Abstract
Females of the genus Mansonia feed on the blood of humans, livestock, and other vertebrates to develop their eggs. The females’ biting behavior may cause severe disturbance to blood hosts, with a negative impact on public health and economics. Certain species have been identified as potential or effective disease vectors. The accurate species identification of field-collected specimens is of paramount importance for the success of monitoring and control strategies. Mansonia (Mansonia) morphological species boundaries are blurred by patterns of intraspecific heteromorphism and interspecific isomorphism. DNA barcodes can help to solve taxonomic controversies, especially if combined with other molecular tools. We used cytochrome c oxidase subunit I (COI) gene 5′ end (DNA barcode) sequences to identify 327 field-collected specimens of Mansonia (Mansonia) spp. The sampling encompassed males and females collected from three Brazilian regions and previously assigned to species based on their morphological characteristics. Eleven GenBank and BOLD sequences were added to the DNA barcode analyses. Initial morphospecies assignments were mostly corroborated by the results of five clustering methods based on Kimura two-parameter distance and maximum likelihood phylogeny. Five to eight molecular operational taxonomic units may represent taxonomically unknown species. The first DNA barcode records for Mansonia fonsecai, Mansonia iguassuensis, and Mansonia pseudotitillans are presented.
1. Introduction
Some Mansonia species have been implicated as vectors of filariasis in Asia [1,2], Africa [3], and the Americas [4]. Many of these species were found to be naturally infected by arboviruses that can infect humans, causing diseases such as chikungunya [5], Zika fever [6], dengue fever [7], West Nile fever [8], Japanese encephalitis [9,10,11,12], Eastern equine encephalitis [13,14], Saint Louis encephalitis [15,16,17], and Venezuelan equine encephalitis [18,19], among others [5]. The subgenus Mansonia Blanchard, 1901 is widely distributed across the Americas, mainly in areas across the neotropical region [20]. Mansonia (Mansonia) dyari Belkin, Heinemann & Page, 1970 and Mansonia (Mansonia) titillans (Walker, 1848) are found from the southern United States to Central and South America, including the Caribbean islands [21]. Mansonia titillans and Mansonia (Mansonia) indubitans Dyar & Shannon, 1925 are vectors of Venezuelan equine encephalitis virus in Peru [22] and Venezuela [23]. Some species classified in the subgenus Mansonia are vectors of the Oriboca and Bussuquara viruses and likely of Wulchereria brancrofti Cobbold, 1877 in South America [5].
In addition, Mansonia mosquitoes can become disturbing pests for humans and livestock in areas where their populations reach high densities, given the voracity of hematophagous females [24]. Thus, monitoring populations of Mansonia species is necessary wherever these mosquitoes may pose a risk to human and animal health.
For the effective design of mosquito surveillance and control programs, it is essential to identify the target species accurately [25]. However, the small size of the specimens, frequent injuries during field collection, inadequate storage, and the growing shortage of well-trained professionals are among the obstacles to morphology-based species identification [26]. Added to these obstacles is the fact that several distinct species belong to morphologically similar complexes. In these complexes, both isomorphic and polymorphic taxa complicate species identification [26], for example, as for Culex pipiens [27] and Anopheles gambiae complexes [28]. Using molecular markers in taxonomy not only contributes to resolving morphology-based species delimitation impasses, but also serves to test species hypotheses, even if they have already been validated [29].
Since the publication of a seminal article by Hebert et al. [30], the 5′ end fragment of 658 base pairs of cytochrome c oxidase subunit I (COI) mitochondrial gene has been increasingly used as a DNA barcode for species identification, including mosquito disease vectors [26,31,32]. This molecular marker has allowed advances in exploring the genetic diversity of the mosquito fauna of Brazil [33,34,35,36,37,38], Argentina [33], Colombia [17,39,40], Ecuador [41], French Guiana [42], Mexico [43,44], Canada [45], the United Kingdom [46], Belgium [47], Sweden [48], Malawi [49], Saudi Arabia [50], Iran [51], Pakistan [52], India [53], Sri Lanka [54], Singapore [55], Turkey [56], China [57], Japan [58], and Australia [59].
The identification of mosquito species using COI sequences is usually in line with that based on morphology at or below genus level, but the DNA barcode region may not be adequate to identify cryptic mosquito species [26]. However, even when the COI gene does not clearly differentiate closely related species with recent divergence history, it demonstrates lineages that are better separated when additional nuclear markers are employed [60,61]. The COI sequences are relatively easy to obtain and abundantly available for a wide variety of taxa in publicly accessible databases such as GenBank and Barcode of Life Database (BOLD). This makes the DNA barcodes interesting as a molecular tool to verify a potential complex of morphologically similar species [42,43,44,57,59,62].
Bibliographic records show some disagreement regarding the taxonomy of the species in the subgenus Mansonia [63,64,65,66,67,68]. This can be explained by gaps in the morphological, biological, and ecological knowledge of most species. The immature stages and adult females of Mansonia (Mansonia) pessoai (Barreto & Coutinho, 1944), Mansonia (Mansonia) cerqueirai (Barreto & Coutinho, 1944), and Mansonia (Mansonia) chagasi (da Costa Lima, 1935) are unknown. The last taxonomic changes in the group were the revalidation of Mansonia (Mansonia) fonsecai (Pinto, 1932) [68]—previously in the synonymy of Ma. indubitans—and description of Mansonia (Mansonia) iguassuensis Barbosa, Navarro-Silva & Sallum, 2007 [69].
The hypothesis that there are species complexes in the subgenus Mansonia is not a novelty [66]. In such cases like, DNA barcodes have been employed to reveal and identify species that are grouped into morphologically similar taxa [26]. However, the results of the DNA barcode region comparisons are more robust and accurate when there is a taxon-specific sequence library [70]. In this investigation, we employed field-collected Mansonia (Mansonia) spp. specimens preliminarily identified at the species level using morphological characteristics. Subsequently, samples of each species, males, and females, and those with noticeable morphological variations, were separated for COI sequencing. The availability of COI sequences generated for the present study will improve the identification of Mansonia (Mansonia) spp. using DNA barcodes.
2. Materials and Methods
2.1. Mosquito Sampling
Adults and immatures of Mansonia species were collected from rural and urban areas in the Brazilian states of São Paulo (SP), Paraná (PR), Rondônia (RO), Acre (AC), Amazonas (AM), Amapá (AP), and Pará (PA), from April 2015 to August 2020 (Figure 1; see Supplementary Table S1 for geographic coordinates). Adults were collected from AC, AM, AP, and RO using automatic CDC light traps (CDC-LT), with manual electric catchers (EC) resting on the vegetation in early morning, human landing catch overnight, and the barrier screen sampling method (BSS) [71]. The CDC-LT and BSS collections were carried out from 6 p.m. to 6 a.m. Males and females were immediately euthanized with ethyl acetate vapor and placed in plastic vials containing silica gel for transport and storage until morphological identification. In the Laboratory of Entomology in Public Health-Culicidae systematics, of the Faculty of Public Health of the University of São Paulo, São Paulo, Brazil, specimens that were morphologically identified at the species level were separated for generation of DNA barcode sequences.
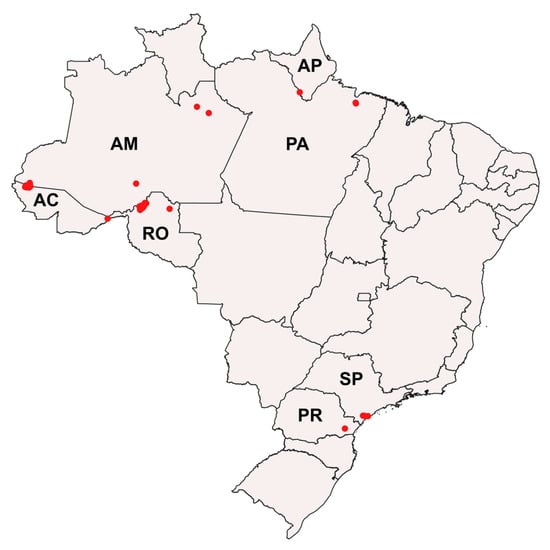
Figure 1.
Distribution of Mansonia (Mansonia) spp. collection areas in the states of Rondônia (RO), Acre (AC), Amazonas (AM), Amapá (AP), Pará (PA), São Paulo (SP), and Paraná (PR), Brazil. Red points correspond to the specimen collection sites.
In addition to adults, larvae, and pupae of Mansonia spp. were sampled from streams, backwaters, ponds, and flooded areas in the Brazilian states of São Paulo, Paraná, Pará, and Rondônia. Each collection site was sampled for three to four hours. Individuals of Eichhornia crassipes (Mart.) Solms (Commelinales: Pontederiaceae) and Pistia stratiotes L. (Alismatales: Araceae) were chosen randomly at different points in the aquatic system and searched for larvae and pupae attached to the roots. The plants were taken from the water and their roots were vigorously shaken in a 20-L bucket containing sieved water from the habitat. The water was visually searched for third- and fourth-instar larvae and pupae. Once found, the specimens were separated using a 3 mL plastic pipette. The immatures were transferred and kept alive in sterile Whirl-Pak® sample bags containing water from the habitat previously sieved to remove any predators. In addition, one or more P. stratiotes plants were included in the sample bag as substrate for larvae and pupae. These plants were the only species used for the transportation of immature culicids and maintenance under laboratory conditions because they fitted into the sample bag and into the laboratory container, unlike E. crassipes. The sample bags were transported to the Laboratory of Entomology in Public Health-Culicidae, Faculty of Public Health of the University of São Paulo, São Paulo, Brazil. Field-collected larvae and pupae were kept individually in colorless 500 mL plastic vials with approximately 300 mL of sieved water from the habitats. Each container was identified according to the sample site and covered with a piece of plastic mesh attached to its top to prevent any disturbances to the developing immature mosquitoes. Adults that emerged in the laboratory were kept alive for at least 12 h before they were euthanized, identified at the species level, and stored individually in small plastic vials in silica gel just as the field-collected adults.
