Expression of Chitinase and shRNA Gene Exhibits Resistance to Fungi and Virus
Abstract
1. Introduction
2. Materials and Methods
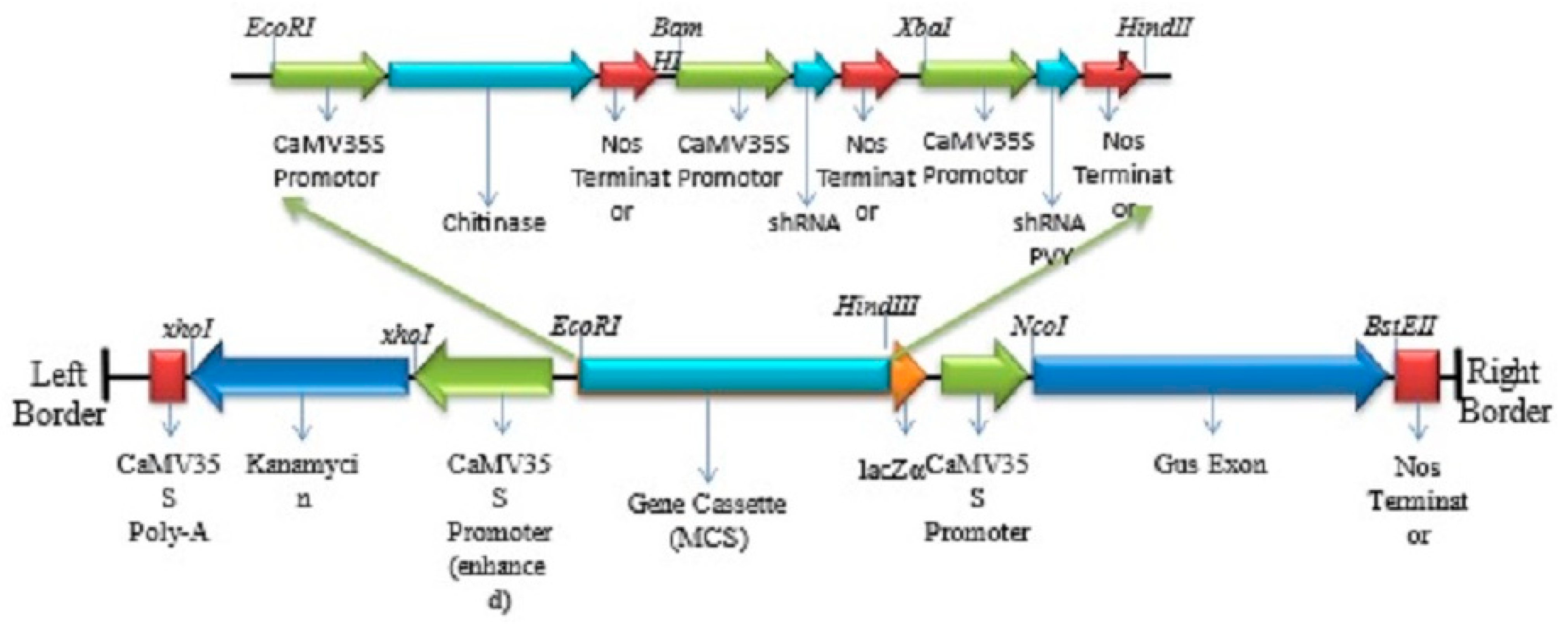
2.1. Construct Development
2.2. Plant Transformation
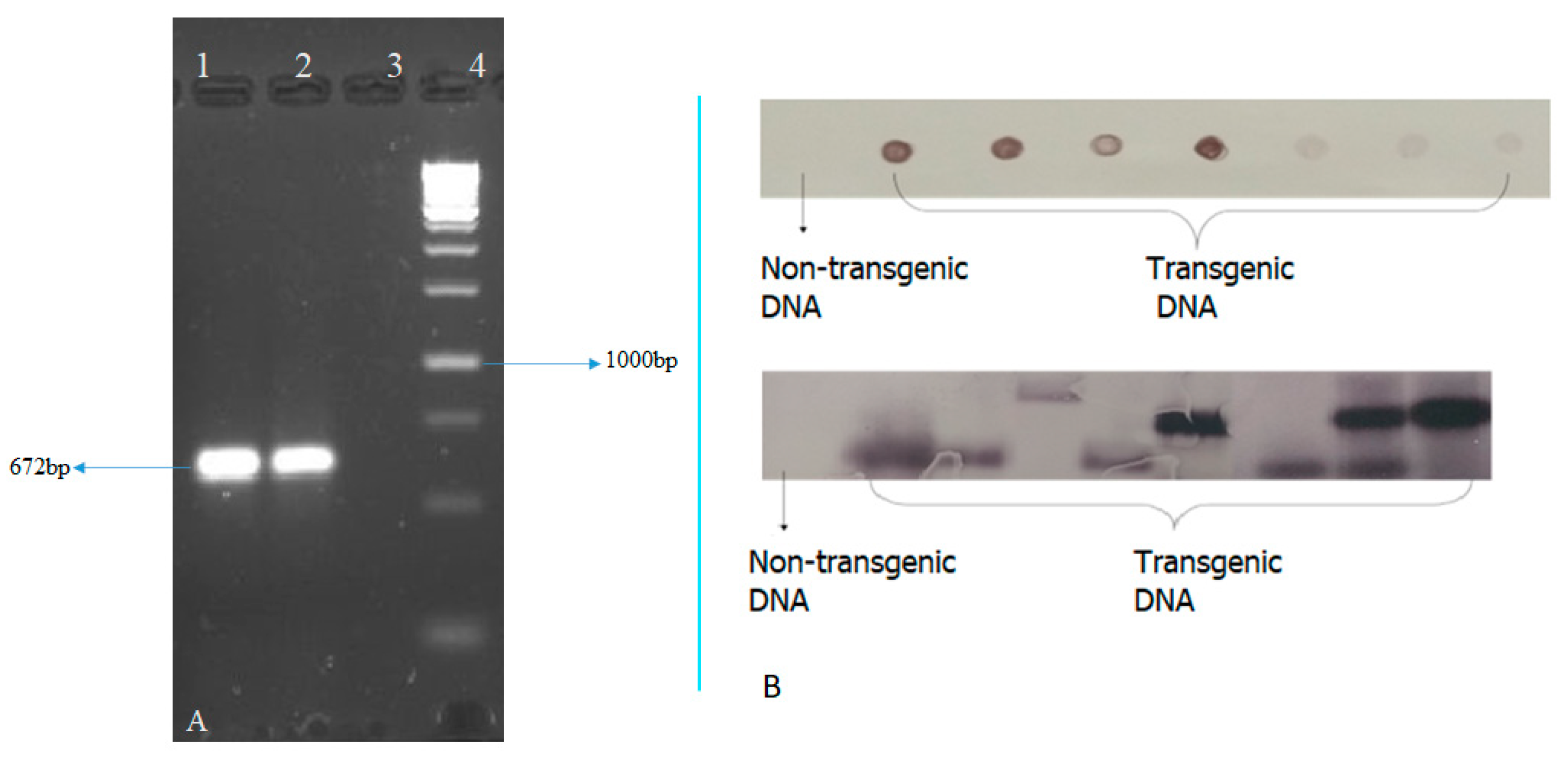
2.3. Molecular Analysis of Transformed Plants
2.4. Southern Blot
2.5. Bioassay for Chitinase Gene
2.5.1. Fungal Inhibition Assay of Crude Plant Extract
2.5.2. Detached Leaf Assay
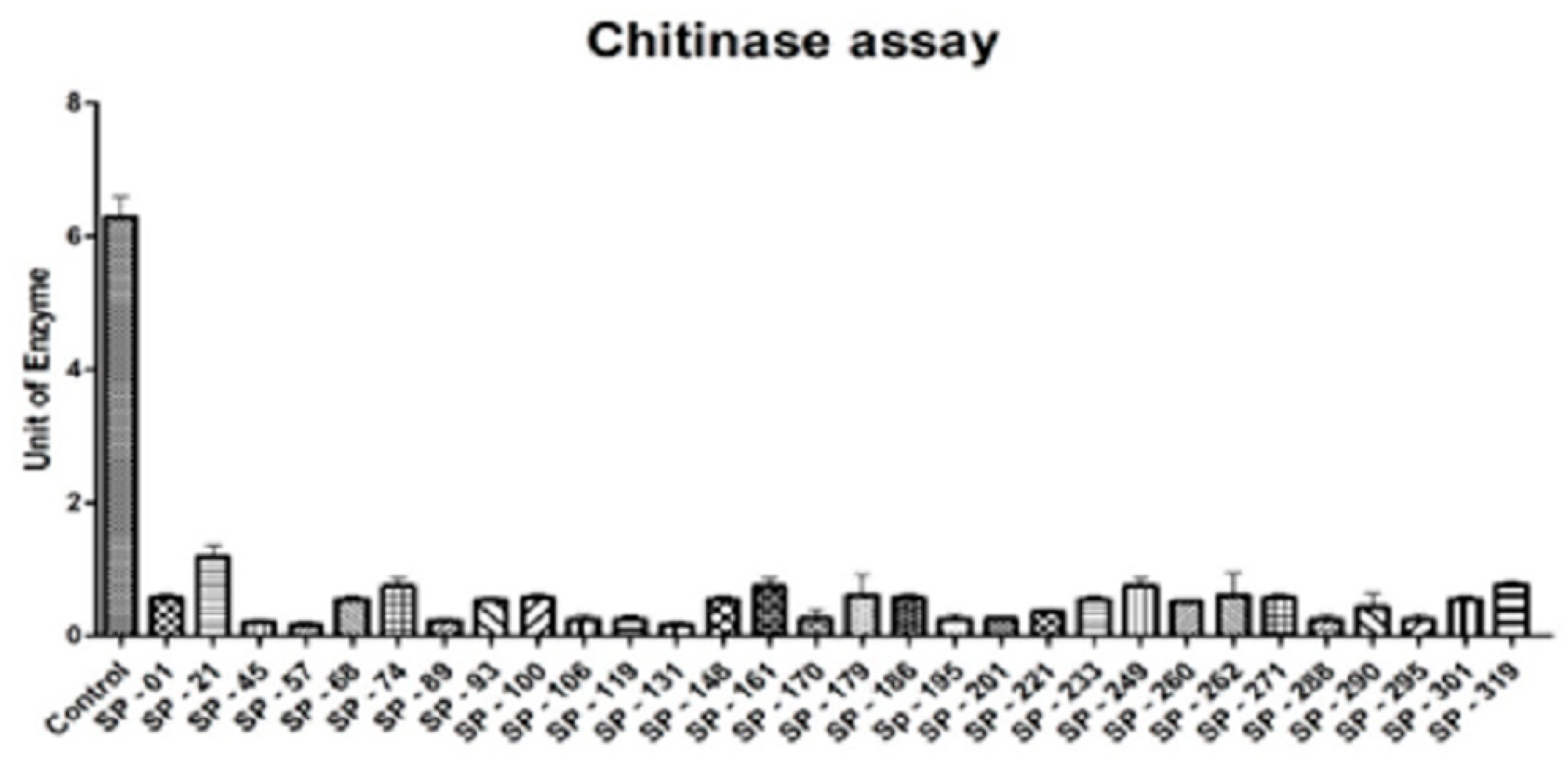
2.5.3. Endo Chitinase Assay
2.6. Bioassays for Potato Viruses
2.6.1. Infestation Assay of PVX
2.6.2. Infestation Assay of PVY
2.6.3. Serological Assay
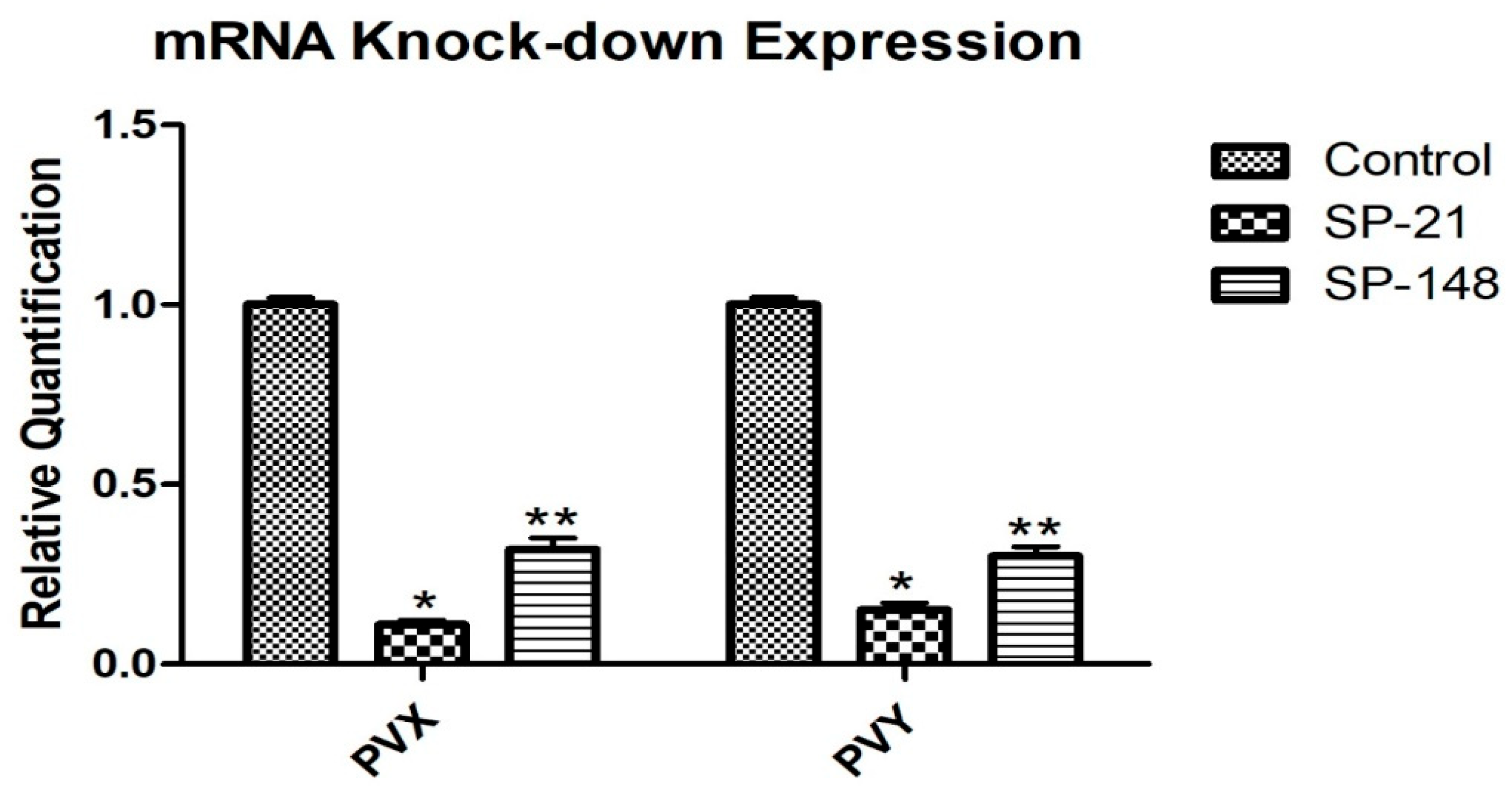
2.7. Expression Analysis Quantitative PCR
3. Results
3.1. Transformation of the pCAMBIA-CXY Construct
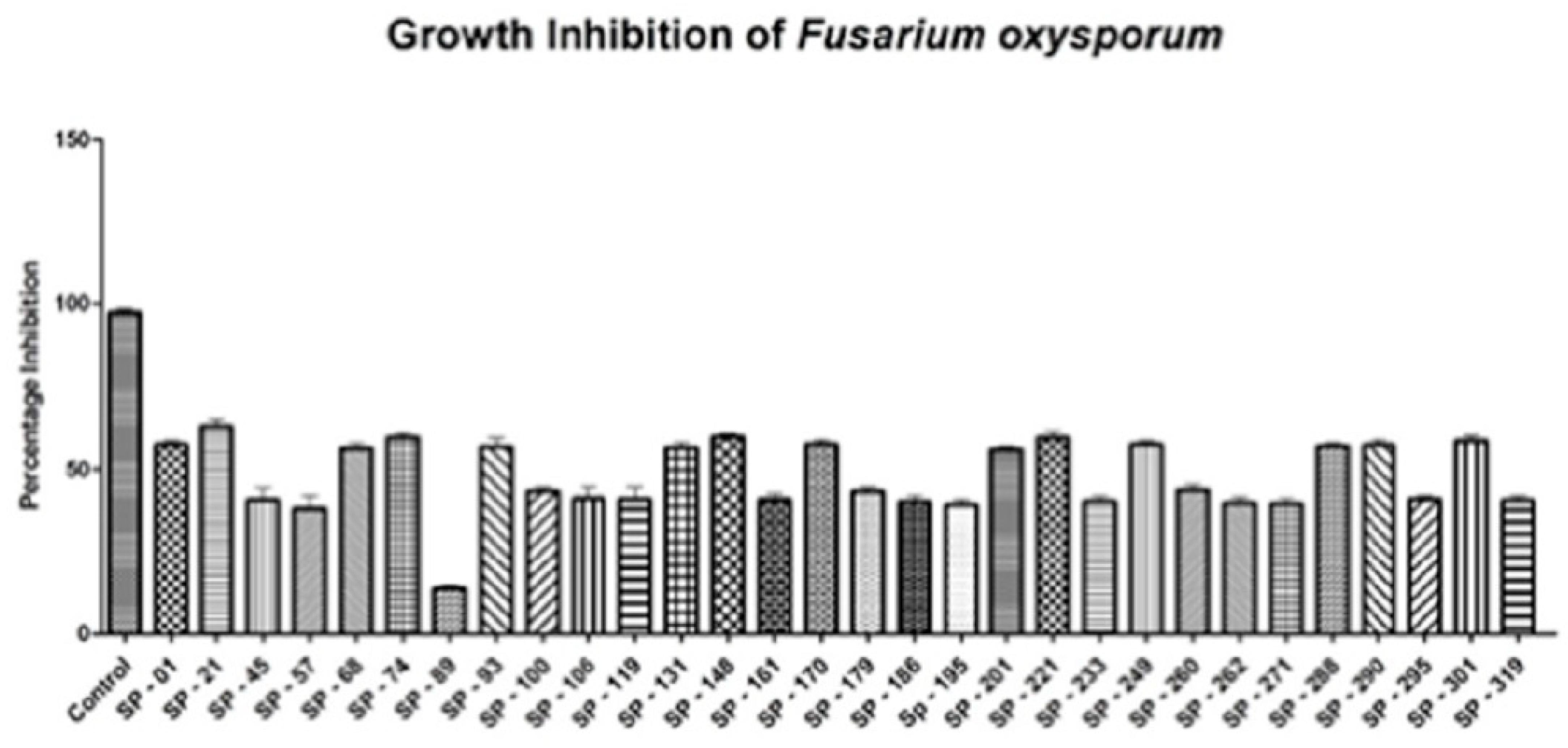
3.2. Fungal Bioassay
In Vitro Antifungal Activity of Transgenic Plants
3.3. Viral Assays of Transgenic Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, S.M.; Metry, E.; Rashed, M.; Ismail, I.; Atta, A.; El-Keredy, A.M.I.R.A. Transformation of Chitinase Gene to Resist Early Blight Disease in Some Potato Virus Resistant Lines. Egypt. J. Genet. Cytol. 2018, 47, 1. [Google Scholar]
