Comparative Analysis of Whole Transcriptome Profiles in Septic Cardiomyopathy: Insights from CLP- and LPS-Induced Mouse Models
Abstract
1. Introduction
2. Materials and Methods
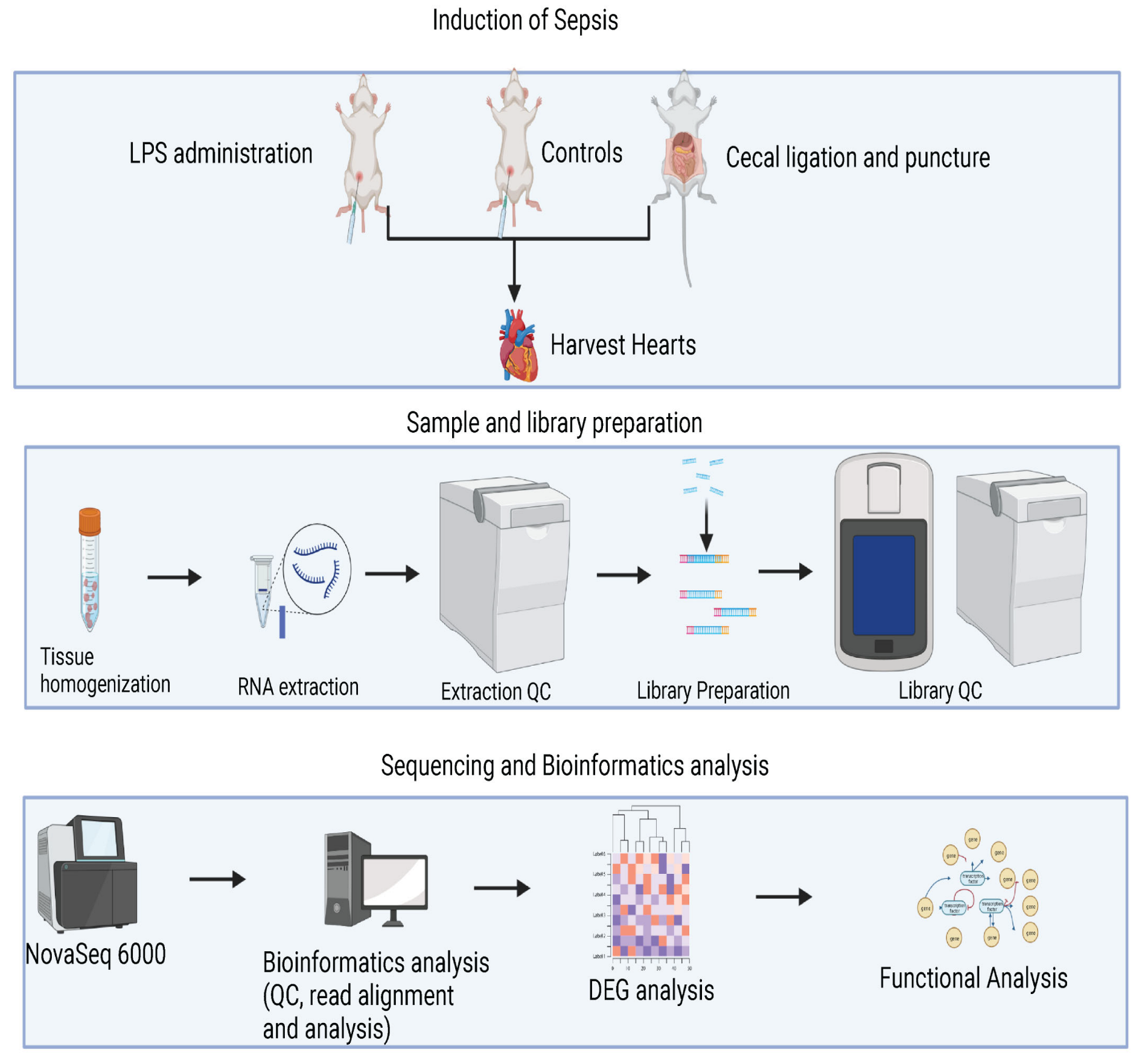
2.1. Animal Models and Experimental Design
2.2. Cecal Ligation and Puncture (CLP) Procedure Induced Sepsis
2.3. Lipopolysaccharide (LPS) Administration Induced Sepsis
2.4. Echocardiographic Image Acquisition
2.5. Sample Collection and RNA Extraction
2.6. RNA-Seq Analysis
3. Results
3.1. CLP- and LPS-Induced Septic Animal Models Developed Cardiac Dysfunction
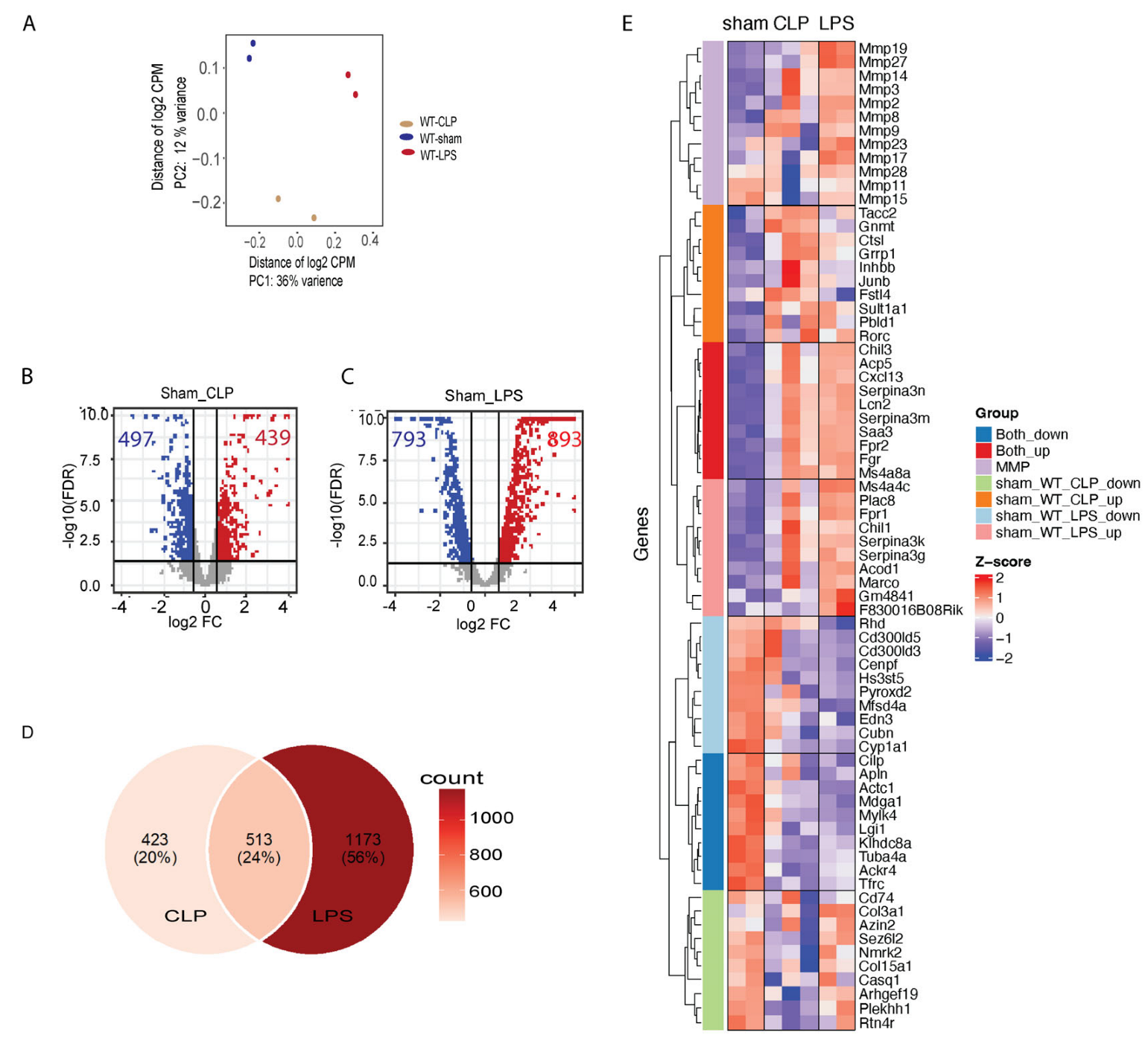
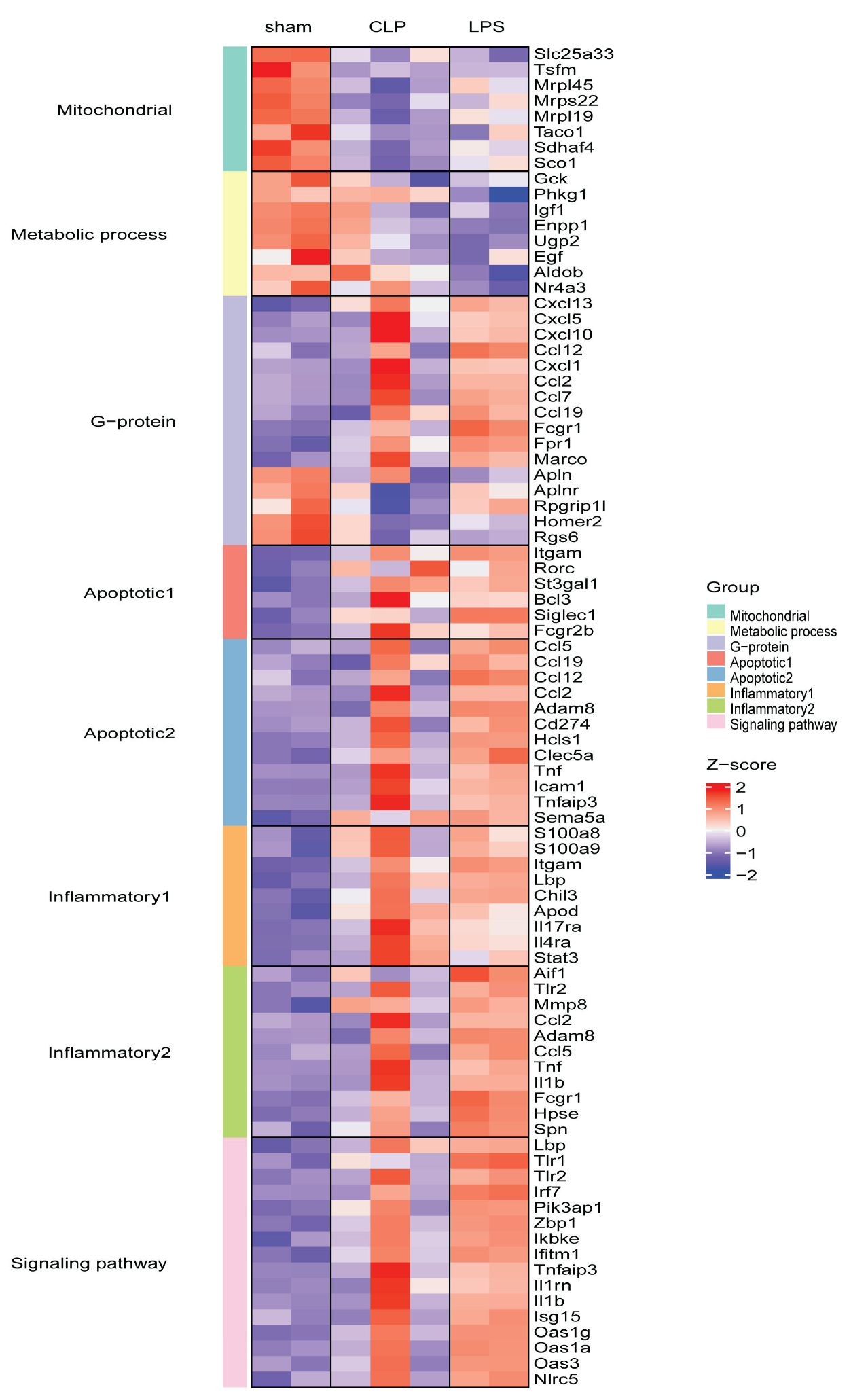
3.2. Transcriptome Changes in Mouse Hearts following Sepsis Induction
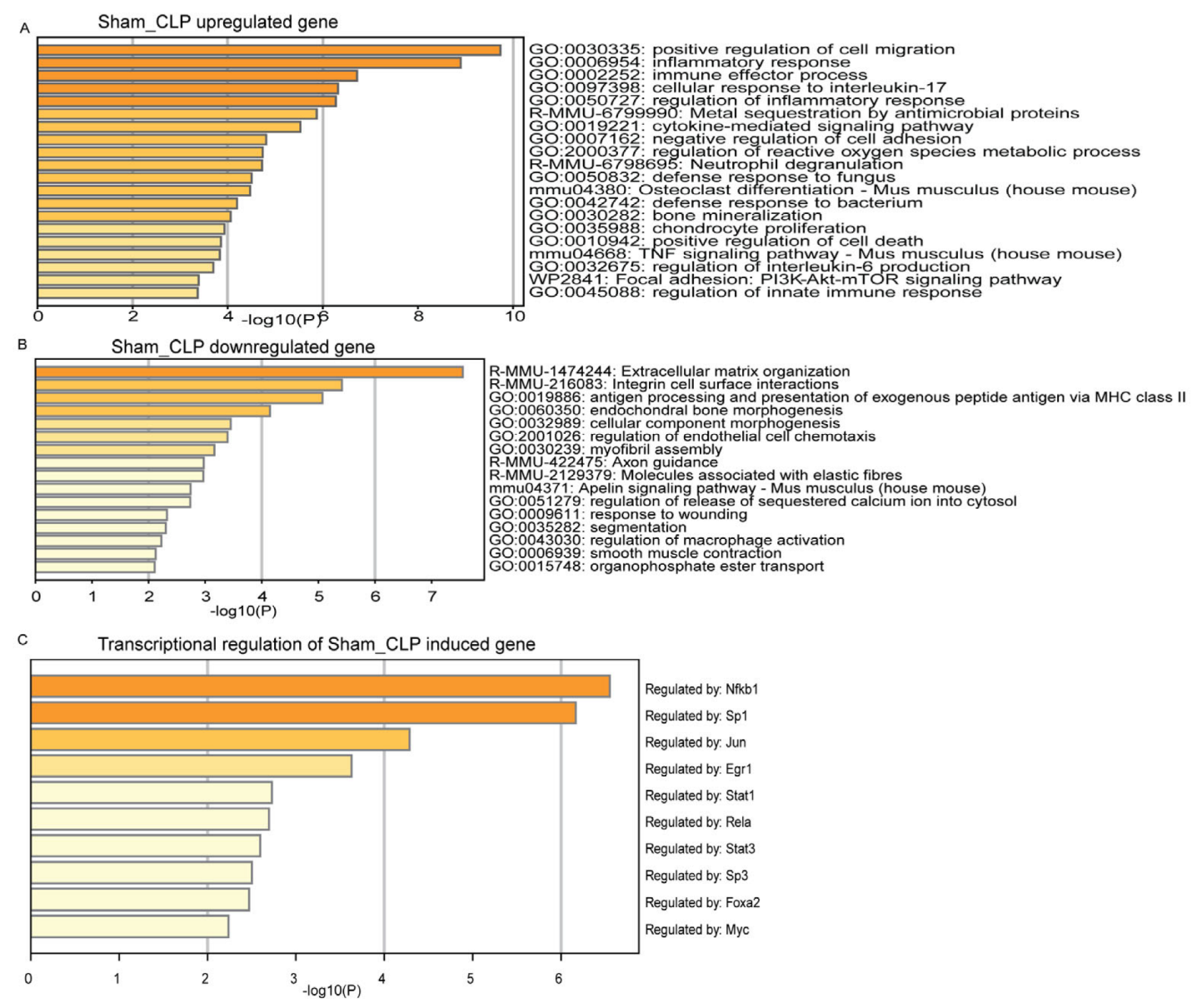
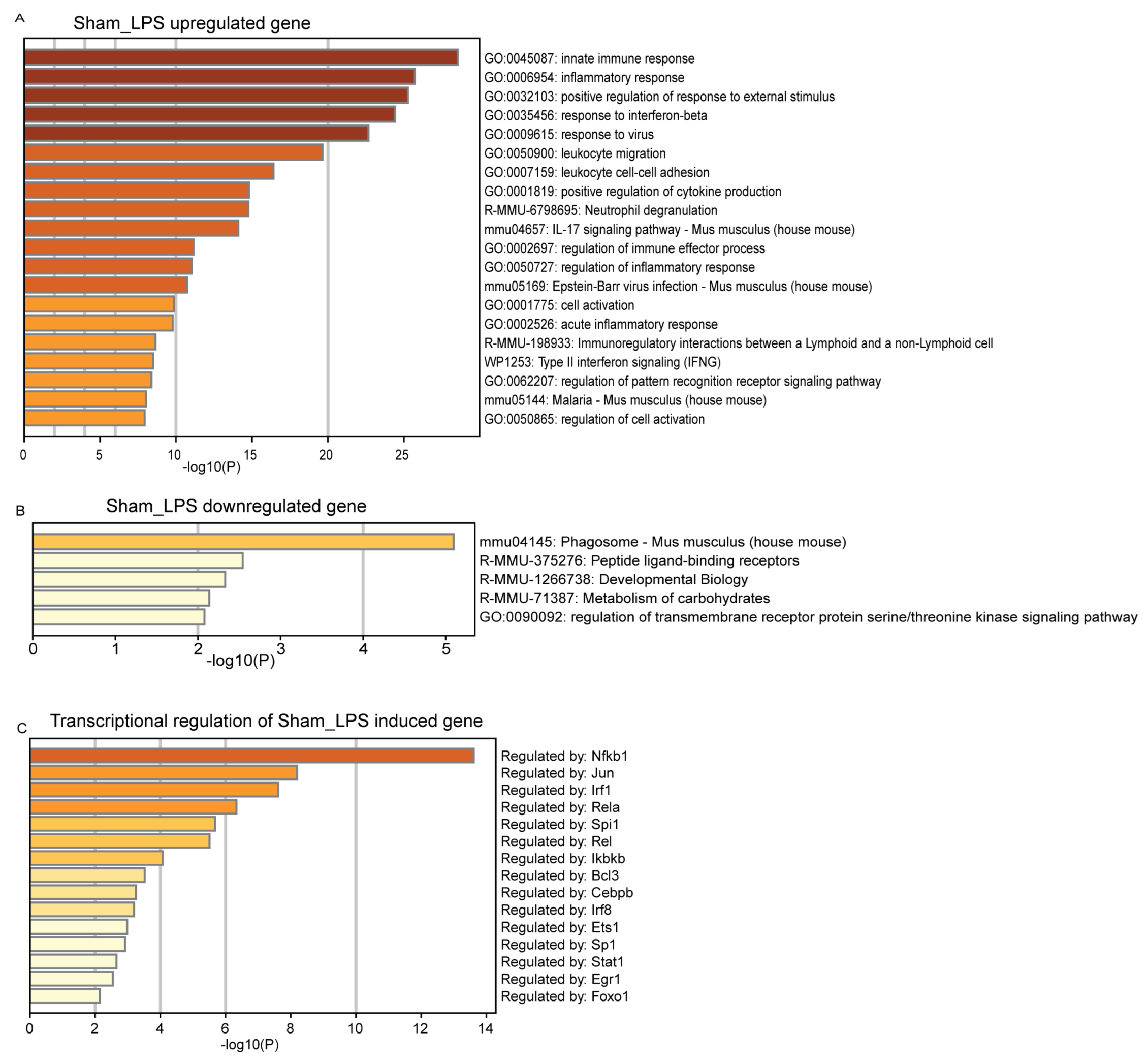
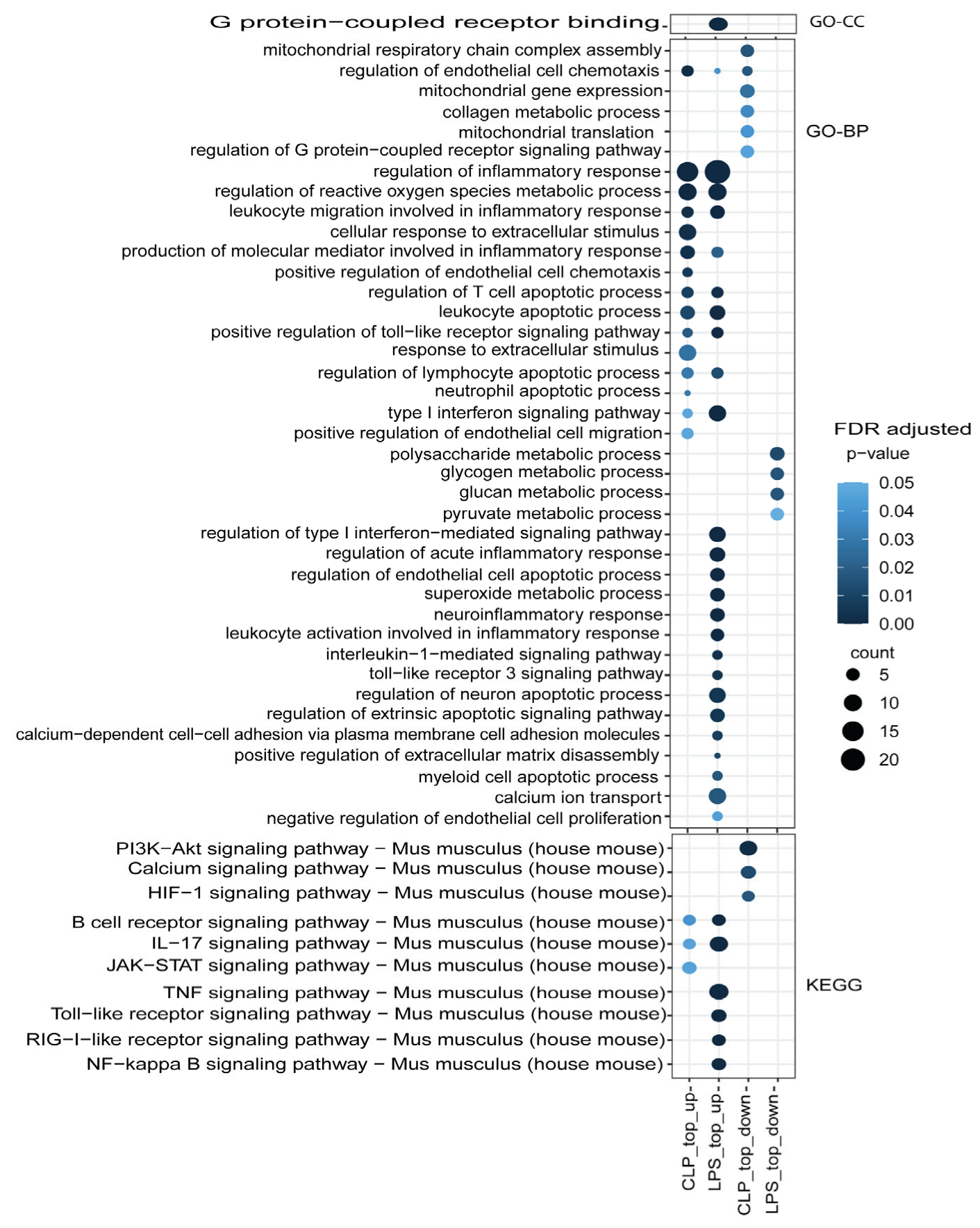
3.3. Functional Mechanism Comparison between CLP- and LPS-Induced Sepsis
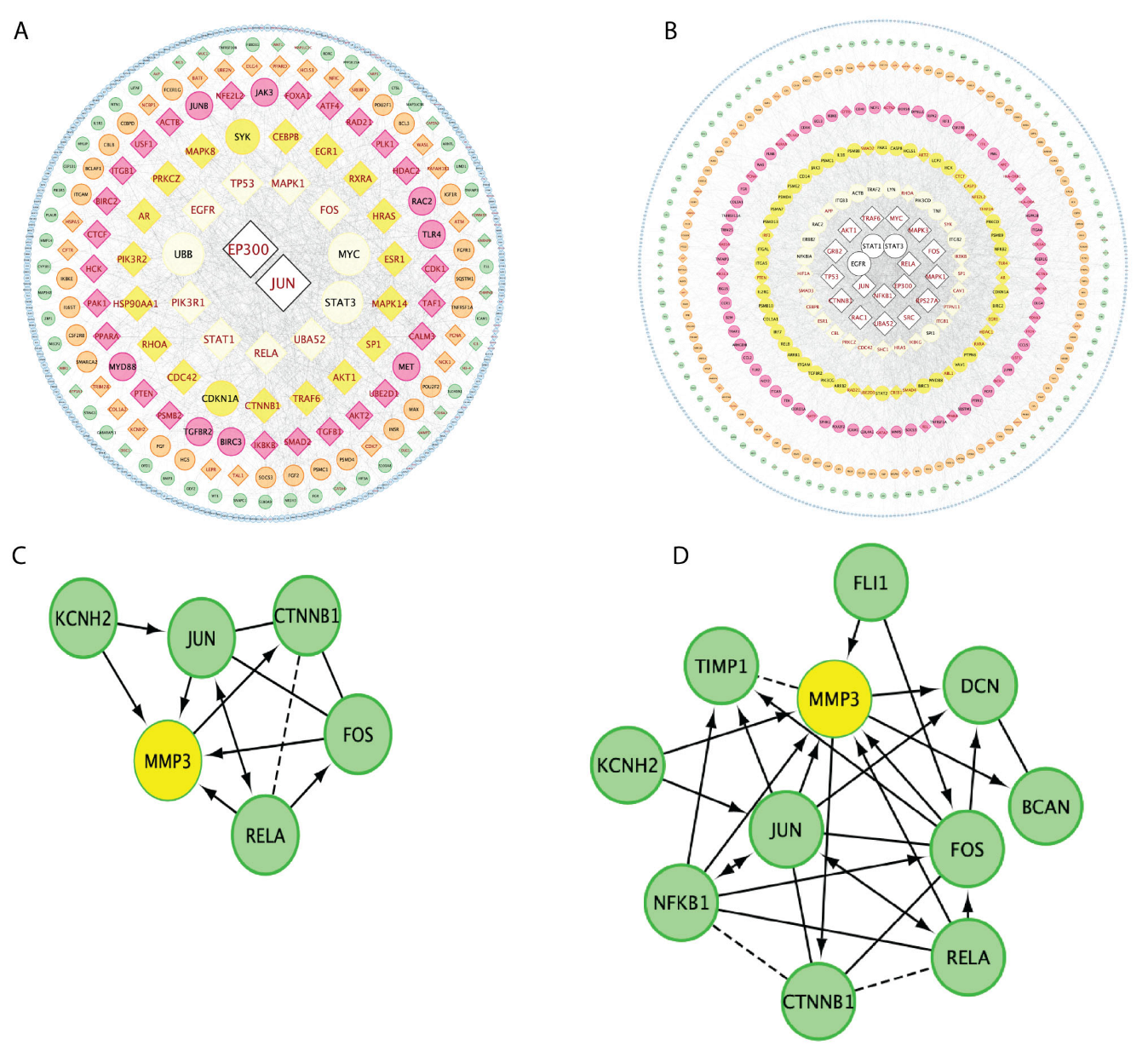
3.4. Gene Regulation Network Comparisons between CLP and LPS Sepsis Inductions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Poveda-Jaramillo, R. Heart Dysfunction in Sepsis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 298–309. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, C.; Bai, J.; Du, X. Macrophage SAMSN1 protects against sepsis-induced acute lung injury in mice. Redox Biol. 2022, 56, 102432. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, H.; Li, X.; Leng, B. Effects of JAK2/STAT3 signaling pathway activation on sepsis-induced kidney injury. Minerva Med. 2022, 113, 350–352. [Google Scholar] [CrossRef] [PubMed]
- de Padua Lucio, K.; Rabelo, A.C.S.; Araujo, C.M.; Brandao, G.C.; de Souza, G.H.B.; da Silva, R.G.; de Souza, D.M.S.; Talvani, A.; Bezerra, F.S.; Cruz Calsavara, A.J.; et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 5048031. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Vardon-Bounes, F.; Merlet-Dupuy, V.; Conil, J.M.; Buleon, M.; Fourcade, O.; Tack, I.; Minville, V. Sepsis modeling in mice: Ligation length is a major severity factor in cecal ligation and puncture. Intensive Care Med. Exp. 2016, 4, 22. [Google Scholar] [CrossRef]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Drosatos, K.; Lymperopoulos, A.; Kennel, P.J.; Pollak, N.; Schulze, P.C.; Goldberg, I.J. Pathophysiology of sepsis-related cardiac dysfunction: Driven by inflammation, energy mismanagement, or both? Curr. Heart Fail. Rep. 2015, 12, 130–140. [Google Scholar] [CrossRef]