2.2. DNA Extraction
Genomic DNA was extracted from one or two legs of each mosquito specimen. For each extraction, the legs were macerated in 10 μL of 0.9% NaCl with an autoclaved pistil and then 20 μL of 5% Chelex-100 was added. The solution was vortexed and incubated at 99 °C for 10 min. After centrifugation at 13,000 rpm at 25 °C for 5 min, the supernatant was recovered, and an aliquot was frozen at −20 °C for PCR amplification of the target COI gene region. The remaining DNA was stored at −70 °C in the frozen entomological collection of the Faculty of Public Health, São Paulo, Brazil.
2.3. DNA Amplification
The primers LCO1490 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ [72] were used to amplify the COI gene barcode region, according to Bourke et al. [35]. Each reaction was performed in a total volume of 25 μL containing 2 μL of DNA, 1× PCR buffer (Invitrogen), 1.5 mM MgCl2 (Invitrogen), 0.2 mM of each dNTP (Amresco), 0.1 μM of each primer, and 0.625 U of Taq Platinum polymerase (Invitrogen), and the remaining volume consisted of ultrapure water. The PCR thermal conditions consisted of 94 °C for 3 min, five cycles of 94 °C for 30 s, 45 °C for 90 s, 68 °C for 60 s, followed by 35 cycles of 94 °C for 30 s, 51 °C for 30 s, 68 °C for 60 s, and a final extension at 68 °C for 10 min. PCR products were purified by PEG precipitation (20% polyethylene glycol 8000/2.5 M NaCl).
2.4. Sequencing
Sequencing reactions were performed in both directions using a Big Dye Terminator cycle sequencing kit v3.1 (Applied Biosystems, Foster City, CA, USA), and the same set of PCR primers. Each sequencing reaction was carried in a total volume of 10 μL containing 1 μL of purified PCR product, 0.5 μL of Big Dye® Terminator v3.1 Ready Reaction Mix (PE Applied Biosystems), 1× sequencing buffer (PE Applied Biosystems), and 3.6 pmol of F or R primer, and the remaining volume consisted of ultrapure water. The sequencing products were purified with Sephadex G50 columns (GE Healthcare) and analyzed in an Applied Biosystems 3130 DNA Analyser (PE Applied Biosystems, Warrington, UK). Sequences were edited using the Sequencher software v. 5.2.4 (Genes Codes Corporation, Ann Arbor, MI, USA) and the primer regions were removed.
2.5. Sample Identification and COI Database
All adult specimens were preliminarily identified at the species level using the identification keys of Barreto and Coutinho [64], Forattini [24], Barbosa [73], and Assumpção [74]. Male specimens had their identification checked by examining their genitalia mounted on microscope slides. The prior morphological identification of mosquitoes was then revised, considering the species geographic distribution records and the analysis of the COI gene sequences.
Searches were performed with the Basic Alignment Search Tool (BLAST), available at https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 8 April 2023), for all DNA barcode sequences to assess their similarities to those available from Mansonia species and to confirm that they were coding data, free of indels, nuclear mitochondrial DNA sequences (NUMTS), or stop codons. All sequences included in the analyses were 88.47–100% similar to other Mansonia (Mansonia) spp. sequences from GenBank ≥ 642 bp in length (Supplementary Table S2). Muscle was implemented in Mega X [75] for the alignment of nucleotide sequences. Under the reference of the Mansonia (Mansonia) amazonensis (Theobald, 1901) mitochondrion complete genome sequence available in GenBank (accession number MK575483), it was possible to verify that the 658 bp fragment of the COI gene corresponds to position 1509–2166. All 327 new sequences obtained for this study were deposited in GenBank with access numbers from OP785292 to OP785618 (Supplementary Table S1).
To reinforce the specific identity of the new DNA barcode sequences, 10 fragments of the COI gene from GenBank and one from BOLD were added to the dataset. GenBank sequences were generated from specimens identified as Mansonia amazonensis (NC044657 and MK575483), Mansonia (Mansonia) flaveola (Coquillett, 1906) (JX260065), Ma. indubitans (MN997669, MN997670, MN997671, and MN997672) and Ma. titillans (MN997665, MN997666, and MN997667). One Ma. dyari sequence was downloaded from BOLD (MOSN659-18.COI-5P). All 338 sequences were aligned using Muscle, as described above. As the Ma. amazonensis sequences obtained from GenBank corresponded to the complete mitochondrial genome, they were trimmed after alignment to match their length to that of the other sequences (658 base pairs).
A descriptive overview of the dataset was provided by MEGA X. The numbers of variable, conserved and parsimony-informative sites were determined. Three algorithms were applied for calculation of genetic distances: Kimura two-parameter (K2p) [76], Jukes-Cantor (J-C) [77], and Tamura three-parameter (T3p) [78]. As the results obtained from the three algorithms did not differ substantially, only K-2-p distances were used for the estimation of a neighbor-joining (NJ) tree–bootstrap method with 1000 replications and other distance-based cluster analyses (see Supplementary Tables S3 and S4 for J-C and T3p distances, respectively). In order to reduce the computation time spent on NJ analysis, the haplotype was identified using TCS version 1.21 [79] and multiple identical conspecific sequences were collapsed. Four sequences available in GenBank were used as outgroups: Mansonia (Mansonioides) africana (Theobald, 1901) (KU380402), Coquillettidia (Rhynchotaenia) venezuelensis (Theobald, 1912) (OP785619), Coquillettidia (Coquillettidia) perturbans (Walker, 1856) (JF867689), and Psorophora (Janthinosoma) longipalpus Randolph & O’Neill, 1944 (JX260114). The NJ tree was rooted in the Ps. longipalpus sequence using Figtree version 1.4. (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 8 April 2023). The topology of the NJ tree supported the grouping of sequences according to their separation into lineages formed by terminal branches present in at least 99% of the bootstrap replication. These lineages were also referred to as molecular operational taxonomic units (MOTUs) [80]. The MOTUs that segregated specimens morphologically assigned to the same species were shown by “G (Group) I”, “G II” and, when necessary, also “G III” and “G IV” following the morphospecies name. Matrices of intra- and inter-MOTU genetic distances are provided in the results.
2.6. Cluster Analyses
Partition groups of all data were inferred from ranked distances using online versions of Automatic Barcode Gap Discovery (ABGD) [81] and Assemble Species by Automatic Partitioning (ASAP) [82]. The settings applied to the ABGD run were Pmin = 0.001, Pmax = 0.1, steps = 10, X = 1.5, and Nb bins = 20. The K2p substitution model was selected for both ABGD and ASAP distance-based methods. Pairwise distances were computed using the Refined Single Linkage (RESL) algorithm implemented on the BOLD Systems workbench to designate MOTUs, as described by Ratnasingham and Hebert [83].
Putative species groups were alternatively inferred based on the number of substitutions using the multi-rate Poisson tree processes (mPTP) version 0.2.4 [84]. A Markov Chain Monte Carlo (MCMC) of 10 million generations, sampling every 10,000 generations, was conducted in four independent runs supported by the mPTP model. Samples generated in the first step of MCMC and branches with lengths smaller than or equal to 0.0029103938 were discarded. As for the outgroup, a 658 bp COI sequence of Ps. longipalpus from GenBank (JX260114) was selected. As input for mPTP, we used a maximum likelihood (ML) best tree result created using RAxML version 8.2.12 [85] with rapid bootstrapping (-f a), random seed (-x 12345), and the GTRCATI substitution model.
3. Results
3.1. Sample Identification and COI Database
The prior morphological identification attributed 209 females and 118 male Mansonia (Mansonia) specimens to eight species: Ma. amazonensis (n = 22), Ma. flaveola (n = 13), Ma. fonsecai (n = 28), Ma. humeralis (n = 64), Ma. iguassuensis (n = 5), Ma. indubitans (n = 79), Mansonia (Mansonia) pseudotitillans (Theobald, 1901) (n = 3), and Ma. titillans (n = 113) (see Supplementary Table S1 for more details on developmental stage, sex, collection date, site, and method concerning each specimen). As the identification of the specimens was essentially based on morphological characters—although their geographical origin was also considered—the taxa were henceforth treated as morphospecies. Considering the 658 bp DNA barcode sequences obtained from 327 analyzed specimens, 436 sites were conserved (66.26%), 212 were variable (33.74%), 205 were parsimony-informative (31.16%), and 17 were singleton sites (2.58%). Sequences were AT rich (average 38.6% T; 17.0% C; 29.3% A; and 15.1% G), especially at the third codon position (average 45.4% T, 7.7% C, 45.0% A and 1.9% G).
3.2. NJ and Distance Analyses
Figure 2 presents an NJ tree with collapsed terminal branches (see Supplementary Figure S1 for non-collapsed branches and Supplementary Table S1 for the correspondence of individual sequences with their respective haplotypes and MOTUs). The estimated NJ topology showed that the sequences of Ma. flaveola, Ma. titillans, and Ma. pseudotitillans were split into two distinct sibling lineages (MOTUs), namely, G I and G II for each morphospecies. Of the 14 sequences of Ma. flaveola analyzed, only one, extracted from a specimen from Puerto Rico, was segregated from others into Ma. flaveola G II, keeping the other 11 haplotypes, originating from the states of Rondônia and Acre, gathered into Ma. flaveola G I.