- Wang, B.; Ma, Y.; Zhang, Z.; Wu, Z.; Wu, Y.; Wang, Q.; Li, M. Potato viruses in China. Crop Prot. 2011, 30, 1117–1123. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, F.; Zhong, Z.; Chen, R.; Wang, Y.; Chang, L.; Bock, R.; Nie, B.; Zhang, J. Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol. J. 2019, 17, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Sajid, I.A.; Tabassum, B.; Yousaf, I.; Khan, A.; Adeyinka, O.S.; Shahid, N.; Nasir, I.A.; Husnain, T. In Vivo Gene Silencing of Potato Virus X by Small Interference RNAs in Transgenic Potato. Potato Res. 2019, 63, 143–155. [Google Scholar] [CrossRef]
- Tabassum, B.; Nasir, I.A.; Khan, A.; Aslam, U.; Tariq, M.; Shahid, N.; Husnain, T. Short hairpin RNA engineering: In planta gene silencing of potato virus Y. Crop Prot. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Pawelzik, E.; Möller, K. Sustainable Potato Production Worldwide: The Challenge to Assess Conventional and Organic Production Systems. Potato Res. 2014, 57, 273–290. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z. Potato production in Pakistan: Challenges and prospective management strategies—A review. Pak. J. Bot. 2018, 50, 2077–2084. [Google Scholar]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato soil-borne diseases. A review. Agron. Sustain. Dev. 2011, 32, 93–132. [Google Scholar] [CrossRef]
- Kolychikhina, M.S.; Beloshapkina, O.; Phiri, C. Change in potato productivity under the impact of viral diseases. IOP Conf. Series: Earth Environ. Sci. 2021, 663, 012035. [Google Scholar] [CrossRef]
- Arif, M.; Azhar, U.; Arshad, M.; Zafar, Y.; Mansoor, S.; Asad, S. Engineering broad-spectrum resistance against RNA viruses in potato. Transgen. Res. 2011, 21, 303–311. [Google Scholar] [CrossRef]
- Chung, B.N.; Yoon, J.-Y.; Palukaitis, P. Engineered resistance in potato against potato leafroll virus, potato virus A and potato virus Y. Virus Genes 2013, 47, 86–92. [Google Scholar] [CrossRef]
- Hameed, A.; Tahir, M.N.; Asad, S.; Bilal, R.; Van Eck, J.; Jander, G.; Mansoor, S. RNAi-Mediated Simultaneous Resistance Against Three RNA Viruses in Potato. Mol. Biotechnol. 2017, 59, 73–83. [Google Scholar] [CrossRef]
