SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment of cGGM Patients
2.2. Identification of SLC5A1 Mutations
2.3. Antibodies and Reagents
2.4. Construction of cDNA Clones
2.5. Cell Culture
2.6. Stable Expression of WT and Mutant SLC5A1 Alleles in CaCo-2 Cells
2.7. Transient Expression of WT and Mutant SLC5A1 Alleles in HEK 293T Cells
2.8. Detergent Fractionation and Immunoblotting
2.9. Deglycosylation of Protein Extracts
2.10. Immunofluorescence and Imaging
2.11. Human SGLT1 Protein Structure Modeling
3. Results
3.1. Clinical Findings
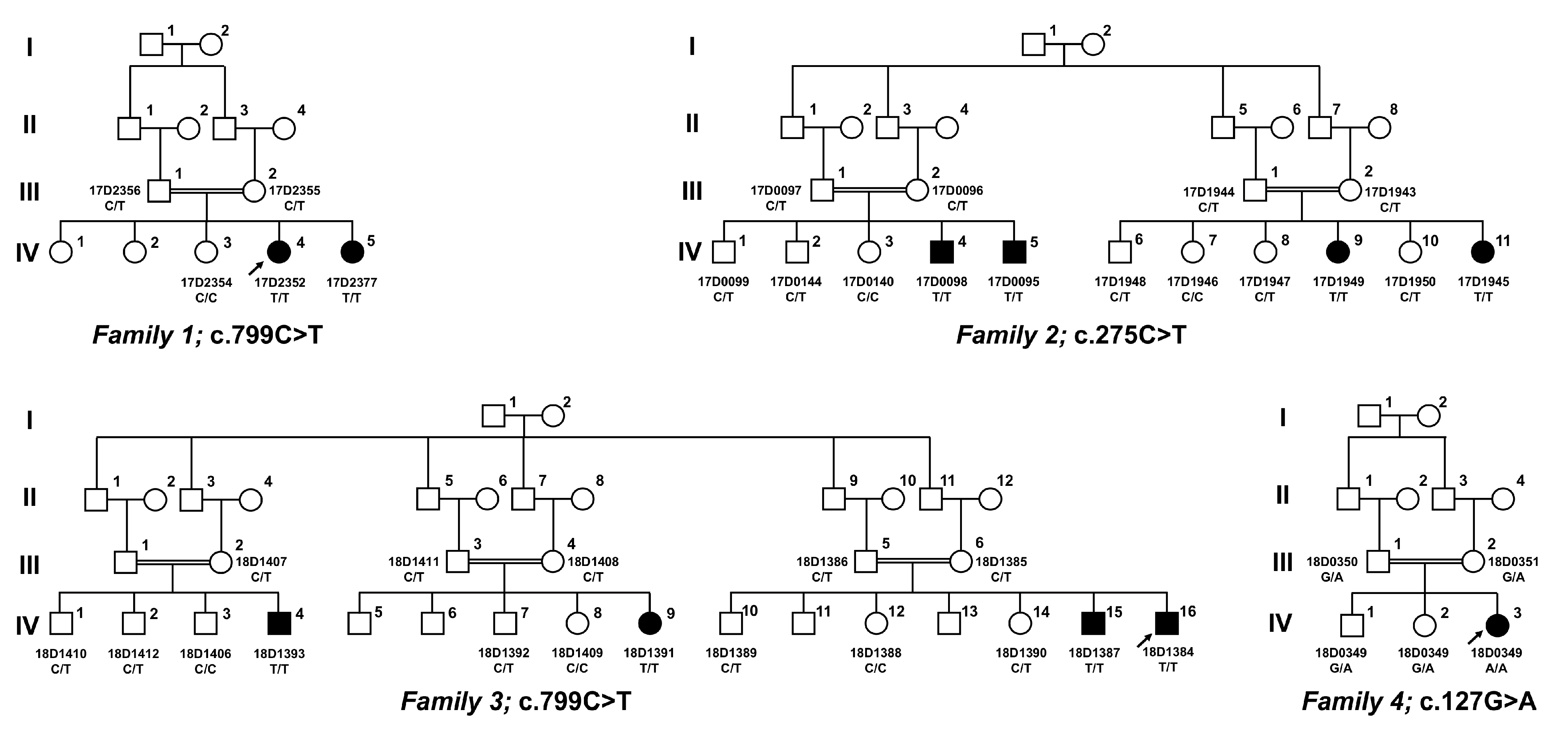
3.2. Identification of Pathogenic and Likely Pathogenic SLC5A1 Variants
- (1)
- p.Arg267* has a very low allele frequency, and p.Ala92Val and p.Gly43Arg are absent from the population database gnomAD, discounting them to represent frequent sequence variants;
- (2)
- All three variants segregate with disease in each family;
- (3)
- p.Arg267* causes an early, premature stop codon, predicting abrogation of protein production; this variant has been reported once, in a homozygous state, in a Turkish patient with cGGM [17];
- (4)
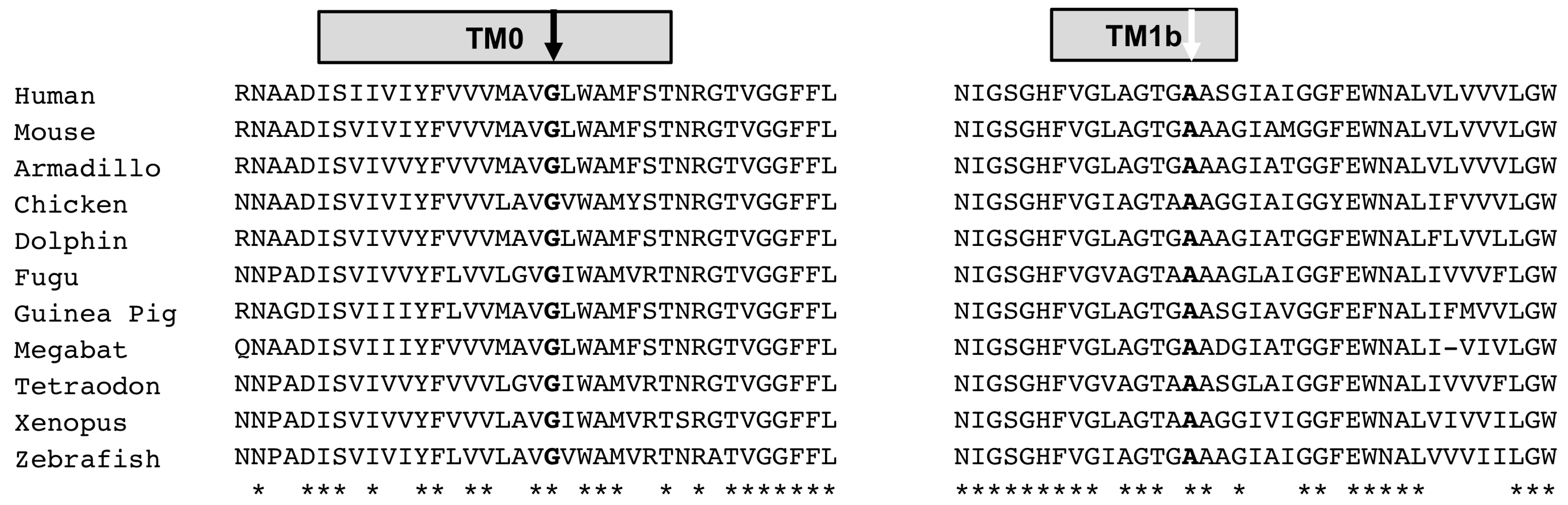
- The novel SLC5A1 variant c.275C>T (p.Ala92Val) was found in the index patient from family two in a homozygous state, and it resided within a large homozygous region of 23 Mb on chromosome 22q12.3, indicating inheritance by identity by descent and providing evidence for its pathogenicity. This novel variant replaces a highly conserved amino acid (Figure 2), and multiple lines of in silico prediction analyses supported its pathogenicity (PolyPhen2 score: 1.00, CADD score: 19, 76, SIFT score: 0.02);
- (5)
- The novel SLC5A1 variant c.127G>A (p.Gly43Arg) was found in the index in family four in the homozygous state, and it resided within a large homozygous region of 17 Mb on chromosome 22q12.3, indicating inheritance by identity by descent and providing evidence for its pathogenicity. This novel variant replaces a nearly invariantly conserved amino acid (Figure 2), and multiple lines of in silico prediction analyses supported its pathogenicity (PolyPhen2 score: 0.998, CADD score: 22, SIFT score: 0); whole-genome sequencing was performed, and did not detect potentially disease-causing SLC5A1 variants in cis;
- (6)
- Moreover, WES did not identify other pathogenic variants related to this disease in other genes in families one, two, and four.