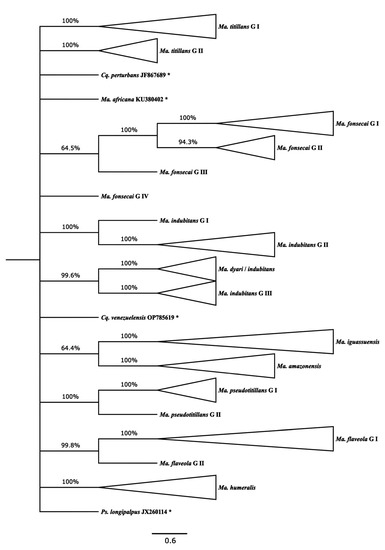
Figure 2.
NJ topology estimated by MEGA X using K2p genetic distances (scale bar) with 124 Mansonia (Mansonia) spp. COI haplotype sequences, eight of which were extracted from Genbank and BOLD (summarized from Supplementary Figure S1). Only branches present in at least 60% of the bootstrap replicates are shown. The tree is rooted on the Ps. longipalpus sequence, which, as with those of the other outgroup members, is indicated by an asterisk. Bootstrap values (1000 replicates) are shown above the branches.
Ma. titillans G I gathered 30 haplotypes, representing specimens from the northern region of Brazil, the states of Acre, Rondônia, and Amapá. An additional 10 haplotypes, from the state of São Paulo, southeastern Brazil, and Rondônia, constituted Ma. titillans G II. Only sequences from Rondônia were included in Ma. pseudotitillans G I and Ma. pseudotitillans G II.
Ma. fonsecai and Ma. indubitans sequences were distributed among four MOTUs for each morphospecies. All Ma. fonsecai sequences were extracted from specimens collected in the state of Paraná, southern Brazil. Ma. fonsecai G I and Ma. fonsecai G II aggregated three and two different haplotypes, respectively. The other two remaining haplotypes for this morphospecies were segregated into two distinct MOTUs, Ma. fonsecai G III and Ma. fonsecai G IV, making it the most divergent among all four.
A single sequence of Ma. indubitans, extracted from a specimen collected in the state of Amazonas, was segregated from all the other sequences into Ma. indubitans G I. Its sister lineage, Ma. indubitans G II, included three other haplotypes from the states of Amazonas and Rondônia. Ma. Indubitans G III, the most divergent among the three MOTUs, assembled 15 haplotypes, seven of which included sequences from the type locality—in the state of Pará—mixed or not with other sequences from the states of Acre and Rondônia. The fourth MOTU included five haplotypes, four of them supposedly Ma. indubitans specimens from Colombia and another from a Puerto Rican specimen identified as Ma. dyari. Therefore, this last MOTU was named Ma. dyari/indubitans.
All Ma. amazonensis, Ma. humeralis, and Ma. iguassuensis sequences, represented by 17, 17, and 4 haplotypes, respectively, were grouped into conspecific distinct lineages defined as MOTUs. Those of Ma. amazonensis were generated from specimens collected in the states of Acre, Amazonas, and Rondônia. The origins of the Ma. humeralis specimens used in the study are in these same three states, in addition to São Paulo, the only state where Ma. iguassuensis was sampled. Finally, no outgroup sequences joined any lineage formed by those outgroups of Mansonia (Mansonia) spp.
Overall, the mean distance among all sequences was approximately 12.75% ± 0.92%. The average of the distances within the morphospecies groups were all >1% just for Ma. amazonensis, Ma humeralis, and Ma. iguassuensis. For the other morphospecies, such values ranged from 2.19% ± 0.46% (Ma. pseudotitillans) to 6.52% ± 0.65% (Ma. fonsecai). After progressively dividing the sequences into groups, corresponding to the lineages seen in the NJ tree topology, the intra-MOTU mean K2p distances were recalculated (Table 1).

Table 1.
Average of intra-MOTU K2p distances computed by MEGA X with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). The number of sequences (n) and corresponding haplotypes (h) per MOTU are given. In the second and third columns, the samples morphologically identified as Ma. flaveola, Ma. fonsecai, Ma. indubitans, Ma. pseudotitillans, and Ma. titillans were subdivided into groups corresponding to the MOTUs shown in the NJ tree topology. It was not possible to calculate the average of distances for MOTUs composed of only one haplotype. Asterisks show the highest value for each column.
At the maximum number of lineages after progressive partitions, Ma. flaveola G I showed the highest value among intra-MOTU mean distances, 0.83% ± 0.20%. This distance is less than half of the lowest one found by comparing the average of distances between different MOTUs, 2.15% ± 0.54%, involving Ma. fonsecai G I and Ma. fonsecai G II (Table 2; see Supplementary Table S5 for pairwise comparisons of all haplotype sequences). Ma. flaveola G I and Ma. indubitans G I were the most genetically divergent MOTUs (19.44% ± 1.95%).

Table 2.
Average of inter-MOTU K2p distances (lower left) and respective standard errors (upper right) computed by MEGA X with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). Asterisk shows the lowest value.
3.3. Cluster Analyses
After including 10 sequences of Ma. amazonensis, Ma. flaveola, Ma. indubitans, and Ma. titillans from GenBank and one of Ma. dyari from BOLD, the automated clustering analyses were carried out with 338 individual sequences. This sequence set was partitioned into subsets (MOTUs) independently by ABGD, ASAP, RESL, and mPTP. The results were very congruent among the four algorithms and in relation to the lineages generated in the NJ analysis (Table 3). The ABDG and ASAP results showed some variations in the numbers of MOTUs, according to the computed prior limit to intraspecific diversity (P) [81] and threshold distance (dT) [82] values, respectively. Thus, Table 3 presents only the more biologically plausible results for P and dT values considered for these two algorithms (see Supplementary Table S6 for complete results). The initial partition of ABGD analysis found 13 MOTUs, independently of the P value, and the computed barcode gap [86] is illustrated by the histogram and the rank of distances (Figure 3, a and b, respectively). The recursive partition generated 14 MOTUs when P was 5.99%, 15 MOTUs when P was 3.59% or 2.15%, and repeatedly 16 MOTUs when P ranged from 0.17% to 1.29%. The barcode gap computed by ASAP did not differ from that of ABGD (Figure 3c,d). The ASAP algorithm segregated the sequences into 17 and 16 MOTUs when the dT values were 1.38% and 2.47%, respectively. The sequence partition with the lowest ASAP score—the best, according to Puillandre et al. [82]—distributed the sequences into 15 MOTUs according to dT = 3.50%. The same sequence clustering pattern was successively maintained by increasing the dT value to the limit of 10.09%.

Table 3.
Comparison of automated clustering methods based on genetic K2p distances (ABGD, ASAP, and RESL) and number of substitutions (mPTP) with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). The dotted lines delimit the MOTUs generated by the different clustering methods (columns).
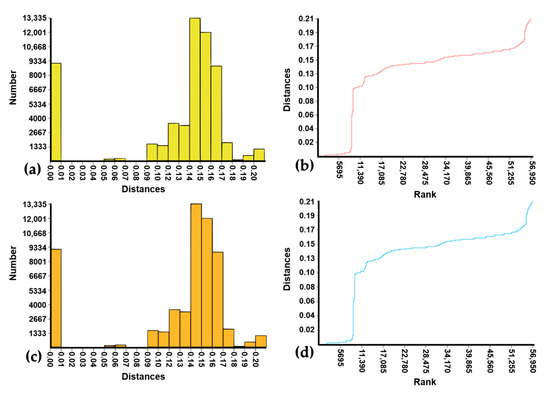
Figure 3.
Histogram and rank of pairwise K2p distances generated by ABDG ((a) and (b), respectively) and ASAP ((c) and (d), respectively) with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). The frequencies of the pairwise distances computed by ABDG and ASAP are shown by yellow columns (a) and orange columns (c), respectively; and ranked ordered pairwise distances computed by ABDG and ASAP are shown by red curve (a) and blue curve (b).
The remaining two clustering methods, mPTP and RESL, generated 16 and 17 MOTUs, respectively. The RESL results are summarized in Table 4, where each MOTU is associated with a corresponding barcode index number (BIN) [83]. The sequence composition of the MOTUs generated by RESL mirrors was exactly that of the NJ analysis. The mean intra-MOTU K2p distances ranged from 0.00% to 0.83%. Only Ma. flaveola G I and Ma. titillans G I, the maximum intra-MOTU distances, exceeded 1%, but none of them reached 1.7%. The smallest inter-MOTU K2p distance occurred between Ma. fonsecai G I and Ma. fonsecai G II (1.83%). All other nearest neighbor (NN) distances ranged from 3.06% to 11.16%.

Table 4.
Mean and maximum (Max.) intra-MOTU and nearest neighbor (NN) K2p distances generated by the RESL algorithm implemented in BOLD Systems workbench with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD).
The results of all clustering methods generated a single MOTU for each of the three morphospecies, Ma. amazonensis, Ma. humeralis, and Ma. iguassuensis, identical to the NJ lineages. They split the Ma. flaveola and Ma. titillans sequences into two MOTUs for each morphospecies, also corresponding exactly to the composition of their respective G I and G II NJ lineages.
