Ancient Mitochondrial Genomes Provide New Clues to the Origin of Domestic Cattle in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Archaeological Background and Samples
2.2. DNA Extraction, Library Construction and High-Throughput Sequencing
2.3. Data Processing
3. Results
3.1. Sequencing Results of Ancient DNA Sequences
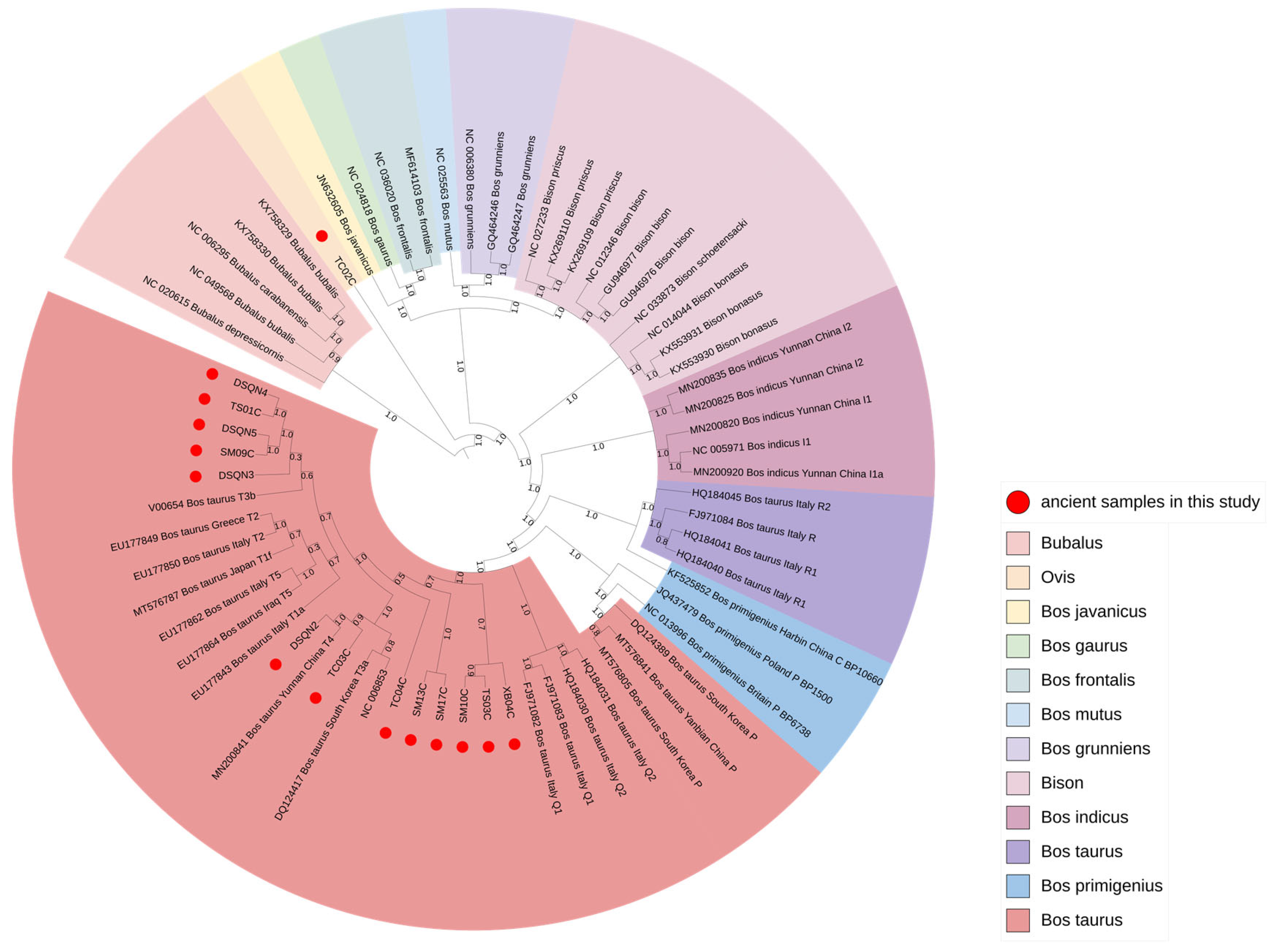
3.2. Bayesian Tree Analysis Based on Mitogenomes
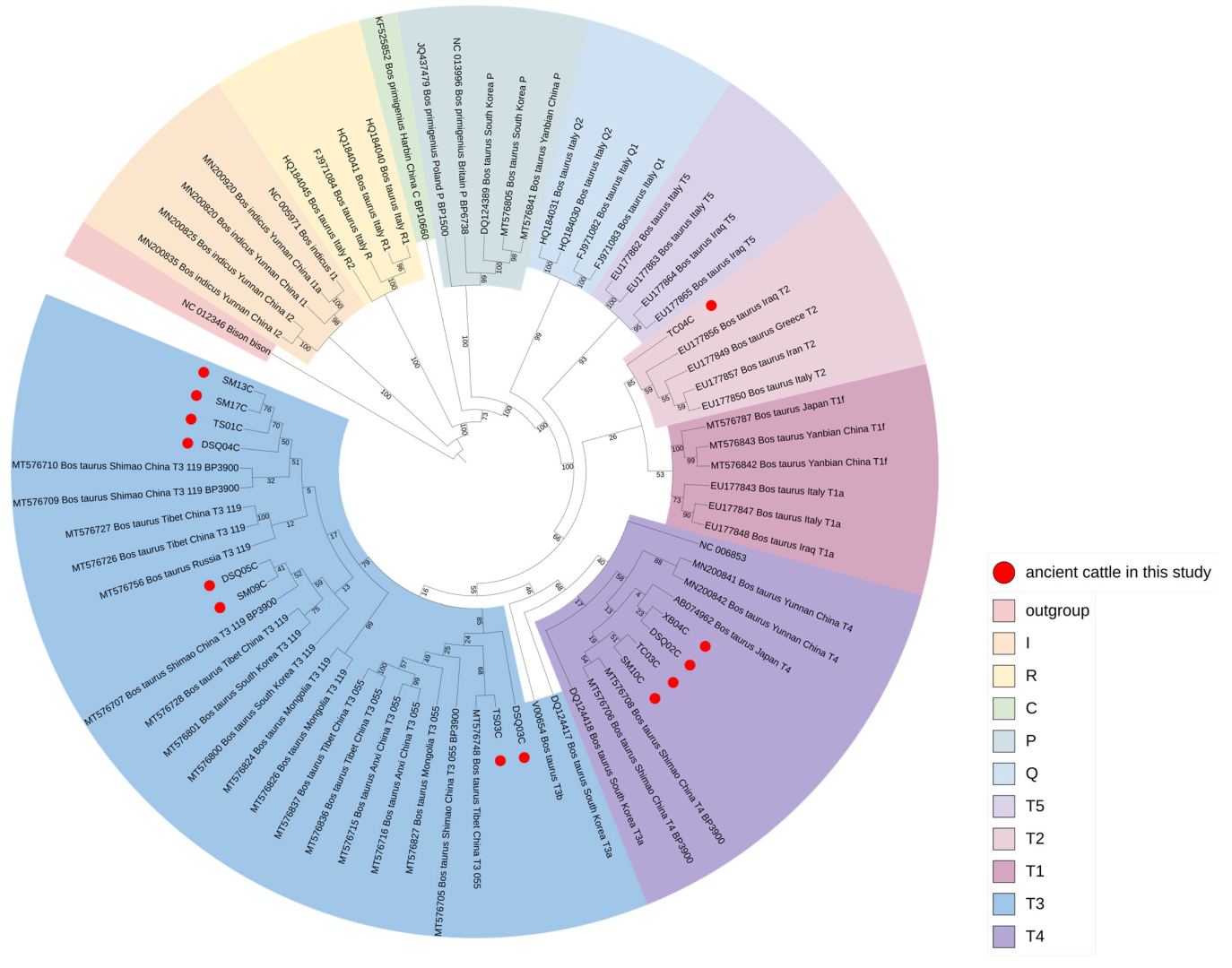
3.3. Maximum Likelihood Tree Analysis Based on Mitogenomes
3.4. TempNet Analysis Based on Mitochondrial Control Regions
3.5. TCS Network Analysis Based on Mitogenomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmer, D.; Gourichon, L.; Monchot, H.; Peters, J.; Segui, S.M. Identifying Early Domestic Cattle from Pre-Pottery Neolithic Sites on the Middle Euphrates Using Sexual Dimorphism. In The First Steps of Animal Domestication: New Archaeozoological Approaches; Oxbow Books: Oxford, UK, 2005; pp. 86–95. ISBN 978-1-84217-121-9. [Google Scholar]
- Ajmone-Marsan, P.; Garcia, J.F.; Lenstra, J.A. On the Origin of Cattle: How Aurochs Became Cattle and Colonized the World. Evol. Anthropol. Issues News Rev. 2010, 19, 148–157. [Google Scholar] [CrossRef]
- Cai, D.W.; Sun, Y.; Tang, Z.W.; Zhou, H. Molecular Archaeological Research on the Origins of Cattle in Northern China. Quat. Sci. 2014, 34, 166–172. (In Chinese) [Google Scholar] [CrossRef]
- Xia, X.-T.; Achilli, A.; Lenstra, J.A.; Tong, B.; Ma, Y.; Huang, Y.-Z.; Han, J.-L.; Sun, Z.-Y.; Chen, H.; Lei, C.-Z.; et al. Mitochondrial Genomes from Modern and Ancient Turano-Mongolian Cattle Reveal an Ancient Diversity of Taurine Maternal Lineages in East Asia. Heredity 2021, 126, 1000–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Hou, L.; Li, H.; Xiang, H.; Zhao, X. Ancient Mitogenomes Reveal the Domestication and Distribution of Cattle During the Longshan Culture Period in North China. Front. Genet. 