in Human Glioma and Its Correlations with Prognosis. Medicine 2015, 94, e979. [Google Scholar] [CrossRef]
- Prescott, J.E.; Osthus, R.C.; Lee, L.A.; Lewis, B.C.; Shim, H.; Barrett, J.F.; Guo, Q.; Hawkins, A.L.; Griffin, C.A.; Dang, C.V. A Novel c-Myc- responsive Gene, JPO1, Participates in Neoplastic Transformation. J. Biol. Chem. 2001, 276, 48276–48284. [Google Scholar] [CrossRef]
- Osthus, R.C.; Karim, B.; Prescott, J.E.; Smith, B.D.; McDevitt, M.; Huso, D.L.; Dang, C.V. The Myc Target Gene JPO1/CDCA7 Is Frequently Overexpressed in Human Tumors and Has Limited Transforming Activity In vivo. Cancer Res. 2005, 65, 5620–5627. [Google Scholar] [CrossRef]
- Goto, Y.; Hayashi, R.; Muramatsu, T.; Ogawa, H.; Eguchi, I.; Oshida, Y.; Yoshida, K. JPO1/CDCA7, a novel transcription factor E2F1-induced protein, possesses intrinsic transcriptional regulator activity. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2006, 1759, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Mao, C.; Yang, R.; Yan, B.; Shi, Y.; Liu, X.; Tao, Y. EGLN1/c-Myc Induced Lymphoid-Specific Helicase Inhibits Ferroptosis through Lipid Metabolic Gene Expression Changes. Theranostics 2017, 7, 3293–3305. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, S.; Xu, M.; Ma, B.; Liu, R.; Xu, Y.; Zhang, Y. TFAP2C-Mediated lncRNA PCAT1 Inhibits Ferroptosis in Docetaxel-Resistant Prostate Cancer Through c-Myc/miR-25-3p/SLC7A11 Signaling. Front. Oncol. 2022, 12, 862015. [Google Scholar] [CrossRef]
- Kuganesan, N.; Dlamini, S.; Tillekeratne, L.M.V.; Taylor, W.R. Tumor suppressor p53 promotes ferroptosis in oxidative stress conditions independent of modulation of ferroptosis by p21, CDKs, RB, and E2F. J. Biol. Chem. 2021, 297, 101365. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Mi, Y.; Qian, H.; Guo, N.; Yan, A.; Zhang, Y.; Gao, X. A Potential Mechanism of Temozolomide Resistance in Glioma–Ferroptosis. Front. Oncol. 2020, 10, 897. [Google Scholar] [CrossRef]
- Shi, J.; Yang, N.; Han, M.; Qiu, C. Emerging roles of ferroptosis in glioma. Front. Oncol. 2022, 12, 993316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fang, Y.; Li, X.; Zhang, Z. Ferroptosis: A novel therapeutic strategy and mechanism of action in glioma. Front. Oncol. 2022, 12, 947530. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Li, X.; Weng, Y.; Guo, D.; Kong, P.; Cui, Y. CDCA7 promotes TGF-β-induced epithelial–mesenchymal transition via transcriptionally regulating Smad4/Smad7 in ESCC. Cancer Sci. 2023, 114, 91–104. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, W.D.; Kim, S.K.; Moon, D.H.; Lee, S.J. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Weng, Y.; Wang, S.; Wang, F.; Wang, Y.; Kong, P.; Zhang, L.; Cheng, C.; Cui, H.; Xu, E.; et al. CDCA7 Facilitates Tumor Progression by Directly Regulating CCNA2 Expression in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 734655. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Miao, C.; Xing, Q.; Wang, Z. High expression of CDCA7 predicts poor prognosis for clear cell renal cell carcinoma and explores its associations with immunity. Cancer Cell Int. 2021, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Qin, M.; Zhang, J.; Liao, C. High Expression of CDCA7 Predicts Tumor Progression and Poor Prognosis in Human Colorectal Cancer. Mol. Med. Rep. 2020, 22, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Li, F.; Song, Y.; Yu, D.; Xiong, Z.; Li, Y.; Shi, T.; Yuan, Z.; Lin, C.; Wu, X.; et al. Overexpression of CDCA7 predicts poor prognosis and induces EZH2-mediated progression of triple-negative breast cancer. Int. J. Cancer 2018, 143, 2602–2613. [Google Scholar] [CrossRef]