The sequences of some morphospecies clustered variably in ABDG and ASAP as the automated algorithm considered different genetic distance limits for the putative species.
Considering the delimitation of groups, those of the NJ lineages Ma. fonsecai G I and Ma. fonsecai G II were merged into a single MOTU in the results of ABGD (when P ≥ 2.15%), ASAP (when dT ranged from 2.47% to 10.09%), and mPTP. However, the splitting of this single sequence subset, generating those corresponding to lineages Ma. fonsecai G I and Ma. fonsecai G II, took place when ABGD’s P value ranged from 0.17% to 1.29%, ASAP’s dT = 1.38, and in the RESL results. The remaining Ma. fonsecai sequences invariably generated two MOTUs that were identical to the NJ lineages Ma. fonsecai G III and Ma. fonsecai G IV. In the same way, all the Ma. pseudotitillans sequences gathered into a single cluster in the ABGD and ASAP results, but for the latter, only if dT ≥ 3.50%. When ASAP’s dT ≤ 2.47%, the Ma. pseudotitillans sequences were divided into two MOTUs, corresponding to the NJ lineages Ma. pseudotitillans G I and Ma. pseudotitillans G II, as well as into mPTP and RESL outcomes. With just one exception, four MOTUs were generated in the segregation of Ma. indubitans sequences, faithfully reflecting the composition of the NJ lineages for this morphospecies, Ma. indubitans G I–G III and Ma. dyari/indubitans. This last MOTU gathered only the GenBank’s Ma. indubitans sequences and that of Ma. dyari from BOLD. However, when P = 5.99%, the ABDG analysis generated only two MOTUs, one of which aggregated the sequences of the NJ lineages Ma. indubitans G I and Ma. indubitans G II and all the remaining sequences of Ma. indubitans and Ma. dyari.
4. Discussion
Although the morphological identification of Mansonia mosquitoes did not prove to be complicated at the genus level, the same cannot be said about species identification. When identifying samples collected with CDC-LT, the difficulties become more prominent because of damage to specimens and loss of characters with taxonomic importance, such as bristles and scales. There is considerable uncertainty about the morphological identification of some specimens assigned to Ma. titillans, Ma. indubitans, and Ma. fonsecai. These species have often been mistaken for one another over the years, and to distinguish them, laborious and time-consuming preparation of the terminalia on microscope slides is needed [63,64,65,66,67,68].
When even after examining morphological, ecological, and geographic data, there is still doubt about the specific identification of specimens, incorporating molecular data into the analyses can help to elucidate species boundaries [87]. Despite the great efforts devoted to the construction of DNA barcode libraries for mosquito species of health interest [26,31,32], some groups remain somewhat neglected, with relatively few sequences available in publicly accessible databases, as is the case of Mansonia (Mansonia) spp.
We analyzed 327 new DNA barcode sequences extracted from mosquitoes of the subgenus Mansonia morphologically assigned to eight species (Supplementary Table S1). There was a notorious A + T nucleotide bias in these sequences, mainly at the 3rd codon position, in agreement with previous analyses of the COI gene sequences of other mosquito genera [33,45,57,59]. In fact, this seems to be a pattern concerning fragments of the mitochondrial genome of insects [88]. The proportions of conserved, variable, parsimony-informative and singleton sites were consistent with those of other previously published Mansonia (Mansonia) spp. DNA barcode sequences [38].
Neighbor-joining analysis based on K2p distances with 1000 replicates segregated the Ma. amazonensis, Ma. humeralis, and Ma. iguassuensis sequences into three well-supported conspecifics (100%) lineages treated here as MOTUs. Their intra-MOTU mean K2p distances ranged from 0.36% ± 0.18% to 0.54% ± 0.13% (Table 1), but the average of all pairwise distances between members of these three MOTUs and those of any other MOTUs was ≥10.39% ± 1.28% (Table 2). Congruently, the maximum K2p distances computed by RESL within MOTUs corresponding to Ma. amazonensis, Ma. humeralis, and Ma. iguassuensis ranged from 0.46% to 0.92%, while the NN distance ranged from 9.48% to 11.16% (Table 4).
Ma. humeralis sequences were extracted from specimens collected from both the Amazon (western, central, and eastern) and from the southeastern coast of Brazil, representing the genetic variability among geographically very distant populations. The results of the four clustering methods implemented, whether based on genetic distances or on the number of nucleotide substitutions, aggregated all these sequences into a single MOTU, corroborating those of the NJ analysis. All specimens from which the sequences in question were extracted showed golden scales symmetrically covering the two anterolateral areas of the scutum and erect scales in the basal portion of the anterior tibiae, typical characters of Ma. humeralis [64,89]. Considering the body of evidence presented here, the molecular identification of Ma. humeralis fully corresponded to that based on morphology.
The results of the molecular analyses for species delimitation confirmed the morphological identification of the specimens morphologically assigned to Ma. amazonensis and Ma iguassuensis. The loss of the golden scales that normally cover the entire scutum of Ma. amazonensis specimens can be mistaken for those of Ma. wilsoni (unfortunately, not sampled in our collections), especially in the case of females [75]. In turn, Ma. iguassuensis was described less than 20 years ago by Barbosa et al. [69], according to whom the species can be misidentified as Ma. titillans, Ma. indubitans, Ma. wilsoni, or Ma. humeralis. Therefore, the possibility of identifying Ma. amazonensis and Ma iguassuensis through DNA barcodes provides an optimistic perspective. Especially the identification of Ma. iguassuensis can be reassessed by using DNA barcode sequences, given its likely preservation in collections misidentified as morphologically similar species. It is fair to declare that there was only one circumstance in which the molecular and morphological identification of the Ma. amazonensis and Ma. iguassuensis specimens did not match. When ASAP based the partition on dT = 11.74%, a single MOTU gathered the sequences of these two species plus those of Ma. fonsecai, Ma. indubitans, Ma. dyari, and Ma. titillans (Supplementary Table S6). The morphological differences between some pairs of these six species [24,64,68,69] and the high score of the ASAP gap width (11.00) make this an unlikely outcome, which will therefore be neglected.
At the opposite extreme, ASAP dT (0.76%) was the only case in which the result of an analysis segregated Ma. flaveola sequences into four MOTUs instead of two, as output by the other analytical methods. According to maximum conspecific distances for various mosquito genera published in the last two decades, this distance threshold was very low to separate non-conspecific sequences [44,45,52,53,57,59]. Thus, it is not credible that the sequences morphologically assigned to Ma. flaveola could really represent four distinct species. When these sequences were analyzed all together, the computed average of pairwise distances was 2.32% ± 0.29%. This value is within the range of those proposed by Hebert et al. [30] as thresholds of intraspecific divergence to delimit vertebrate and insect species—2% and 3%, respectively. According to these criteria, one might accept that all Ma. flaveola sequences belong to a single species. However, if the Ma. flaveola G I sequence (Amazonian) is analyzed separately, the mean pairwise distances decrease to 0.83% ± 0.20%, a value that is almost one third of the previous one and approaches those obtained for Ma. amazonensis, Ma. humeralis, and Ma. iguassuensis MOTUs (Table 1 and Table 4). Scarpassa et al. [38] found some divergence between sequences of supposed Ma. flaveola from Argentina and Puerto Rico averaging 4.60% ± 0.01% but classified only as “hidden genetic variation”. As the mean pairwise distance between the Ma. flaveola GI and Ma. flaveola GII was 9.76% ± 1.23% (Table 2), an expected value for pairs of congeneric Diptera species [90], we believe it is reasonable to suspect that they are different species, mainly in view of the strong geographic barriers separating their populations. In that case, it is more plausible to believe that the authentic, or stricto sensu (s.s.), Ma. flaveola is represented by lineage G II, whose member is topotypical for the species [91]. A more comprehensive sampling is necessary to verify this hypothesis.
The best ASAP partition and two others generated by ABGD considered the three Ma. pseudotitillans sequences analyzed as members of a single MOTU (Supplementary Table S6) with pairwise NJ distance ranging from 0.15% ± 0.15% to 3.29% ± 0.70% (Supplementary Table S5). In contrast, the RESL and mPTP algorithms equally divided the sequences into two MOTUs, Ma. pseudotitillans G I and Ma. pseudotitillans G II. According to the NJ and RESL analyses, the mean divergence between these sequence subsets is >3% (Table 2 and Table 4). According to the criteria proposed by Hebert et al. [30], these findings lead to an ambiguity that prevents the delimitation of a clear species boundary. Even though the very small sample size may eventually explain the inconclusive finding by itself, it is important to emphasize that Ma. pseudotitillans is present in a much larger area than that of the eastern Amazon, where the three specimens under analysis were collected. There are records of this species in different biomes in Brazil [92] and in various other South American and Caribbean countries [93]. Thus, future sampling efforts aimed at a better understanding of Ma. pseudotitillans taxonomy should seek to cover a wider geographic and ecosystem range.