3.3. Modeling SLC5A1 Variant p.Ala92Val Provides Evidence for Its Pathogenicity
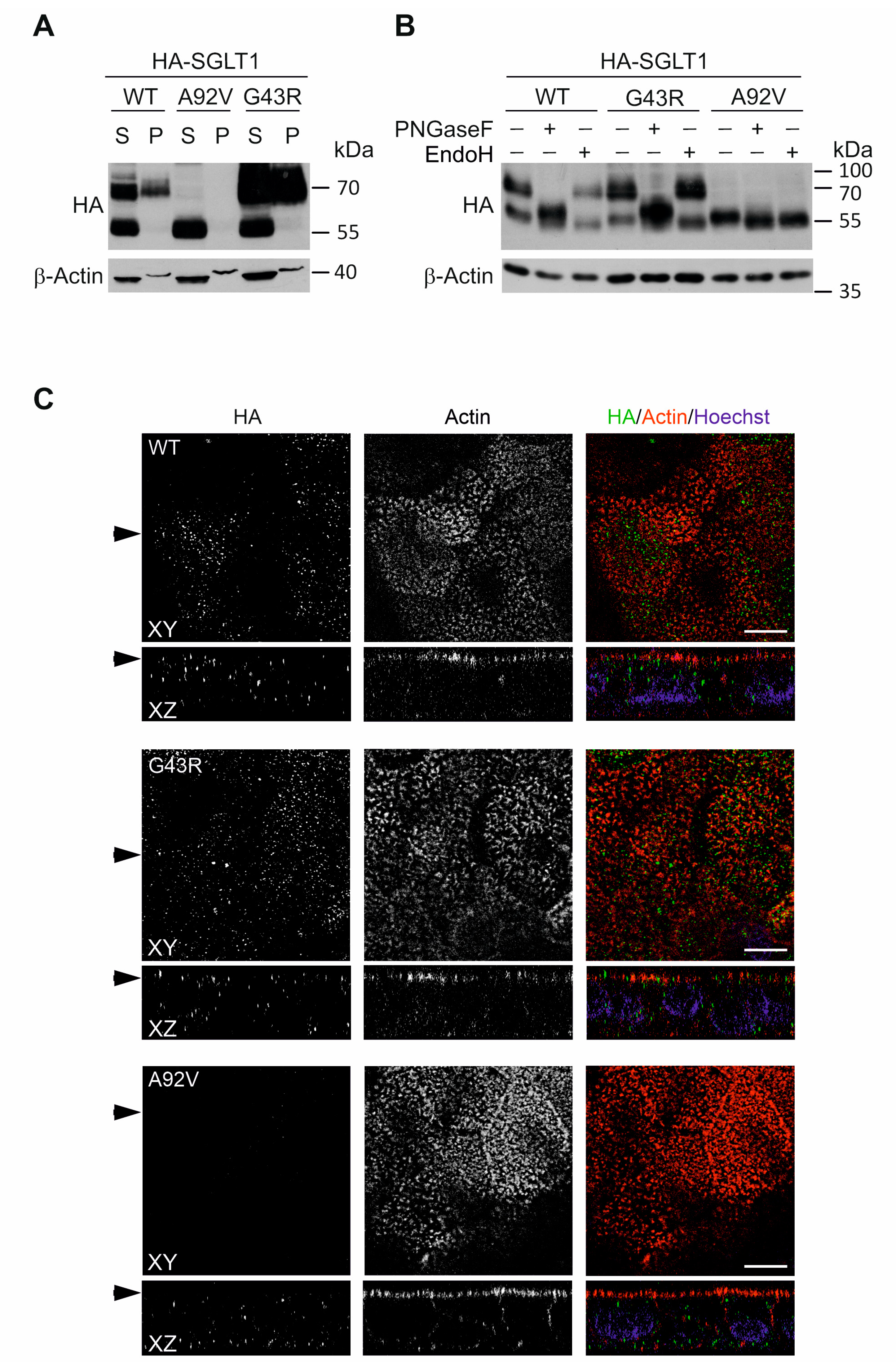
3.4. Transient and Stable Expression of Novel SLC5A1 Variants in HEK293T and CaCo-2 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Ma, M. Literature review on congenital glucose–galactose malabsorption from 2001 to 2019. J. Paediatr. Child Health 2020, 56, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.M.A.; El-Mouzan, M.I.; Shiekh, O.K.E.; Mazyad, A.A. Congenital glucose-galactose malabsorption in Arab children. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Zabel, B.; Mundlos, S.; Dyer, J.; Wright, E.M. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991, 350, 354–356. [Google Scholar] [CrossRef]
- Lostao, M.P.; Loo, D.D.; Hernell, O.; Meeuwisse, G.; Martin, M.G.; Wright, E.M. The Molecular Basis of Glucose Galactose Malabsorption in a Large Swedish Pedigree. Function 2021, 2, zqab040. [Google Scholar] [CrossRef]
- Han, L.; Qu, Q.; Aydin, D.; Panova, O.; Robertson, M.J.; Xu, Y.; Dror, R.O.; Skiniotis, G.; Feng, L. Structure and mechanism of the SGLT family of glucose transporters. Nature 2022, 601, 274–279. [Google Scholar] [CrossRef]
- Al-Suyufi, Y.; ALSaleem, K.; Al-Mehaidib, A.; Banemai, M.; Aldekhail, W.M.; Al-Muhandes, A.; Mohammed, M.; Allam, R.; Jambi, A.; Ramzan, K.; et al. SLC5A1 Mutations in Saudi Arabian Patients with Congenital Glucose-Galactose Malabsorption. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 250–252. [Google Scholar] [CrossRef]
- Chan, A.P.; Namjoshi, S.S.; Jardack, P.M.; Maloney, L.; Ardjmand, A.; Jackson, N.N.; Martin, M.G. Long-Term Dietary Changes in Subjects with Glucose Galactose Malabsorption Secondary to Biallelic Mutations of SLC5A1. Dig. Dis. Sci. 2021, 66, 4414–4422. [Google Scholar] [CrossRef]
- Saadah, O.I.; Alghamdi, S.A.; Sindi, H.H.; Alhunaitti, H.; Bin-Taleb, Y.Y.; Alhussaini, B.H. Congenital glucose–galactose malabsorption: A descriptive study of clinical characteristics and outcome from Western Saudi Arabia. Arab J. Gastroenterol. 2014, 15, 21–23. [Google Scholar] [CrossRef]