- Han, H.; Lee, S.; Lee, I. NGSEA: Network-based gene set enrichment analysis for interpreting gene expression phenotypes with functional gene sets. Bioinformatics 2019, 42, 579–588. [Google Scholar] [CrossRef]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef]
- Wang, L.; Ge, J.; Lan, Y.; Shi, Y.; Luo, Y.; Tan, Y.; Liang, M.; Deng, S.; Zhang, X.; Wang, W.; et al. Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer 2020, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, M.A.; Oliveira-Costa, J.P.; Carter, B.S.; Stott, S.L.; Nahed, B.V. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro Oncol. 2018, 20, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Z.; Lv, P.; Zhan, Y.; Zhong, Q. SCAMP3 Promotes Glioma Proliferation and Indicates Unfavorable Prognosis via Multiple Pathways. OncoTargets Ther. 2020, 13, 3677–3687. [Google Scholar] [CrossRef]
- Kristensen, B.; Priesterbach-Ackley, L.; Petersen, J.; Wesseling, P. Molecular pathology of tumors of the central nervous system. Ann. Oncol. 2019, 30, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.C.; Wu, F.; Wang, Q.X.; Zhang, S.; Zhang, K.N.; Liu, Y.Q.; Zhao, Z.; Jiang, T.; Wang, Y.Z.; Kang, C.S. m6A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gliomas. Aging 2019, 11, 1204–1225. [Google Scholar] [CrossRef]
- Calvert, A.E.; Chalastanis, A.; Wu, Y.; Hurley, L.A.; Kouri, F.M.; Bi, Y.; Kachman, M.; May, J.L.; Bartom, E.; Hua, Y.; et al. Cancer-Associated IDH1 Promotes Growth and Resistance to Targeted Therapies in the Absence of Mutation. Cell Rep. 2017, 19, 1858–1873. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, T.J.; Zeller, K.I.; Osthus, R.C.; Wonsey, D.R.; Dang, C.V. A strategy for identifying transcription factor binding sites reveals two classes of genomic c-Myc target sites. Proc. Natl. Acad. Sci. USA 2003, 100, 5313–5318. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.; Gomez-Roman, N.; Hochegger, H.; Chalmers, A.J. Differential sensitivity of Glioma stem cells to Aurora kinase A inhibitors: Implications for stem cell mitosis and centrosome dynamics. Stem Cell Res. 2014, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz-Krzeminska, I.; Sarasquete, M.E.; Quwaider, D.; Krzeminski, P.; Ticona, F.V.; Paino, T.; Delgado, M.; Aires, A.; Ocio, E.M.; Garcia-Sanz, R.; et al. Restoration of microRNA-214 expression reduces growth of myeloma cells through positive regulation of P53 and inhibition of DNA replication. Haematologica 2013, 98, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, J.M.; Ramirez, F.G.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2015, 112, 1779–1784. [Google Scholar] [CrossRef]
- Silva, R.D.F.E.; Dos Santos, N.F.G.; Pereira, V.R.A.; Amaral, A. Simultaneous Analysis of P53 Protein Expression and Cell Proliferation in Irradiated Human Lymphocytes by Flow Cytometry. Dose-Response 2014, 12, 110–120. [Google Scholar] [CrossRef]