When all 116 sequences assigned to Ma. titillans COI were treated as a single hypothetical taxonomic unit, the pairwise distances averaged 4.39% ± 0.54% (Table 1), exceeding the 3% limit proposed for insects [30]. In fact, two MOTUs, namely, Ma. titillans G I and Ma. titillans G II, were generated when sequences were analyzed regardless of the clustering method (Table 3). When the distance analysis was performed separately for these two MOTUs using MEGA X, pairwise distances for Ma. titillans G I and Ma. titillans G II averaged 0.49% ± 0.11% and 0.31% ± 0.11%, respectively, while the inter-MOTU mean pairwise distance was 10.65% ± 1.34%. These genetic distance results were congruent with those obtained from RESL (Table 4). By assuming that an average interspecific genetic distance 10 times greater than the mean intraspecific distances indicates the existence of species complexes [94], such results may strongly support the hypothesis of a species complex in a henceforth lato sensu (l.s.) Ma. titillans. Only Ma. titillans G I included sequences from specimens collected in a location geographically close to the type locality, which therefore apparently indicates Ma. titillans s.s. Differently represented by specimens from southeastern Brazil, Ma. titillans G II is likely to be an unknown species. This hypothesis is supported by differences we found in the male genitalia morphology of Ma. titillans G I (Supplementary Figure S2a,b) and Ma. titillans G II (Supplementary Figure S2c,d). The main perceived differences were related to the shape of the dorsal margin, basolateral lobe, gonostillar claw, and contour of the aedeagus. In the early 1970s, variations observed in morphological characters of immature Ma. titillans had already led to the hypothesis that it was a species complex [66].
The average of the pairwise K2p genetic distances computed by the NJ analysis with all sequences morphologically assigned to Ma. indubitans (5.74% ± 0.57%, Table 1) also exceeded the 3% intraspecific threshold [30]. By separating them according to the MOTUs defined in the topology of the NJ tree, the mean intra-MOTU distances for Ma. indubitans G II and Ma. indubitans G III were 0.20% ± 0.14% and 0.32% ± 0.08% (Table 1), respectively, while the inter-MOTU averaged 13.04% ± 1.41 (Table 2). Aggregated as a single MOTU, as suggested by the ABGD result under P = 5.99% (Supplementary Table S6), the Ma. indubitans G I and Ma indubitans G II sequences diverged by an average of 2.12% ± 0.41% (Table 2). This last genetic distance could justify the conspecificity inference [30]. However, the single Ma. indubitans G I sequence was about 20 times more divergent from all Ma indubitans G II sequences than those seen for any pair of the latter (Supplementary Table S5). Such set of genetic distances can generate some controversy, even more so with sympatric populations (Ma. indubitans haplotypes 1 and 4, in Supplementary Table S1). Therefore, it is prudent to increase the sampling from which Ma. indubitans G I and Ma. indubitans G II specimens are obtained for reanalysis before concluding whether they are part of one or more species.
None of the 70 individual sequences of Ma. indubitans G III (15 haplotypes) diverged less than 11.47% ± 1.33% from any other in Ma. indubitans G I and Ma. indubitans G II (Supplementary Table S5). Among those 70 specimens from which the sequences were extracted and whose genetic distances averaged 0.32% ± 0.08% (Table 1), 22 were topotypes collected from the metropolitan area of Belém in the state of Pará (Supplementary Table S1). Therefore, it seems appropriate to assume that Ma. indubitans is a species complex that comprises at least two isomorphic species—Ma. indubitans G I and Ma. indubitans G II—and Ma. indubitans s.s. (Ma. indubitans G III).
Neighbor joining and all other clustering methods unexpectedly assembled the supposed Ma. indubitans GenBank sequences from Colombia (MN997669–MN997672) and the Ma. dyari BOLD sequence from Puerto Rico in a MOTU named Ma. dyari/indubitans. Scarpassa et al. [38] obtained the same finding when they used NJ analysis and Bayesian inference sequences to compare Ma. dyari from North, Central, and South America and sequences from Colombia assigned to Ma. indubitans, all downloaded from GenBank. For Ma. dyari/indubitans, the intra-MOTU genetic K2p distances averaged 0.37% ± 0.17% (Table 1 and Table 4) and its NN—Ma. indubitans G III—diverged by more than 15 times (Table 2 and Table 4). Ma. dyari, morphologically very similar to Ma. indubitans, was described in samples collected from Jamaica [66], and its known distribution extends from the southern United States to Colombia [93]. Thus, it is reasonable to believe that the Colombian specimens assigned to Ma. indubitans we analyzed are actually Ma. dyari, which would explain the relatively high genetic divergence in relation to our Ma. indubitans sequences.
In addition, when comparing our findings to those of Scarpassa et al. [38], there is an important disagreement to highlight. The authors inferred that certain Mansonia (Mansonia) specimens they sampled in Porto Velho in the state of Rondônia belonged to a cryptic species more closely related to Ma. dyari and provisionally named “Ma. near dyari”. However, we also assembled collections in Porto Velho during practically the same periods and believe that the “Ma. near dyari” of Scarpassa et al. [38] may actually be Ma. indubitans s.s., because: (i) there is no previous record of Ma. dyari in Brazil; (ii) adult females of Ma. indubitans can be easily misidentified as Ma. dyari because of the high degree of morphological similarity between these species; (iii) 21.5% of the mosquitoes we sampled in Porto Velho were morphologically assigned to Ma. indubitans, while Scarpassa et al. [38] recorded only 2% of the species in their sample; (iv) the mean genetic K2p distance reported by Scarpassa et al. [38] for the “Ma. near dyari” DNA barcode sequences (0.10% ± 0.10%) was compatible with the mean intra-MOTU divergence we found for Ma. indubitans G III DNA barcode sequences (0.32% ± 0.08%, Table 1), some of which are topotypical; and (v) Scarpassa et al.’s. “Ma. near dyari” and “original” Ma. dyari are mutually NN [38] (mean K2p distance = 6.6% ± 1.70%), as well as our Ma. indubitans G III and Ma. dyari/indubitans (mean K2p distance = 6.42% ± 0.96%) (Table 2).
Ma. fonsecai was primarily described by Pinto [95] in specimens collected from eastern Bolivia, but shortly afterwards, the classification changed to Ma. indubitans synonymy [63]. About 70 years later, Barbosa et al. [68] revalidated Ma. fonsecai after morphologically comparing its holotype and paratypes with mosquito samples from southern Brazil. It has been suspected that different species could have been misidentified as Ma. indubitans in South America because of morphological variations in the gonostylus detected in a priori Ma. indubitans populations from Colombia [66]. We also found morphological variations in the gonostylus of males morphologically assumed as Ma. fonsecai. This was more conspicuous when comparing the gonostylus of Ma. fonsecai G I and Ma. fonsecai G III (Supplementary Figure S3). These morphological variations were congruent with the genetic divergences among the Ma. fonsecai related MOTUs.
The genetic K2p distance among the seven haplotypes of the specimens morphologically assigned to Ma. fonsecai ranged from 0.15% ± 0.15% to 12.39% ± 1.42% (Supplementary Table S5). Haplotypes 1 and 7 were quite divergent among themselves and in relation to the others (≥9.93% ± 1.26%, Supplementary Table S5). This result corroborates their isolations into two MOTUs—Ma. fonsecai G III and Ma. fonsecai G IV, respectively—according to all automated clustering methods (Table 3). The hypothesis that Ma. fonsecai is a species complex becomes even more plausible given that the Ma. fonsecai G III and Ma. fonsecai G IV populations are separated from that explored in Curitiba for Ma. fonsecai revalidation [68] by approximately 160 km and the Serra do Mar, a mountain range with peaks that rise over 1800 m above sea level [96]. It is more likely that the Ma. fonsecai s.s. is represented here by specimens linked to MOTUs Ma. fonsecai G I and/or Ma. fonsecai G II, all of which practically originate from Curitiba. The only exception is a single Ma. fonsecai G II specimen from the state of São Paulo (Supplementary Table S1).
Five remaining Ma. fonsecai haplotypes were grouped into a single MOTU by most clustering methods (Table 3), including mPTP, which, unlike ABGD, ASAP and RESL, is based on phylogenetic assumptions [84]. Only RESL separated these sequences into two different MOTUs, Ma. fonsecai G I and Ma. fonsecai G II, mutually NN to each other (K2p distance = 1.83%). Their mean inter-MOTU pairwise K2p genetic distance was less than 10 times higher than the Ma. fonsecai G I mean intra-MOTU divergence (Table 1, Table 2 and Table 4), discrediting the hypothesis that they are two species [94]. Moreover, the average of pairwise distances computed for the above-mentioned Ma. fonsecai G I + G II single MOTU (1.39% ± 0.35%, Table 1) was lower than the 3% threshold for insect intraspecific divergence [30]. Two examples from the literature based on K2p distances corroborate the hypothesis generated by the ABGD, ASAP, and mPTP analyses, according to which Ma. fonsecai G I and Ma. fonsecai G II are most likely the same species. Cywinska et al. [45] sampled 37 mosquito species in Canada and found that 98% of the conspecific sequences diverged <2% from each other, although Beebe [26] suggested that the small divergence may be related to possible sampling limitations. Similarly, Wang et al. [57] analyzed 122 mosquito species from China and reported that about 98% of the conspecific sequences diverged ≤1.67%, while more than 98% of interspecific divergences ranged from 2.3% to 21.8%.
Despite the evidence for conspecificity of Ma. fonsecai G I and Ma. fonsecai G II, some caution is still necessary regarding this issue. This is so because there may be genetic exchange among lineages that have diverged abruptly or recently, obscuring their relationships [97]. In principle, by improving the sampling design and/or using additional molecular markers, it is possible to overcome controversies regarding the barcode gap [26]. Nevertheless, the Ma. fonsecai s.s. is more likely represented here by specimens linked to MOTUs Ma. fonsecai G I and/or Ma. fonsecai G II, all of which practically originate from Curitiba. The only exception is a single Ma. fonsecai G II specimen from the state of São Paulo (Supplementary Table S1).