2021, 12, 759827. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.L.; Zhang, D.H. A brief report on excavations at the Tao Temple site, Xiangfen County, Shanxi. Archaeology 1980, 18. (In Chinese) [Google Scholar]
- He, N.; Gao, J.T. Learning Torch Passed to Seek the Ancient City of Yaodu-Brief Explanation the History of Forty Years on Excavation and Research the Site of Tao Si. Cult. Relics South China 2018, 26–40. (In Chinese) [Google Scholar] [CrossRef]
- Cao, Y.P. Taosi Culture Research. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2009. (In Chinese). [Google Scholar]
- Fu, K.L. A case study of the changing animal use in the Late Neolithic of China—A study of the fauna of the Late Longshan Age at the Taosi site in Shanxi Province. Three Gener. Archaeol. 2011, 129–182. (In Chinese) [Google Scholar]
- Cai, L.Z.; Qiu, S.H. Carbon 13 Determination and Ancient Recipes Research. Archaeology 1984, 949–955. (In Chinese) [Google Scholar]
- Zhao, Z.J.; He, N. Floatation Results from the Remains Excavated on the Taosi City-site in 2002 and Their Analysis. Archaeology 2006, 77–86. (In Chinese) [Google Scholar]
- Zhou, Y.M.; Liu, M.M.; Feng, C.Y.; Han, C.S. Morphological analysis of skulls of the Longshan period residents from the Xubao site. J. Acta Anthropol. Sin. 2018, 37, 18–28. (In Chinese) [Google Scholar] [CrossRef]
- Fang, Y.S. Excavation Brief of Erlitou Site, Yanshi, Henan. Archaeology 1965, 3–7+215–224. (In Chinese) [Google Scholar]
- Qiu, S.H.; Cai, L.Z. 14C Chronological Framework in the “Xia-Shang-Zhou Chronology Project”. Archaeology 2001, 90–100. (In Chinese) [Google Scholar]