- Atay, F.Y.; Derme, T.; Uras, N.; Ceylaner, G.; Ceylaner, S.; Sari, F.N.; Oguz, S.S. Congenital Glucose–Galactose Malabsorption in a Turkish Newborn: A Novel Mutation of Na+/Glucose Cotransporter Gene. Dig. Dis. Sci. 2017, 62, 280–281. [Google Scholar] [CrossRef]
- Zufferey, R.; Dull, T.; Mandel, R.J.; Bukovsky, A.; Quiroz, D.; Naldini, L.; Trono, D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998, 72, 9873–9880. [Google Scholar] [CrossRef] [PubMed]
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris zofingiensis Targeting TNFR/NF-κB Signaling at Different Levels. Cells 2022, 11, 1407. [Google Scholar] [CrossRef]
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef]
- Jubb, H.C.; Higueruelo, A.P.; Ochoa-Montano, B.; Pitt, W.R.; Ascher, D.B.; Blundell, T.L. Arpeggio: A Web Server for Calculating and Visualising Interatomic Interactions in Protein Structures. J. Mol. Biol. 2017, 429, 365–371. [Google Scholar] [CrossRef]
- Frappier, V.; Najmanovich, R.J. A coarse-grained elastic network atom contact model and its use in the simulation of protein dynamics and the prediction of the effect of mutations. PLoS Comput. Biol. 2014, 10, e1003569. [Google Scholar] [CrossRef] [PubMed]
- Akduman, H.; Dilli, D.; Ceylaner, S. A Case of Congenital Glucose Galactose Malabsorption with a New Mutation in the SLC5A1 Gene. J. Pediatr. Genet. 2022, 11, 317–319. [Google Scholar] [CrossRef]
- Soylu, O.B.; Ecevit, C.; Altinoz, S.; Ozturk, A.A.; Temizkan, A.K.; Maeda, M.; Kasahara, M. Nephrocalcinosis in glucose-galactose malabsorption: Nephrocalcinosis and proximal tubular dysfunction in a young infant with a novel mutation of SGLT1. Eur. J. Pediatr. 2008, 167, 1395–1398. [Google Scholar] [CrossRef]
- Kipp, H.; Khoursandi, S.; Scharlau, D.; Kinne, R.K.H. More than apical: Distribution of SGLT1 in Caco-2 cells. Am. J. Physiol. Cell Physiol. 2003, 285, C737–C749. [Google Scholar] [CrossRef]
- Babcock, S.J.; Flores-Marin, D.; Thiagarajah, J.R. The genetics of monogenic intestinal epithelial disorders. Hum. Genet. 2022, 142, 613–654. [Google Scholar] [CrossRef]
- Posovszky, C. Congenital intestinal diarrhoeal diseases: A diagnostic and therapeutic challenge. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 187–211. [Google Scholar] [CrossRef]