- Valkonen, J.P. Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato. Breed. Sci. 2015, 65, 69–76. [Google Scholar] [CrossRef]
- Hussain, T.; Khan, A.A. Bacillus subtilis HussainT-AMU and its Antifungal activity against Potato Black scurf caused by Rhizoctonia solani on seed tubers. Biocatal. Agric. Biotechnol. 2020, 23, 101443. [Google Scholar] [CrossRef]
- Głowacka, K.; Kromdijk, J.; Leonelli, L.; Niyogi, K.K.; Clemente, T.E.; Long, S.P. An evaluation of new and established methods to determine T-DNA copy number and homozygosity in transgenic plants. Plant Cell Environ. 2016, 39, 908–917. [Google Scholar] [CrossRef]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the Global Food Demand of the Future by Engineering Crop Photosynthesis and Yield Potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef]
- Moravčíková, J.; Matusikova, I.; Libantová, J.; Bauer, M.; Mlynárová, L. Expression of a cucumber class III chitinase and Nicotiana plumbaginifoliaclass I glucanase genes in transgenic potato plants. Plant Cell Tissue Organ Cult. (PCTOC) 2004, 79, 161–168. [Google Scholar] [CrossRef]
- Wang, M.-B.; Waterhouse, P.M. Application of gene silencing in plants. Curr. Opin. Plant Biol. 2002, 5, 146–150. [Google Scholar] [CrossRef]
- Missiou, A.; Kalantidis, K.; Boutla, A.; Tzortzakaki, S.; Tabler, M.; Tsagris, M. Generation of transgenic potato plants highly resistant to potato virus Y (PVY) through RNA silencing. Mol. Breed. 2004, 14, 185–197. [Google Scholar] [CrossRef]
- Bhatti, M.U.; Riaz, S.; Toufiq, N.; Adeyinka, O.S.; Khan, A.; Yousaf, I.; Tariq, M.; Murtaza, S.; Nasir, I.A.; Tabassum, B. The potential and efficacy of Allium sativum leaf lectin (ASAL) against sap-sucking insect pests of transgenic maize. Biologia 2020, 75, 2351–2358. [Google Scholar] [CrossRef]
- Khan, A.; Nasir, I.A.; Tabassum, B.; Aaliya, K.; Tariq, M.; Rao, A.Q. Expression studies of chitinase gene in transgenic potato against Alternaria solani. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 563–576. [Google Scholar] [CrossRef]