- Merdji, H.; Schini-Kerth, V.; Meziani, F.; Toti, F. Long-term cardiovascular complications following sepsis: Is senescence the missing link? Ann. Intensive Care 2021, 11, 166. [Google Scholar] [CrossRef]
- Ren, J.; Wu, S. A burning issue: Do sepsis and systemic inflammatory response syndrome (SIRS) directly contribute to cardiac dysfunction? Front. Biosci. 2006, 11, 15–22. [Google Scholar] [CrossRef]
- Hunter, J.D.; Doddi, M. Sepsis and the heart. Br. J. Anaesth. 2010, 104, 3–11. [Google Scholar] [CrossRef]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Pittman, J.E.; Hirshberg, E.L.; Jones, J.P.; Lanspa, M.J.; Kuttler, K.G.; Litwin, S.E.; Grissom, C.K. Diastolic dysfunction and mortality in early severe sepsis and septic shock: A prospective, observational echocardiography study. Crit. Ultrasound J. 2012, 4, 8. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O.; Dogo, D.; Nikos, E.M. Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases. Inflammopharmacology 2023, 31, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Duburcq, T.; Dekeyser, T.; Neviere, R.; Howsam, M.; Favory, R.; Preau, S. Involvement of Mitochondrial Disorders in Septic Cardiomyopathy. Oxid. Med. Cell. Longev. 2017, 2017, 4076348. [Google Scholar] [CrossRef]