Ultimately, no overlap was observed between maximum intra-MOTU and minimum inter-MOTU K2p genetic distances, which ranged from about four to >20 times the former. The existence of clear barcode gaps [86,98] means that DNA barcodes are useful to distinguish congeneric species [99]. In addition, the ratio of the number of BINs generated by RESL (Table 4) to the number of recognized morphospecies (BIN/SP ≅ 2) indicates that there are species neglected by the current taxonomic system, as is ordinary for poorly known taxa [100] such as Mansonia (Mansonia). As isomorphic unrecognized species gathered into single morphological taxa may differ in pathogen transmission potentials [101], these findings deserve attention.
5. Conclusions
The results of the analyses carried out with DNA barcode sequences made it possible to unequivocally distinguish all eight initially listed Mansonia (Mansonia) morphospecies from each other. Unprecedented records of DNA barcode sequences assigned to Ma. fonsecai, Ma. iguassuensis, and Ma. pseudotitillans were presented. Ma. amazonensis, Ma. flaveola, Ma. humeralis, Ma. indubitans, and Ma. titillans sequences substantially complement the DNA barcode library for Mansonia (Mansonia) spp., providing greater reliability to its molecular specific identification [29,70].
The molecular evidence gathered here suggests the existence of isomorphic species complexes related to Ma. flaveola, Ma. fonsecai, Ma. indubitans, Ma. pseudotitillans, and Ma. titillans. However, these findings should be treated as preliminary for the delimitation of Mansonia (Mansonia) species [82]. Regardless of reformulation of sampling strategies, it is recommended that new species hypotheses be tested through the association of other molecular markers [102]. Beebe [26] provides an online supplementary table that compiles several molecular markers from the literature applied to the barcoding of mosquito genera.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14061127/s1. Figure S1: NJ topology estimated by MEGA X using K2p genetic distances (scale bar) with 124 Mansonia (Mansonia) spp. COI haplotype sequences, eight of which represent sequences extracted from GenBank and BOLD (asterisk). The tree is rooted on the Ps. longipalpus sequence, which, similar to those of the other outgroup members, is highlighted in red. Bootstrap values (1000 replicates) are shown above or to the left of the branches; Figure S2: Morphological differences between gonostylus and aedeagus of Ma. titillans G I (a and b, respectively) and Ma. titillans G II (c and d, respectively); Figure S3: Morphological differences between gonostylus of Ma. fonsecai G I (a) and Ma. fonsecai G III (b); Table S1: Additional individual data on 327 Mansonia (Mansonia) spp. specimens from which new DNA barcode sequences were extracted for the study and another 11 sequences downloaded from GenBank or BOLD. Adult collections made by active search, human attraction, barrier screen method, and Shannon light trap were supported by electric catchers. Dashes indicate absence of specific data; Table S2: Similarities between DNA barcode sequences of Mansonia spp. from GenBank database and 327 Mansonia (Mansonia) spp. sample; Table S3: Average of inter-MOTU Jukes–Cantor distances (lower left) and respective standard errors (upper right) computed by MEGA X with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). Asterisk shows the lowest value; Table S4: Average of inter-MOTU Tamura three-parameter distances (lower left) and respective standard errors (upper right) computed by MEGA X with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of them from GenBank and BOLD). Asterisk shows the lowest value; Table S5: All pairwise K2p distances (lower left) and standard errors (upper right) computed by MEGA X for 124 Mansonia (Mansonia) spp. haplotype sequences; Table S6: Comparison of all automated clustering methods results based on genetic K2p distances (ABGD, ASAP and RESL) and number of substitutions (mPTP) with 338 Mansonia (Mansonia) spp. DNA barcode sequences (11 of which from GenBank and BOLD). The rows delimit the MOTUs generated by the different clustering methods (columns). The partitions of ABGD and ASAP are shown for different values of prior limit to intraspecific diversity (P) and threshold distance (dT).
Author Contributions
Conceptualization, J.A.A. and M.A.M.S.; methodology, J.A.A. and M.A.M.S.; validation, J.A.A. and M.A.M.S.; formal analysis, J.A.A.; investigation, J.A.A., T.M.P.d.O., I.L.R.d.S. and T.P.d.S.; resources, M.A.M.S.; data curation, J.A.A., T.M.P.d.O. and I.L.R.d.S.; writing—original draft preparation, J.A.A.; writing—review and editing, J.A.A., T.M.P.d.O., I.L.R.d.S., T.P.d.S. and M.A.M.S.; visualization, J.A.A.; supervision, M.A.M.S.; project administration, M.A.M.S.; funding acquisition, M.A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Research and Development project from Santo Antonio Energia (ANEEL project CT.PD.124.2018); and CNPq grant no. 303382/2022-8 to M.A.M.S.
Institutional Review Board Statement
Sampling was undertaken using SISBIO scientific permit number 67383-1.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Bento Pereira da Silva and his family for the logistical and operational support for field activities in Porto Velho; Mario Antonio Navarro da Silva and Nicholas Brady Thrun for logistical and operational support for field and laboratory activities in Curitiba; Roger W. Hutchings and Rosa S. G. Hutchings for allowing access to mosquitoes deposited in the Invertebrate Collection of the Instituto Nacional de Pesquisas da Amazônia, Manaus; and Luis Carlos de Oliveira, Rui Lima and Jair Donizete for the logistical and operational support for field activities in Pariquera-Açu and Iguape.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wharton, R.H. Studies on filariasis in Malaya: Notes on the breeding of Mansonia (Mansonioides) mosquitoes in the laboratory. Ann. Trop. Med. Parasitol. 1957, 51, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S. Vectors of Wuchereria bancrofti and Brugia malayi in the South and Southeast Asian regions: Their distribution, biology and control. Ceylon J. Med. Sci. 1975, 24, 1–27. [Google Scholar]
- Ughasi, J.; Bekard, H.E.; Coulibaly, M.; Adabie-Gomez, D.; Gyapong, J.; Appawu, M.; Wilson, M.D.; Boakye, D.A. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit. Vectors 2012, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Attack on the vector of filariasis in British Guiana. Public Health Rep. 1964, 79, 137–143. [Google Scholar] [CrossRef] [PubMed]
- White, G.B.; Faust, C. Medical acarology and entomology. In Manson’s Tropical Diseases, 23rd ed.; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; Elsevier Health Sciences: Beijing, China, 2014; pp. 1258–1272. [Google Scholar]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Cropp, C.B.; Daffner, J.F.; Calisher, C.H.; Jakob, W.L.; Christensen, H.A. Arbovirus investigations in Argentina, 1977–1980. II. Arthropod collections and virus isolations from Argentine mosquitoes. Am. J. Trop. Med. Hyg. 1985, 34, 945–955. [Google Scholar] [CrossRef]
- Unlu, I.; Kramer, W.L.; Roy, A.F.; Foil, L.D. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. J. Med. Entomol. 2010, 47, 625–633. [Google Scholar] [CrossRef]
- Simpson, D.I.H.; Bowen, E.T.W.; Platt, G.S.; Way, H.; Smith, C.E.G.; Peto, S.; Kamath, S.; Liat, L.B.; Wah, L.T. Japanese encephalitis in Sarawak: Virus isolation and serology in a Land Dyak village. Trans. R. Soc. Trop. Med. Hyg. 1970, 64, 503–510. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; Amerasinghe, P.H.; Amerasinghe, F.P.; Calisher, C.H.; Perera, L.P.; Arunagiri, C.H.; Munasingha, N.B.; Karunaratne, S.H.P.P. Viruses isolated from mosquitoes collected in Sri Lanka. Am. J. Trop. Med. Hyg. 1994, 51, 154–161. [Google Scholar] [CrossRef]
- Dhanda, V.; Thenmozhi, V.; Kumar, N.P.; Hiriyan, J.; Arunachalam, N.; Balasubramanian, A.; Ilango, A.; Gajanana, A. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian J. Med. Res. 1997, 106, 4–6. [Google Scholar]
- van den Hurk, A.F.; Nisbet, D.J.; Hall, R.A.; Kay, B.H.; Mackenzie, J.S.; Ritchie, S.A. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J. Med. Entomol. 2003, 40, 82–90. [Google Scholar] [CrossRef]
- Gonzalez, O.F.; Rodriguez, M.C.; Mendoza, J.L.; Negrin, E.M. Distribuicion de las principales especies de culicidos de importância medica em la Isla de la Juventud. Rev. Cubana Med. Trop. 1989, 41, 200–207. [Google Scholar]
- Vasconcelos, P.F.C.; Rosa, J.F.S.T.; Rosa, A.P.A.T.; Dégallier, N.; Pinheiro, F.P.; Sá Filho, G.C. Epidemiologia das encefalites por arbovírus na Amazônia brasileira. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 465–476. [Google Scholar] [CrossRef]
- Gorgas Memorial Laboratory. 50th Annual Report of the Gorgas Memorial Laboratory, Fiscal Year 1978; U.S. Government Printing Office: Washington, DC, USA, 1979. [Google Scholar]
- Beranek, M.D.; Gallardo, R.; Almirón, W.R.; Contigiani, M.S. First detection of Mansonia titillans (Diptera: Culicidae) infected with St. Louis encephalitis virus (Flaviviridae: Flavivirus) and Bunyamwera serogroup (Peribunyaviridae: Orthobunyavirus) in Argentina. J. Vector Ecol. 2018, 43, 340–343. [Google Scholar] [CrossRef]
- Hoyos-López, R.; Soto, S.U.; Rúa-Uribe, G.; Gallego-Gómez, J.C. Molecular identification of Saint Louis encephalitis virus genotype IV in Colombia. Mem. Inst. Oswaldo Cruz 2015, 110, 719–725. [Google Scholar] [CrossRef]
- Gilyard, R.T. Mosquito transmission of Venezuelan virus equine encephalo-myelitis in Trinidad. Bull. U. S. Army Med. Dep. 1944, 1, 96–107. [Google Scholar]
- Sudia, W.D.; Lord, R.D.; Newhouse, V.F.; Miller, D.L.; Kissling, R.E. Vector-host studies of an epizootic of Venezuelan equine encephalomyelitis in Guatemala, 1969. Am. J. Epidemiol. 1971, 93, 137–143. [Google Scholar] [CrossRef]
- Wilkerson, R.C.; Linton, Y.M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, ML, USA, 2021; Volume 1. [Google Scholar]
- Harbach, R.E. Culicidae Classification. Mosquito Taxonomic Inventory. Available online: https://mosquito-taxonomic-inventory.myspecies.info/simpletaxonomy/term/6045 (accessed on 23 January 2022).