- Hwang, C.-I.; Matoso, A.; Corney, D.C.; Flesken-Nikitin, A.; Körner, S.; Wang, W.; Boccaccio, C.; Thorgeirsson, S.S.; Comoglio, P.M.; Hermeking, H.; et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc. Natl. Acad. Sci. USA 2011, 108, 14240–14245. [Google Scholar] [CrossRef]
- Jin, Y.; Xiao, W.; Song, T.; Feng, G.; Dai, Z. Expression and Prognostic Significance of p53 in Glioma Patients: A Meta-analysis. Neurochem. Res. 2016, 41, 1723–1731. [Google Scholar] [CrossRef]
- Nieder, C.; Petersen, S.; Petersen, C.; Thames, H. The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat. Rev. 2000, 26, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Xu, L.; Cui, H.; Xu, M.; Yi, L. MicroRNAs and cell cycle of malignant glioma. Int. J. Neurosci. 2015, 126, 1–9. [Google Scholar] [CrossRef]
- Ren, D.; Zhuang, X.; Lv, Y.; Zhang, Y.; Xu, J.; Gao, F.; Chen, D.; Wang, Y. FAM84B promotes the proliferation of glioma cells through the cell cycle pathways. World J. Surg. Oncol. 2022, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, A.; Li, Y.; Yu, G.; Yan, X. XPA Enhances Temozolomide Resistance of Glioblastoma Cells by Promoting Nucleotide Excision Repair. Cell Transplant. 2022, 31, 096368972210927. [Google Scholar] [CrossRef]
- Lim, Y.C.; Roberts, T.L.; Day, B.W.; Stringer, B.W.; Kozlov, S.; Fazry, S.; Bruce, Z.C.; Ensbey, K.S.; Walker, D.G.; Boyd, A.W.; et al. Increased sensitivity to ionizing radiation by targeting the homologous recombination pathway in glioma initiating cells. Mol. Oncol. 2014, 8, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Sun, W.; Zhang, J.; Xu, Q.; Zhou, X.; Mao, L. Ferroptosis in colorectal cancer: Potential mechanisms and effective therapeutic targets. Biomed. Pharmacother. 2022, 153, 113524. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Horky, P.; Abbas, S.Q.; Majid, M.; Bilal, A.H.M.; Ali, F.; Behl, T.; Hassan, S.S.U.; Bungau, S. Ferroptosis: A New Road towards Cancer Management. Molecules 2022, 27, 2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, C.; Liu, J.; Meng, C.; Gu, Q.; Du, X.; Yan, M.; Yu, Y.; Liu, F.; Xia, C. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharm. Res. 2023, 187, 106563. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Guo, S.; Xue, X.; Wang, Y.; Qiu, S.; Cui, J.; Ma, L.; Zhang, X.; Wang, J. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 2020, 20, 587. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Chang, Y.; Wang, K.; Kang, X.; Huang, R.; Zhang, Y.; Chen, J.; Zeng, F.; Wu, F.; et al. EFEMP2 indicates assembly of M0 macrophage and more malignant phenotypes of glioma. Aging 2020, 12, 8397–8412. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, K.; Dong, W.; Hu, F.; Zhao, S.; Liu, S. γδ T Cells in Peripheral Blood of Glioma Patients. Med. Sci. Monit. 2018, 24, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, J.; Su, H.; Luo, S.; Chen, J.; Qiu, S.; Chi, Y.; Lin, J.; Xu, X.; Zheng, D. Antitumor CD8 T cell responses in glioma patients are effectively suppressed by T follicular regulatory cells. Exp. Cell Res. 2021, 407, 112808. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, R.; Zhao, P.; Yu, X.; Li, J.; Ma, G.; Tang, J.; Zhang, L.; Feng, L.; Sun, L.; et al. Proinflammatory follicular helper T cells promote immunoglobulin G secretion, suppress regulatory B cell development, and correlate with worse clinical outcomes in gastric cancer. Tumor Biol. 2017, 39, 101042831770574. [Google Scholar] [CrossRef]