- Lebenthal, E.; Garti, R.; Mathoth, Y.; Cohen, B.E.; Katzenelson, D. Glucose-galactose malabsorption in an Oriental-Iraqui Jewish family. J. Pediatr. 1971, 78, 844–850. [Google Scholar] [CrossRef]
- Xin, B.; Wang, H. Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin. Genet. 2011, 79, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.T.; Martı́n, M.G.; Turk, E.; Hirayama, B.A.; Bosshard, N.U.; Steinmann, B.; Wright, E.M. Missense mutations in SGLT1 cause glucose–galactose malabsorption by trafficking defects. Biochim. Biophys. Acta 1999, 1453, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Matsuzaki, T.; Hagiwara, H.; Aoki, T.; Tajika-Takahashi, Y.; Takata, K. Apical localization of sodium-dependent glucose transporter SGLT1 is maintained by cholesterol and microtubules. Acta Histochem. Cytochem. 2006, 39, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Calmettes, G.; Morand, P.; Ribalet, B.; John, S. Real-time imaging of sodium glucose transporter (SGLT1) trafficking and activity in single cells. Physiol. Rep. 2017, 5, e13062. [Google Scholar] [CrossRef]
- Ridsdale, A.; Denis, M.; Gougeon, P.-Y.; Ngsee, J.K.; Presley, J.F.; Zha, X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol. Biol. Cell 2006, 17, 1593–1605. [Google Scholar] [CrossRef]
- Alamoudi, L.O.; Alfaraidi, A.T.; Althagafi, S.S.; Al-Thaqafy, M.S.; Hasosah, M. Congenital Glucose-Galactose Malabsorption: A Case with a Novel SLC5A1 Mutation in a Saudi Infant. Cureus 2021, 13, e18440. [Google Scholar] [CrossRef]


| Patient | Gender | Age at Onset (d) | Current Age (y) | Birth Weight (gr) | Weight (kg) (SD) | Height (cm) (SD) | WBC ×103/µL | Hb g/dL | Platelet ×103/µL | BUN mg/dL | Creatinine mg/dL | Na mEq/L | K mEq/L | CI mEq/L | HCO3 mmol/L | Stool Reducing Substances (Grade 1 to 4 Positive) | Clinical Assessment of Dehydration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 3 | 10 | 3000 | 23.5 (−1.41) | 129 (−0.87) | 13.8 | 12.1 | 314 | 49 | 0.76 | 151 | 3.0 | 129 | 7.02 | + | Severe |
| 2 | F | 4 | 7 | 3200 | 13.4 (−4.31) | 98 (−5.26) | 12.6 | 15.3 | 239 | 42 | 0.85 | 149 | 3.2 | 117 | 7.17 | + | Severe |
| 3 | M | 15 | 9 | 3000 | 22.5 (−1.64) | 128 (−0.75) | 14.14 | 12.9 | 524 | 51 | 0.47 | 148 | 2.78 | 103.6 | 7.1 | + | Moderate |