- Yang, J. Zoological Archaeology of the Erlitou Site. Master’s Thesis, Graduate School of Chinese Academy of Social Sciences, Beijing, China, 2006. (In Chinese). [Google Scholar]
- Si, Y.; Li, Z.P.; Hu, Y.W.; Yuan, J.; Wang, C.S. Hydrogen and Oxygen Stable Isotopic Analysis of Animal Bone Collagen from Erlitou Site, Yanshi, Henan Province. Quat. Sci. 2014, 196–203. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Z.J.; Liu, C. Analysis and Discussion on Flotation Results from the Erlitou Site in Yanshi City. Agric. Archaeol. 2019, 7–20. (In Chinese) [Google Scholar]
- General Editorial Committee of the Encyclopedia of China. Encyclopedia of China Archaeology, 1st ed.; Encyclopedia of China Press: Beijing/Shanghai, China, 1986; p. 571. (In Chinese) [Google Scholar]
- Zhu, Y.P.; Guo, Z.Z.; Wang, L.X. Excavation Brief of the Dashanqian Site in 1996, Karachin Banner, Inner Mongolia. Archaeology 1998, 43–49. (In Chinese) [Google Scholar]
- Wang, L.X. The Economic Pattern and Environmental Context of Lower Xiajiadian Culture Reflected by the Excavation Data of Dashanqian Site. Res. China’s Front. Archaeol. 2007, 350–357. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Z.Y.; Shao, J.; Di, N. A Synthesis of the Archaeological Discovery and Research of the Shimao Site. Cult. Relics Cent. China 2020, 39–62. (In Chinese) [Google Scholar]
- Hu, S.M.; Yang, M.M.; Sun, Z.Y.; Shao, J. Research on Faunal Remains from the 2012~2013 Season Excavation at the Shimao Site in Shenmu, Shaanxi. Archaeol. Cult. Relics 2016, 109–121. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, C.Y.; Hu, S.M.; Sun, Z.Y.; Shao, J.; Yang, M.M. Strontium Isotopic Analysis of Teeth Dental Tissues from the Houyangwan Locus at the Shimao Site in Shenmu, Shaanxi. Archaeol. Cult. Relics 2016, 128–133. (In Chinese) [Google Scholar] [CrossRef]