- D’Elia, J.A.; Weinrauch, L.A. Calcium Ion Channels: Roles in Infection and Sepsis Mechanisms of Calcium Channel Blocker Benefits in Immunocompromised Patients at Risk for Infection. Int. J. Mol. Sci. 2018, 19, 2465. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Keskey, R.; Thewissen, R. Can the Cecal Ligation and Puncture Model Be Repurposed To Better Inform Therapy in Human Sepsis? Infect. Immun. 2020, 88, e00942-19. [Google Scholar] [CrossRef]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef]
- Wu, R.; Chang, H.C.; Khechaduri, A.; Chawla, K.; Tran, M.; Chai, X.; Wagg, C.; Ghanefar, M.; Jiang, X.; Bayeva, M.; et al. Cardiac-specific ablation of ARNT leads to lipotoxicity and cardiomyopathy. J. Clin. Investig. 2014, 124, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wyatt, E.; Chawla, K.; Tran, M.; Ghanefar, M.; Laakso, M.; Epting, C.L.; Ardehali, H. Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Mol. Med. 2012, 4, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 May 2023).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. iMeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wu, G.; Dawson, E.; Duong, A.; Haw, R.; Stein, L. ReactomeFIViz: A Cytoscape app for pathway and network-based data analysis. F1000Research 2014, 3, 146. [Google Scholar]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.F.; Orekhov, A.N. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, J.M.; Lee, J.; Kim, S.K.; Lee, T. Positive Role of Delta Neutrophil Index (DNI) as a Prodiagnostic Marker in Cecal Ligation and Puncture (CLP)-Induced Sepsis Murine Model. Medicina 2022, 58, 369. [Google Scholar] [CrossRef] [PubMed]
- Anter, A.; Ahmed, A.F.; Hammad, A.S.A.; Almalki, W.H.; Abdel Hafez, S.M.N.; Kasem, A.W.; El-Moselhy, M.A.; Alrabia, M.W.; Ibrahim, A.R.N.; El-Daly, M. Corrigendum: The severity of acute kidney and lung injuries induced by cecal ligation and puncture is attenuated by menthol: Role of proliferating cell nuclear antigen and apoptotic markers. Front. Med. 2022, 9, 1024554. [Google Scholar] [CrossRef]
- Siempos, I.I.; Lam, H.C.; Ding, Y.; Choi, M.E.; Choi, A.M.; Ryter, S.W. Cecal ligation and puncture-induced sepsis as a model to study autophagy in mice. J. Vis. Exp. 2014, e51066. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, S.J.; Kim, J.E.; Kang, M.J.; Bae, S.J.; Choi, Y.J.; Gong, J.E.; Kim, K.S.; Jung, Y.S.; Cho, J.Y.; et al. Comparison of response to LPS-induced sepsis in three DBA/2 stocks derived from different sources. Lab. Anim. Res. 2021, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Harashima, A.; Saito, H.; Tsuneyama, K.; Munesue, S.; Motoyoshi, S.; Han, D.; Watanabe, T.; Asano, M.; Takasawa, S.; et al. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J. Immunol. 2011, 186, 3248–3257. [Google Scholar] [CrossRef]
- Backes, Y.; van der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef]
- Brenner, T.; Rosenhagen, C.; Steppan, J.; Lichtenstern, C.; Weitz, J.; Bruckner, T.; Martin, E.O.; Hoffmann, U.; Weigand, M.A.; Hofer, S. Redox responses in patients with sepsis: High correlation of thioredoxin-1 and macrophage migration inhibitory factor plasma levels. Mediat. Inflamm. 2010, 2010, 985614. [Google Scholar] [CrossRef]
- Vaschetto, R.; Nicola, S.; Olivieri, C.; Boggio, E.; Piccolella, F.; Mesturini, R.; Damnotti, F.; Colombo, D.; Navalesi, P.; Della Corte, F.; et al. Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 2008, 34, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, M.; Sader, S. Matrix metalloproteinases in cardiovascular disease. Can. J. Cardiol. 2006, 22 (Suppl. B), 25B–30B. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.R.; Jacob, A.; Zhou, M.; Wang, P. Modulation of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in sepsis. Int. J. Clin. Exp. Med. 2010, 3, 180–185. [Google Scholar]
- Chen, C.; Weng, J.; Fang, D.; Chen, J.; Chen, M. Transcriptomic study of lipopolysaccharide-induced sepsis damage in a mouse heart model. Exp. Ther. Med. 2020, 20, 3782–3790. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.L.; Han, X.; Li, P.B.; Guo, S.B.; Li, H.H. Time Series Transcriptomic Analysis by RNA Sequencing Reveals a Key Role of PI3K in Sepsis-Induced Myocardial Injury in Mice. Front. Physiol. 2022, 13, 903164. [Google Scholar] [CrossRef]
- Langston, J.C.; Rossi, M.T.; Yang, Q.; Ohley, W.; Perez, E.; Kilpatrick, L.E.; Prabhakarpandian, B.; Kiani, M.F. Omics of endothelial cell dysfunction in sepsis. Vasc. Biol. 2022, 4, R15–R34. [Google Scholar] [CrossRef]
- Kurmann, L.; Okoniewski, M.; Ogunshola, O.O.; Leeners, B.; Imthurn, B.; Dubey, R.K. Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function. Cells 2021, 10, 963. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Zhou, T.; Xu, Y.; Zhan, J.; Wu, J. CXCL13 Is Involved in the Lipopolysaccharide-Induced Hyperpermeability of Umbilical Vein Endothelial Cells. Inflammation 2020, 43, 1789–1796. [Google Scholar] [CrossRef]