- Turell, M.J.; Jones, J.W.; Sardelis, M.R.; Dohm, D.J.; Coleman, R.E.; Watts, D.M.; Fernandez, R.; Calampa, C.; Klein, T.A. Vector competence of Peruvian mosquitoes (Diptera: Culicidae) for epizootic and enzootic strains of Venezuelan equine encephalomyelitis virus. J. Med. Entomol. 2000, 37, 835–839. [Google Scholar] [CrossRef]
- Méndez, W.; Liria, J.; Navarro, J.C.; Garcia, C.Z.; Freier, J.E.; Salas, R.; Weaver, S.C.; Barrera, R. Spatial dispersion of adult mosquitoes (Diptera: Culicidae) in a sylvatic focus of Venezuelan equine encephalitis virus. J. Med. Entomol. 2001, 38, 813–821. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica; EDUSP: Sao Paulo, Brazil, 2002; Volume 2. [Google Scholar]
- WHO (World Health Organization). Vector Control for Malaria and Other Mosquito-Borne Diseases; Report of a WHO Study Group; World Health Organ: Geneva, Switzerland, 1995; Volume 857, pp. 1–91. [Google Scholar]
- Beebe, N.W. DNA barcoding mosquitoes: Advice for potential prospectors. Parasitology 2018, 145, 622–633. [Google Scholar] [CrossRef]
- Harbach, R.E. Culex pipiens: Species versus species complex–taxonomic history and perspective. J. Am. Mosq. Control Assoc. 2012, 28, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G. Anopheles gambiae complex. Nature 1962, 196, 907. [Google Scholar] [CrossRef]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. Lond. 2005, 85, 407–415. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ondrejicka, D.A.; Locke, S.A.; Morey, K.; Borisenko, A.V.; Hanner, R.H. Status and prospects of DNA barcoding in medically important parasites and vectors. Trends Parasitol. 2014, 30, 582–591. [Google Scholar] [CrossRef]
- Dhakane, R.; Ahirrao, K.; Shinde, A.; Kotule, J. DNA-based identification and control of disease spreading mosquito species: A review. J. Appl. Biol. Biotechnol. 2021, 9, 124–134. [Google Scholar]
- Laurito, M.; de Oliveira, T.M.; Almirón, W.R.; Sallum, M.A.M. COI barcode versus morphological identification of Culex (Culex) (Diptera: Culicidae) species: A case study using samples from Argentina and Brazil. Mem. Inst. Oswaldo Cruz. 2013, 108, 110–122. [Google Scholar] [CrossRef]
- Torres-Gutierrez, C.; Bergo, E.S.; Emerson, K.J.; de Oliveira, T.M.; Greni, S.; Sallum, M.A.M. Mitochondrial COI gene as a tool in the taxonomy of mosquitoes Culex subgenus Melanoconion. Acta Trop. 2016, 164, 137–149. [Google Scholar] [CrossRef]
- Bourke, B.P.; Conn, J.E.; de Oliveira, T.M.P.; Chaves, L.S.; Bergo, E.S.; Laporta, G.Z.; Sallum, M.A.M. Exploring malaria vector diversity on the Amazon Frontier. Malar. J. 2018, 17, 342. [Google Scholar] [CrossRef]
- Saraiva, J.F.; Souto, R.N.P.; Scarpassa, V.M. Molecular taxonomy and evolutionary relationships in the Oswaldoi-Konderi complex (Anophelinae: Anopheles: Nyssorhynchus) from the Brazilian Amazon region. PLoS ONE 2018, 13, e0193591. [Google Scholar] [CrossRef]
- Andrade, D.C.; Morais, S.A.; Marteis, L.S.; Gama, R.A.; Freire, R.C.D.M.; Rekowski, B.S.; Ueno, H.M.; la Corte, R. Diversity of mosquitoes (Diptera: Culicidae) in the Caatinga Biome, Brazil, from the widespread to the eemic. Insects 2020, 11, 468. [Google Scholar] [CrossRef]
- Scarpassa, V.M.; Batista, E.T.; Ferreira, V.C.; Santos Neto, V.A.; Roque, R.A.; Tadei, W.P.; Ferreira, F.A.Z.; Costa, F.M. DNA barcoding suggests new species for the Mansonia subgenus (Mansonia, Mansoniini, Culicidae, Diptera) in the area surrounding the Jirau hydroelectric dam, Porto Velho municipality, Rondônia State, Brazil. Acta Trop. 2022, 233, 106574. [Google Scholar] [CrossRef]
- Rozo-Lopez, P.; Mengual, X. Mosquito species (Diptera, Culicidae) in three ecosystems from the Colombian Andes: Identification through DNA barcoding and adult morphology. ZooKeys 2015, 513, 39–64. [Google Scholar] [CrossRef]
- Muñoz-Gamba, A.S.; Laiton-Donato, K.; Perdomo-Balaguera, E.; Castro, L.R.; Usme-Ciro, J.A.; Parra-Henao, G. Molecular characterization of mosquitoes (Diptera: Culicidae) from the Colombian rainforest. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e24. [Google Scholar] [CrossRef]
- Linton, Y.M.; Pecor, J.E.; Porter, C.H.; Mitchell, L.B.; Garzón-Moreno, A.; Foley, D.H.; Pecor, D.B.; Wilkerson, R.C. Mosquitoes of eastern Amazonian Ecuador: Biodiversity, bionomics and barcodes. Mem. Inst. Oswaldo Cruz 2013, 108, 100–109. [Google Scholar] [CrossRef]
- Talaga, S.; Leroy, C.; Guidez, A.; Dusfour, I.; Girod, R.; Dejean, A.; Murienne, J. DNA reference libraries of French Guianese mosquitoes for barcoding and metabarcoding. PLoS ONE 2017, 12, e0176993. [Google Scholar] [CrossRef]
- Chan-Chable, R.J.; Martínez-Arce, A.; Mis-Avila, P.C.; Ortega-Morales, A.I. DNA barcodes and evidence of cryptic diversity of anthropophagous mosquitoes in Quintana Roo, Mexico. Ecol. Evol. 2019, 9, 4692–4705. [Google Scholar] [CrossRef]
- Adeniran, A.A.; Hernández-Triana, L.M.; Ortega-Morales, A.I.; Garza-Hernández, J.A.; de la Cruz-Ramos, J.; Chan-Chable, R.J.; Vazquez-Marroquín, R.; Huerta-Jiménez, H.; Nikolova, N.I.; Fooks, A.R.; et al. Identification of mosquitoes (Diptera: Culicidae) from Mexico State, Mexico using morphology and COI DNA barcoding. Acta Trop. 2021, 213, 105730. [Google Scholar] [CrossRef]
- Cywinska, A.; Hunter, F.F.; Hebert, P.D. Identifying Canadian mosquito species through DNA barcodes. Med. Vet. Entomol. 2006, 20, 413–424. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Brugman, V.A.; Nikolova, N.I.; Ruiz-Arrondo, I.; Barrero, E.; Thorne, L.; e Marco, M.F.; Krüeger, A.; Lumley, S.; Johnson, N.; et al. DNA barcoding of British mosquitoes (Diptera, Culicidae) to support species identification, discovery of cryptic genetic diversity and monitoring invasive species. ZooKeys 2019, 832, 57–76. [Google Scholar] [CrossRef]
- Versteirt, V.; Nagy, Z.T.; Roelants, P.; Denis, L.; Breman, F.C.; Damiens, D.; Dekoninck, W.; Backeljau, T.; Coosemans, M.; van Bortel, W. Identification of Belgian mosquito species (Diptera: Culicidae) by DNA barcoding. Mol. Ecol. Resour. 2015, 15, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, C.; Larsson, P.; Näslund, J.; Bravo, M.; Evander, M.; Lundström, J.O.; Ahlm, C.; Bucht, G. Identification of Swedish mosquitoes based on molecular barcoding of the COI gene and SNP analysis. Mol. Ecol. Resour. 2014, 14, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Pemba, D.; Kumala, J.; Gowelo, S.; Higa, Y.; Futami, K.; Sawabe, B.; Tsuda, Y. DNA barcoding of mosquitoes collected through a nationwide survey in 2011 and 2012 in Malawi, Southeast Africa. Acta Trop. 2021, 213, 105742. [Google Scholar] [CrossRef] [PubMed]
- Noureldin, E.; Tan, D.; Daffalla, O.; Almutairi, H.; Ghzwani, J.; Torno, M.; Mashi, O.; Hobani, Y.; Ding, H.; Alamri, A.; et al. DNA Barcoding of Potential Mosquito Disease Vectors (Diptera, Culicidae) in Jazan Region, Saudi Arabia. Pathogens 2022, 11, 486. [Google Scholar] [CrossRef]
- Azari-Hamidian, S.; Yaghoobi-Ershadi, M.R.; Javadian, E.; Abai, M.R.; Mobedi, I.; Linton, Y.M.; Harbach, R.E. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med. Vet. Entomol. 2009, 23, 111–121. [Google Scholar] [CrossRef]
- Ashfaq, M.; Hebert, P.D.; Mirza, J.H.; Khan, A.M.; Zafar, Y.; Mirza, M.S. Analyzing mosquito (Diptera: Culicidae) diversity in Pakistan by DNA barcoding. PLoS ONE 2014, 9, e97268. [Google Scholar] [CrossRef]