- Yang, R.C.; Di, N.; Jia, X.; Yin, D.; Gao, S.; Shao, J.; Sun, Z.Y.; Hu, S.M.; Zhao, Z.J. Subsistence Strategies of Early Xia Period: Analysis of Flotation Results from the Shiamao Site in Yulin Area, Shaanxi Province. Quat. Sci. 2022, 101–118. (In Chinese) [Google Scholar] [CrossRef]
- Chen, N.; Cai, Y.; Chen, Q.; Li, R.; Wang, K.; Huang, Y.; Hu, S.; Huang, S.; Zhang, H.; Zheng, Z.; et al. Whole-Genome Resequencing Reveals World-Wide Ancestry and Adaptive Introgression Events of Domesticated Cattle in East Asia. Nat. Commun. 2018, 9, 2337. [Google Scholar] [CrossRef] [PubMed]
- Sergey, S. Minyaev Xiongnu Royal Tomb Complex in the Tsaraam Valley. Int. J. Eurasian Stud. 2011, 153–180. [Google Scholar]
- Yang, D.Y.; Eng, B.; Waye, J.S.; Dudar, J.C.; Saunders, S.R. Improved DNA Extraction from Ancient Bones Using Silica-Based Spin Columns. Am. J. Phys. Anthropol. 1998, 105, 539–543. [Google Scholar] [CrossRef]
- Fu, Q.; Meyer, M.; Gao, X.; Stenzel, U.; Burbano, H.A.; Kelso, J.; Pääbo, S. DNA Analysis of an Early Modern Human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. USA 2013, 110, 2223–2227. [Google Scholar] [CrossRef]
- Schubert, M.; Ermini, L.; Sarkissian, C.D.; Jónsson, H.; Ginolhac, A.; Schaefer, R.; Martin, M.D.; Fernández, R.; Kircher, M.; McCue, M.; et al. Characterization of Ancient and Modern Genomes by SNP Detection and Phylogenomic and Metagenomic Analysis Using PALEOMIX. Nat. Protoc. 2014, 9, 1056–1082. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid Adapter Trimming, Identification, and Read Merging. BMC Res. Notes 2016, 9, 88. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Broad Institute Picard Toolkit. Available online: https://broadinstitute.github.io/picard/ (accessed on 15 January 2023).