| 4 | M | 6 | 4 | 3500 | 14.5 (−0.91) | 101 (−0.25) | 13.650 | 8.5 | 572 | 39 | 0.47 | 147 | 117 | 22 | + | Moderate | |
| 5 | M | 9 | 10 | 3000 | 29 (−0.44) | 132 (−0.42) | 12.8 | 19 | 260 | 329 | 2.06 | 176 | 4.37 | 151 | 12.2 | + | Severe |
| 6 | M | 11 | 8 | 3400 | 19 (−1.95) | 115 (−2.44) | 30.64 | 20.9 | 194 | 344 | 5.37 | 196 | 8.13 | 166 | 4.3 | + | Severe |
| 7 | M | 3 | 4 | 3800 | 19 (−1.95) | 115 (−2.44) | 12.92 | 20.2 | 353 | 65 | 1.42 | 166 | 3.87 | 138 | 13.4 | ++++ | Severe |
| 8 | F | 2 | 3 | 3060 | 12 (−0.88) | 82 (−2.65) | 20.6 | 19.8 | 213 | 38 | 1.46 | 186 | 8.26 | 144 | 11.4 | +++ | Severe |
| Family ID Affected Individual(s) | Variant (SLC5A1, NM_000343.3) | Location | Zygosity | GnomAD (v.2.1.1) | Long Contiguous Stretches of Homozygosity |
|---|---|---|---|---|---|
| 1 2 | c.799C>T p.Arg267* + | Exon 8 | Homozygous | Not observed in homozygous state ** | 23.5 Mb on 22q12.3 |
| 2 4 | c.275C>T p.Ala92Val | Exon 3 | Homozygous | Not observed | 23 Mb on 22q12.3 |
| 3 4 | c.799C>T p.Arg267* + | Exon 8 | Homozygous | Not observed in homozygous state ** | n.d. |
| 4 1 | c.127G>A p.Gly43Arg | Exon 1 | Homozygous | Not observed | 17 Mb on 22q |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoşnut, F.Ö.; Janecke, A.R.; Şahin, G.; Vogel, G.F.; Lafcı, N.G.; Bichler, P.; Müller, T.; Huber, L.A.; Valovka, T.; Aksu, A.Ü. SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Genes 2023, 14, 1359. https://doi.org/10.3390/genes14071359
Hoşnut FÖ, Janecke AR, Şahin G, Vogel GF, Lafcı NG, Bichler P, Müller T, Huber LA, Valovka T, Aksu AÜ. SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Genes. 2023; 14(7):1359. https://doi.org/10.3390/genes14071359
Chicago/Turabian StyleHoşnut, Ferda Ö., Andreas R. Janecke, Gülseren Şahin, Georg F. Vogel, Naz G. Lafcı, Paul Bichler, Thomas Müller, Lukas A. Huber, Taras Valovka, and Aysel Ü. Aksu. 2023. "SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption" Genes 14, no. 7: 1359. https://doi.org/10.3390/genes14071359
APA StyleHoşnut, F. Ö., Janecke, A. R., Şahin, G., Vogel, G. F., Lafcı, N. G., Bichler, P., Müller, T., Huber, L. A., Valovka, T., & Aksu, A. Ü. (2023). SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Genes, 14(7), 1359. https://doi.org/10.3390/genes14071359