- Kumar, N.P.; Rajavel, A.R.; Natarajan, R.; Jambulingam, P. DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 1–7. [Google Scholar] [CrossRef]
- Weeraratne, T.C.; Surendran, S.N.; Karunaratne, S.P. DNA barcoding of morphologically characterized mosquitoes belonging to the subfamily Culicinae from Sri Lanka. Parasit. Vectors 2018, 11, 266. [Google Scholar] [CrossRef]
- Chan, A.; Chiang, L.P.; Hapuarachchi, H.C.; Tan, C.H.; Pang, S.C.; Lee, R.; Lee, K.S.; Ng, L.C.; Lam-Phua, S.G. DNA barcoding: Complementing morphological identification of mosquito species in Singapore. Parasit. Vectors 2014, 7, 569. [Google Scholar] [CrossRef]
- Gunay, F.; Alten, B.; Simsek, F.; Aldemir, A.; Linton, Y.M. Barcoding Turkish Culex mosquitoes to facilitate arbovirus vector incrimination studies reveals hidden diversity and new potential vectors. Acta Trop. 2015, 143, 112–120. [Google Scholar] [CrossRef]
- Wang, G.; Li, C.; Guo, X.; Xing, D.; Dong, Y.; Wang, Z.; Zhang, Y.; Liu, M.; Zheng, Z.; Zhang, H.; et al. Identifying the main mosquito species in China based on DNA barcoding. PLoS ONE 2012, 7, e47051. [Google Scholar] [CrossRef]
- Taira, K.; Toma, T.; Tamashiro, M.; Miyagi, I. DNA barcoding for identification of mosquitoes (Diptera: Culicidae) from the Ryukyu Archipelago, Japan. Med. Entomol. Zool. 2012, 63, 289–306. [Google Scholar] [CrossRef]
- Batovska, J.; Blacket, M.J.; Brown, K.; Lynch, S.E. Molecular identification of mosquitoes (Diptera: Culicidae) in southeastern Australia. Ecol. Evol. 2016, 6, 3001–3011. [Google Scholar] [CrossRef]
- Hemmerter, S.; Šlapeta, J.; van den Hurk, A.F.; Cooper, R.D.; Whelan, P.I.; Russell, R.C.; Johansen, C.A.; Beebe, N.W. A curious coincidence: Mosquito biodiversity and the limits of the Japanese encephalitis virus in Australasia. BMC Evol. Biol. 2007, 7, 100. [Google Scholar] [CrossRef]
- Hemmerter, S.; Šlapeta, J.; Beebe, N.W. Resolving genetic diversity in Australasian Culex mosquitoes: Incongruence between the mitochondrial cytochrome c oxidase I and nuclear acetylcholine esterase 2. Mol. Phylogenet. Evol. 2009, 50, 317–325. [Google Scholar] [CrossRef]
- Delgado-Serra, S.; Viader, M.; Ruiz-Arrondo, I.; Miranda, M.A.; Barceló, C.; Bueno-Mari, R.; Hernández-Triana, L.M.; Miquel, M.; Lester, K.; Jurado-Rivera, J.A.; et al. Molecular characterization of mosquito diversity in the Balearic Islands. J. Med. Entomol. 2021, 58, 608–615. [Google Scholar] [CrossRef]
- Lima, A.C. Especies de Taeniorhynchus (Taeniorhynchus) (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 1935, 30, 453–469. [Google Scholar] [CrossRef]
- Barreto, M.P.; Coutinho, J.O. Sobre o gênero Taeniorhynchus Arribálzaga, 1891, com descrição de três novas espécies do subgênero Taeniorhynchus (Diptera, Culicidae). Arq. Hig. Saude Publica 1944, 9, 51–86. [Google Scholar]
- Pratt, H.D. Notes on American Mansonia mosquitoes. Proc. Entomol. Soc. Wash. 1953, 55, 9–19. [Google Scholar]
- Belkin, J.N.; Heinemann, S.J.; Page, W.A. The Culicidae of Jamaica (Mosquito Studies. XXI). Contrib. Amer. Ent. Inst. 1970, 6, 1–458. [Google Scholar]
- Lane, C.J. On a collection of Culicinae (Diptera: Culicidae) from Brazil. Mosq. Syst. 1992, 24, 16–22. [Google Scholar]
- Barbosa, A.A.; Navarro-Silva, M.A.; Sallum, M.A.M. Description and revalidation of Mansonia (Mansonia) fonsecai (Pinto) (Diptera: Culicidae). Zootaxa 2005, 905, 1–11. [Google Scholar] [CrossRef]
- Barbosa, A.A.; Navarro-Silva, M.A.; Sallum, M.A.M. Mansonia (Mansonia) iguassuensis sp. nov. (Diptera: Culicidae) from Brazil. Zootaxa 2007, 1527, 45–52. [Google Scholar] [CrossRef]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K.L. DNA Barcoding and Taxonomy in Diptera: A Tale of High Intraspecific Variability and Low Identification Success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Burkot, T.R.; Russell, T.L.; Reimer, L.J.; Bugoro, H.; Beebe, N.W.; Cooper, R.D.; Sukawati, S.; Collins, F.H.; Lobo, N.F. Barrier screens: A method to sample blood-fed and host-seeking exophilic mosquitoes. Malar. J. 2013, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Barbosa, A.A. Revisão do Subgênero Mansonia Blanchard, 1901 (Diptera, Culicidae) e Estudo Filogenético de Mansoniini. Doctoral Thesis, Faculdade de Saude Publica da Universidade de Sao Paulo, Sao Paulo, Brazil, 2007. [Google Scholar]
- Assumpção, I.C.D. Chave de Identificaçao Pictorica para o Subgenero Mansonia Blanchard, 1901 (Diptera, Culicidae) da Regiao Neotropical. Undergraduate Thesis, Universidade Federal do Parana, Curitiba, Brazil, 2009. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Floyd, R.; Abebe, E.; Papert, A.; Blaxter, M. Molecular barcodes for soil nematode identification. Mol. Ecol. 2002, 11, 839–850. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PloS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for singlelocus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef]
- Goldstein, P.Z.; DeSalle, R. Integrating DNA barcode data and taxonomic practice: Determination, discovery, and description. Bioessays 2011, 33, 135–147. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, Weighting, and Phylogenetic Utility of Mitochondrial Gene Sequences and a Compilation of Conserved Polymerase Chain Reaction Primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Dyar, H.G.; Knab, F. Eggs and Ovipositon in Certain Species of Mansonia (Diptera; Culicidae). Insecutor Lnscitiae Menstruus 1916, 4, 61–68. [Google Scholar]
- Hebert, P.D.N.; Ratnasingham, S.; DeWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B 2003, 270, S96–S99.
- Coquillett, D.W. New Culicidae from the West Indies and Central America. Proc. Entomol. Soc. Wash. 1906, 7, 182–186. [Google Scholar]
- SIBBr (Sistema de Informação Sobre a Biodiversidade Brasileira). Available online: https://sibbr.gov.br (accessed on 15 December 2022).
- Gaffigan, T.V.; Wilkerson, R.C.; Pecor, J.E.; Stoffer, J.A.; Anderson, T. WRBU Systematic Catalog of Culicidae. Available online: http://www.mosquitocatalog.org/ (accessed on 15 December 2022).
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C. Alguns mosquitos do Brasil e do oriente da Bolivia (Diptera. Culicidae). Rev. Med.-Cir. Do Bras. 1932, 40, 235–309. [Google Scholar]
- Almeida, F.F.M.; Carneiro, C.D.R. Origem e evolução da Serra do Mar. Rev. Bras. De Geociências 1998, 28, 135–150. [Google Scholar] [CrossRef]
- Mallet, J.; Besansky, N.; Hahn, M.W. How reticulated are species? Bioessays 2016, 38, 140–149. [Google Scholar] [CrossRef]
- Meier, R.; Zhang, G.Y.; Ali, F. The Use of mean instead of smallest interspecific distances exaggerates the size of the ‘barcoding gap’ and leads to misidentification. Syst. Biol. 2008, 57, 809–813. [Google Scholar] [CrossRef]
- Gibbs, J. DNA barcoding a nightmare taxon: Assessing barcode index numbers and barcode gaps for sweat bees. Genome 2018, 61, 21–31. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; DeWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef]
- Beebe, N.W.; Russell, T.; Burkot, T.R.; Cooper, R.D. Anopheles punctulatus group: Evolution, distribution, and control. Annu. Rev. Entomol. 2015, 60, 335–350. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Song, S.; Tornabene, L.; Chabarria, R.; Naylor, G.J.; Li, C. Multilocus DNA barcoding—Species identification with multilocus data. Sci. Rep. 2017, 7, 16601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).