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Jónsson, H.; Ginolhac, A.; Schubert, M.; Johnson, P.L.F.; Orlando, L. MapDamage2.0: Fast Approximate Bayesian Estimates of Ancient DNA Damage Parameters. Bioinformatics 2013, 29, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Korneliussen, T.S.; Albrechtsen, A.; Nielsen, R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinform. 2014, 15, 356. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772. [Google Scholar] [CrossRef] [PubMed]
- Cubric-Curik, V.; Novosel, D.; Brajkovic, V.; Rota Stabelli, O.; Krebs, S.; Sölkner, J.; Šalamon, D.; Ristov, S.; Berger, B.; Trivizaki, S.; et al. Large-scale Mitogenome Sequencing Reveals Consecutive Expansions of Domestic Taurine Cattle and Supports Sporadic Aurochs Introgression. Evol. Appl. 2022, 15, 663–678. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. RaxmlGUI 2.0: A Graphical Interface and Toolkit for Phylogenetic Analyses Using RAxML. Methods Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v3: An Online Tool for the Display and Annotation of Phylogenetic and Other Trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Prost, S.; Anderson, C.N.K. TempNet: A Method to Display Statistical Parsimony Networks for Heterochronous DNA Sequence Data. Methods Ecol. Evol. 2011, 2, 663–667. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Cai, D.; Sun, Y.; Tang, Z.; Hu, S.; Li, W.; Zhao, X.; Xiang, H.; Zhou, H. The Origins of Chinese Domestic Cattle as Revealed by Ancient DNA Analysis. J. Archaeol. Sci. 2014, 41, 423–434. [Google Scholar] [CrossRef]
- Cai, D.W.; Zhang, N.F.; Zhu, C.S.; Zhu, S.Q.; Guo, J.L.; Shao, X.Y.; Guo, Y.Q.; Yang, D.Y. Ancient DNA Analysis of Cattle Remains Recovered from Archaeological Sites of the Late Neolithic to the Spring-Autumn Period in Ningxia, Northwest China. Res. China’s Front. Archaeol. 2018, 315–329+413. (In Chinese) [Google Scholar]
- Dai, X.M. Prehistoric Society Stages in China and the Formation of Early States. Acta Archaeol. Sin. 2020, 309–336. (In Chinese) [Google Scholar]
- Institute of Archaeology of Chinese Academy of Social Sciences. A new understanding of settlement patterns and social changes in the late Taosi culture. In Chinese Archaeology in the New Century (Continued)—Essays on the 90th Birthday of Mr. Wang Zhongshu; Science: Beijing, China, 2015; pp. 158–171. (In Chinese) [Google Scholar]