- Schiffer, L.; Kielstein, J.T.; Haubitz, M.; Luhrs, H.; Witte, T.; Haller, H.; Kumpers, P.; Schiffer, M. Elevation of serum CXCL13 in SLE as well as in sepsis. Lupus 2011, 20, 507–511. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, K.; Li, Y.; Lin, Q.; Pan, K.; Nguyen, T.; Aniruddhsingh, S.; Su, Q.; Sharp, W.; Wu, R. Comparative Analysis of Whole Transcriptome Profiles in Septic Cardiomyopathy: Insights from CLP- and LPS-Induced Mouse Models. Genes 2023, 14, 1366. https://doi.org/10.3390/genes14071366
Ullah K, Li Y, Lin Q, Pan K, Nguyen T, Aniruddhsingh S, Su Q, Sharp W, Wu R. Comparative Analysis of Whole Transcriptome Profiles in Septic Cardiomyopathy: Insights from CLP- and LPS-Induced Mouse Models. Genes. 2023; 14(7):1366. https://doi.org/10.3390/genes14071366
Chicago/Turabian StyleUllah, Karim, Yan Li, Qiaoshan Lin, Kaichao Pan, Tu Nguyen, Solanki Aniruddhsingh, Qiaozhu Su, Willard Sharp, and Rongxue Wu. 2023. "Comparative Analysis of Whole Transcriptome Profiles in Septic Cardiomyopathy: Insights from CLP- and LPS-Induced Mouse Models" Genes 14, no. 7: 1366. https://doi.org/10.3390/genes14071366
APA StyleUllah, K., Li, Y., Lin, Q., Pan, K., Nguyen, T., Aniruddhsingh, S., Su, Q., Sharp, W., & Wu, R. (2023). Comparative Analysis of Whole Transcriptome Profiles in Septic Cardiomyopathy: Insights from CLP- and LPS-Induced Mouse Models. Genes, 14(7), 1366. https://doi.org/10.3390/genes14071366