- Kwart, D.; He, J.; Srivatsan, S.; Lett, C.; Golubov, J.; Oswald, E.M.; Poon, P.; Ye, X.; Waite, J.; Zaretsky, A.G.; et al. Cancer cell-derived type I interferons instruct tumor monocyte polarization. Cell Rep. 2022, 41, 111769. [Google Scholar] [CrossRef]
- Mir, M.A.; Agrewala, J.N. Signaling through CD80: An approach for treating lymphomas. Expert Opin. Ther. Targets 2008, 12, 969–979. [Google Scholar] [CrossRef]
- Dai, L.; Guo, X.; Xing, Z.; Tao, Y.; Liang, W.; Shi, Z.; Hu, W.; Zhou, S.; Wang, X. Multi-omics analyses of CD276 in pan-cancer reveals its clinical prognostic value in glioblastoma and other major cancer types. BMC Cancer 2023, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Zhu, D.; Wang, X. High expression of cluster of differentiation 276 indicates poor prognosis in glioma. Clin. Med. Insights Oncol. 2021, 15, 11795549211032330. [Google Scholar] [CrossRef]
- Dai, L.; Huang, Z.; Li, W. Analysis of the PD-1 Ligands Among Gastrointestinal Cancer Patients: Focus on Cancer Immunity. Front. Oncol. 2021, 11, 637015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Zheng, J.; Wang, S.; Liu, C.; Yao, X.; Ren, Y.; Wang, X. Integrative study reveals the prognostic and immunotherapeutic value of CD274 and PDCD1LG2 in pan-cancer. Front. Genet. 2022, 13, 990301. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ma, L.; Sun, Y.; Li, X.; Ren, H.; Tang, S.-C.; Sun, X. The immunotherapy candidate TNFSF4 may help the induction of a promising immunological response in breast carcinomas. Sci. Rep. 2021, 11, 18587. [Google Scholar] [CrossRef]



| Gene Set Name | NES | Nominal p-Value | FDR q-Value |
|---|---|---|---|
| KEGG_NUCLEOTIDE_EXCISION_REPAIR | 2.27 | 0.000 | 0.000 |
| KEGG_CELL_CYCLE | 2.21 | 0.000 | 0.001 |
| KEGG_P53_SIGNALING_PATHWAY | 2.04 | 0.000 | 0.003 |
| KEGG_SPLICEOSOME | 2.23 | 0.000 | 0.000 |
| KEGG_HOMOLOGOUS_RECOMBINATION | 2.09 | 0.000 | 0.001 |
| Gene Set Name | NES | Nominal p-Value | FDR q-Value |
|---|---|---|---|
| KEGG_CELL_CYCLE | 2.34 | 0.000 | 0.000 |
| KEGG_NUCLEOTIDE_EXCISION_REPAIR | 2.16 | 0.000 | 0.005 |
| KEGG_P53_SIGNALING_PATHWAY | 2.13 | 0.000 | 0.006 |
| KEGG_HOMOLOGOUS_RECOMBINATION | 2.01 | 0.000 | 0.014 |
| KEGG_PYRIMIDINE_METABOLISM | 2.12 | 0.000 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, Y.; Zhang, Z.; Zhang, J.; Xu, Q.; Zhou, X.; Mao, L. High Expression of CDCA7 in the Prognosis of Glioma and Its Relationship with Ferroptosis and Immunity. Genes 2023, 14, 1406. https://doi.org/10.3390/genes14071406
Wang Y, Zhao Y, Zhang Z, Zhang J, Xu Q, Zhou X, Mao L. High Expression of CDCA7 in the Prognosis of Glioma and Its Relationship with Ferroptosis and Immunity. Genes. 2023; 14(7):1406. https://doi.org/10.3390/genes14071406
Chicago/Turabian StyleWang, Yunhan, Yu Zhao, Zongying Zhang, Jie Zhang, Qiuyun Xu, Xiaorong Zhou, and Liming Mao. 2023. "High Expression of CDCA7 in the Prognosis of Glioma and Its Relationship with Ferroptosis and Immunity" Genes 14, no. 7: 1406. https://doi.org/10.3390/genes14071406
APA StyleWang, Y., Zhao, Y., Zhang, Z., Zhang, J., Xu, Q., Zhou, X., & Mao, L. (2023). High Expression of CDCA7 in the Prognosis of Glioma and Its Relationship with Ferroptosis and Immunity. Genes, 14(7), 1406. https://doi.org/10.3390/genes14071406