- Xue, J.; Wang, W.; Shao, J.; Dai, X.; Sun, Z.; Gardner, J.D.; Chen, L.; Guo, X.; Di, N.; Pei, X.; et al. Ancient Mitogenomes Reveal the Origins and Genetic Structure of the Neolithic Shimao Population in Northern China. Front. Genet. 2022, 13, 909267. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.H.; Cai, L.Z.; Zhang, X.L. On the Dating of the Erlitou Culture. In Proceedings of the International Symposium on China·the Eritou Site and Erlitou Culture, Yanshi, China, 18 October 2005; pp. 321–332. (In Chinese). [Google Scholar]
- Qin, X.L. On the Production and Circulation of Turquoise Ornaments of the Erlitou Period. Cult. Relics Cent. China 2022, 64–74. (In Chinese) [Google Scholar]
- Verdugo, M.P.; Mullin, V.E.; Scheu, A.; Mattiangeli, V.; Daly, K.G.; Maisano Delser, P.; Hare, A.J.; Burger, J.; Collins, M.J.; Kehati, R.; et al. Ancient Cattle Genomics, Origins, and Rapid Turnover in the Fertile Crescent. Science 2019, 365, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Olivieri, A.; Pellecchia, M.; Uboldi, C.; Colli, L.; Al-Zahery, N.; Accetturo, M.; Pala, M.; Kashani, B.H.; Perego, U.A.; et al. Mitochondrial Genomes of Extinct Aurochs Survive in Domestic Cattle. Curr. Biol. 2008, 18, R157–R158. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Qu, K.; Li, F.; Jia, P.; Chen, Q.; Chen, N.; Zhang, J.; Chen, H.; Huang, B.; Lei, C. Abundant Genetic Diversity of Yunling Cattle Based on Mitochondrial Genome. Animals 2019, 9, 641. [Google Scholar] [CrossRef]
- Sagart, L.; Jacques, G.; Lai, Y.; Ryder, R.J.; Thouzeau, V.; Greenhill, S.J.; List, J.-M. Dated Language Phylogenies Shed Light on the Ancestry of Sino-Tibetan. Proc. Natl. Acad. Sci. USA 2019, 116, 10317–10322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Lu, Y.; Zhang, C.; Wei, L.-H.; Yan, S.; Huang, Y.-Z.; Wang, C.-C.; Mallick, S.; Wen, S.-Q.; Jin, L.; et al. Reconstruction of Y-Chromosome Phylogeny Reveals Two Neolithic Expansions of Tibeto-Burman Populations. Mol. Genet. Genom. 2018, 293, 1293–1300. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Liu, K.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X.; et al. Earliest Domestication of Common Millet (Panicum Miliaceum) in East Asia Extended to 10,000 Years Ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Kong, Z.C.; Lang, S.D. Exploration of Agricultural Plant Remains and the Environment of Human Survival at Dadiwan Site. Cult. Relics Cent. China 2004, 26–30. (In Chinese) [Google Scholar] [CrossRef]
- D’Alpoim Guedes, J.; Lu, H.; Li, Y.; Spengler, R.N.; Wu, X.; Aldenderfer, M.S. Moving Agriculture onto the Tibetan Plateau: The Archaeobotanical Evidence. Archaeol Anthr. Sci. 2014, 6, 255–269. [Google Scholar] [CrossRef]
- Hong, L.Y.; Cui, J.F.; Wang, H.; Chen, J. Analysis and Discussion of the Origin of the Majiayao Type Painted Pottery in the Western Sichuan Basin. South Ethnol. Archaeol. 2011, 7, 1–58. (In Chinese) [Google Scholar]



| Site | Lab Code | Element | Archaeological Code | Age (yBP) | Morphology | DNA |
|---|---|---|---|---|---|---|
| Taosi | TS01C | Bone | 03JXT Ih T5126 FJ1 slope | 4450–4250 | B.t | B.t |
| Taosi | TS02C | Bone | 03JXT Ih T5126 H38 (7) | 4250–4050 | B.t | —— |
| Taosi | TS03C | Bone | 03JXT Ih T5126 H42 (3) | 4250–4050 | B.t | B.t |
| Xubao | XB01C | Bone | 2006JWXIIIT0402H87 | 4500–4000 | B.t | —— |
| Xubao | XB02C | Bone | 2006JWXIIIT0501H232 | 4500–4000 | B.t | —— |
| Xubao | XB03C | Bone | 2006JWXIIIT0802H117 | 4500–4000 | B.t | —— |
| Xubao | XB04C | Bone | 2006JWXIIIT0602H301 | 4500–4000 | B.t | B.t |
| Erlitou | ELT01C | Tooth | 2000YLIII T4 (11) | 3510–3470 | B.t | —— |
| Erlitou | ELT02C | Tooth | 2004YLV T83 H267 | 3510–3470 | B.t | —— |
| Erlitou | ELT06C | Tooth | 2004YLV T85 H330 | 3510–3470 | B.t | —— |
| Dashanqian | DSQ01C | Tooth | T432H501② | 3950–3450 | B.t | —— |
| Dashanqian | DSQ02C | Tooth | 96KDIT422H34⑤ | 3950–3450 | B.t | B.t |
| Dashanqian | DSQ03C | Tooth | 96KDIT433H195 | 3950–3450 | B.t | B.t |
| Dashanqian | DSQ04C | Tooth | T432H501③ | 3950–3450 | B.t | B.t |
| Dashanqian | DSQ05C | Tooth | T337H437③ | 3950–3450 | B.t | B.t |
| Shimao | SM09C | Bone | T2E②: D16 | 3975–3835 | B.t | B.t |
| Shimao | SM10C | Bone | T2E③: D15 | 3975–3835 | B.t | B.t |
| Shimao | SM11C | Bone | Y1: D12 | 3975–3835 | B.t | —— |
| Shimao | SM12C | Bone | Q1③: D5 | 3975–3835 | B.t | —— |
| Shimao | SM13C | Bone | Q1④A: D27 | 3975–3835 | B.t | B.t |
| Shimao | SM14C | Bone | Q2: D3 | 3975–3835 | B.t | —— |
| Shimao | SM15C | Bone | 1#③: D2 | 3975–3835 | B.t | —— |
| Shimao | SM16C | Bone | F1③: D2 | 3975–3835 | B.t | —— |
| Shimao | SM17C | Bone | F7③: D1 | 3975–3835 | B.t | B.t |
| Tsaraam | TC01C | Bone | The barrow 7, northern corridor | 1920–1830 | B.t | —— |
| Tsaraam | TC02C | Bone | The barrow 7, rober’s passage | 1920–1830 | B.t | Ovis |
| Tsaraam | TC03C | Tooth | II-3 | 1920–1830 | B.t | B.t |
| Tsaraam | TC04C | Tooth | II-5 | 1920–1830 | B.t | B.t |
| Tsaraam | TC05C | Tooth | IY-1 | 1920–1830 | B.t | —— |
| Tsaraam | TC06C | Tooth | IY-4 | 1920–1830 | B.t | —— |
| Tsaraam | TC07C | Tooth | IY-7 | 1920–1830 | B.t | —— |
| Tsaraam | TC08C | Tooth | Y-b | 1920–1830 | B.t | —— |
| Tsaraam | TC09C | Tooth | 25 | 1920–1830 | B.t | —— |
| Tsaraam | TC10C | Bone | 67 | 1920–1830 | B.t | —— |
| Lab Code | Raw Reads | Mapped Reads | Endogenous DNA (%) | Mean Coverage of mtDNA (×) | Average Fragment Length (bp) |
|---|---|---|---|---|---|
| TS01C | 22,745,624 | 779,903 | 10.95 | 7.78 | 86.9 |
| TS02C | 30,901,492 | 2740 | 0.03 | 0.08 | 81.22 |
| TS03C * | 2,556,798 | 271,410 | 50.50 | 1972.32 | 118.59 |
| XB01C | 40,817,588 | 1303 | 0.02 | 0.03 | 62.58 |
| XB02C | 19,946,750 | 16,836 | 0.34 | 0.34 | 71.58 |
| XB04C | 11,133,132 | 38,825 | 3.29 | 1.14 | 62.43 |
| ELT01C | 34,706,768 | 575 | 0.01 | 0.01 | 64.41 |
| ELT02C | 27,681,056 | 18,889 | 0.24 | 0.29 | 63.19 |
| ELT06C | 32,832,508 | 10,632 | 0.11 | 0.19 | 74.98 |
| DSQ02C | 42,502,200 | 1,552,224 | 11.63 | 8.51 | 83.77 |
| DSQ03C | 45,448,022 | 1,754,952 | 13.06 | 20.96 | 77.28 |
| DSQ04C | 529,493,626 | 77,142,265 | 49.45 | 739.94 | 79.66 |
| DSQ05C | 318,052,316 | 7,024,230 | 19.64 | 54.59 | 87.86 |
| SM09C * | 8,465,472 | 56,684 | 89.37 | 415.03 | 117.63 |
| SM10C | 18,359,180 | 216,909 | 5.00 | 0.66 | 54.77 |
| SM11C | 19,631,054 | 47,306 | 1.11 | 0.17 | 43.76 |
| SM13C | 59,758,906 | 63,784 | 0.34 | 2.38 | 81.29 |
| SM14C | 74,341,456 | 1822 | 0.01 | 0.01 | 66.78 |
| SM17C | 24,226,842 | 47,258 | 0.56 | 1.41 | 94.64 |
| TC02C | 283,500,054 | 19,274,310 | 29.80 | 2.97 | 87.03 |
| TC03C | 35,269,106 | 279,837 | 3.56 | 4.10 | 54.38 |
| TC04C | 30,827,314 | 116,631 | 1.56 | 0.86 | 62.88 |
| TC05C | 25,639,742 | 33,511 | 0.79 | 0.03 | 53.53 |
| TC06C | 18,656,896 | 44,970 | 0.98 | 0.03 | 62.15 |
| TC07C | 47,617,164 | 71,265 | 0.69 | 0.04 | 70.79 |
| TC08C | 41,519,052 | 85,679 | 1.04 | 0.04 | 63.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Shao, X.; Guo, Y.; Zhang, X.; Zhou, Y.; Yuan, J.; Tang, Z.; Hu, S.; Minyaev, S.S.; Cai, D. Ancient Mitochondrial Genomes Provide New Clues to the Origin of Domestic Cattle in China. Genes 2023, 14, 1313. https://doi.org/10.3390/genes14071313
Zhang N, Shao X, Guo Y, Zhang X, Zhou Y, Yuan J, Tang Z, Hu S, Minyaev SS, Cai D. Ancient Mitochondrial Genomes Provide New Clues to the Origin of Domestic Cattle in China. Genes. 2023; 14(7):1313. https://doi.org/10.3390/genes14071313
Chicago/Turabian StyleZhang, Naifan, Xinyue Shao, Yaqi Guo, Xinyu Zhang, Yawei Zhou, Jing Yuan, Zhuowei Tang, Songmei Hu, Sergey Stepanovich Minyaev, and Dawei Cai. 2023. "Ancient Mitochondrial Genomes Provide New Clues to the Origin of Domestic Cattle in China" Genes 14, no. 7: 1313. https://doi.org/10.3390/genes14071313
APA StyleZhang, N., Shao, X., Guo, Y., Zhang, X., Zhou, Y., Yuan, J., Tang, Z., Hu, S., Minyaev, S. S., & Cai, D. (2023). Ancient Mitochondrial Genomes Provide New Clues to the Origin of Domestic Cattle in China. Genes, 14(7), 1313. https://doi.org/10.3390/genes14071313