- Aaliya, K.; Nasir, I.A.; Khan, A.; Toufiq, N.; Yousaf, I.; Adeyinka, O.S.; Iftikhar, S.; Farooq, A.M.; Tabassum, B. Expression of ice recrystallization inhibition protein in transgenic potato lines associated with reduced electrolyte leakage and efficient recovery post freezing injury. J. Biotechnol. 2021, 327, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, S.; Tabassum, B.; Tariq, M.; Riaz, S.; Yousaf, I.; Jabbar, B.; Khan, A.; Samuel, A.O.; Zameer, M.; Nasir, I.A. Silencing a Myzus persicae Macrophage Inhibitory Factor by Plant-Mediated RNAi Induces Enhanced Aphid Mortality Coupled with Boosted RNAi Efficacy in Transgenic Potato Lines. Mol. Biotechnol. 2022, 64, 1152–1163. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Wonglom, P.; Suwannarach, N.; Kumla, J.; Thaochan, N.; Chomnunti, P.; Pitija, K.; Sunpapao, A. Volatile Organic Compound from Trichoderma asperelloides TSU1: Impact on Plant Pathogenic Fungi. J. Fungi 2021, 7, 187. [Google Scholar] [CrossRef]
- Khan, A.; Nosheen, F.; Tabassum, B.; Adeyinka, O.S.; Shehzad, K.; Shahid, N.; Khan, A.M.; Nasir, I.A. Comparative Silencing Effect of Different siRNA Fragments on Potato Virus X Coat Protein in Transient Transfection Assays. Pak. J. Zool. 2022, 54, 1–7. [Google Scholar] [CrossRef]
- Fatima, N.; Tabassum, B.; Yousaf, I.; Malik, M.; Khan, A.; Sajid, I.A.; Tariq, M.; Toufiq, N.; Riaz, S.; Nasir, I.A. Potential of endochitinase gene to control Fusarium wilt and early blight disease in transgenic potato lines. J. Plant Prot. Res. 2023, 59, 376–382. [Google Scholar] [CrossRef]
- Tsror, L. Biology, epidemiology and management of Rhizoctonia solani on potato. J. Phytopathol. 2010, 158, 649–658. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan Stimulates Defense Reactions in Grapevine Leaves and Inhibits Development of Botrytis Cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413. [Google Scholar] [CrossRef]
- Harveson, R.M.; Smith, J.A.; Stroup, W.W. Improving Root Health and Yield of Dry Beans in the Nebraska Panhandle with a New Technique for Reducing Soil Compaction. Plant Dis. 2005, 89, 279–284. [Google Scholar] [CrossRef]
- Cumagun, C.J.R. Managing plant diseases and promoting sustainability and productivity with trichoderma: The philippine experience (review articles). J. Agr. Sci. Tech. 2012, 14, 699–714. [Google Scholar]
- Punja, Z.K. Genetic engineering of plants to enhance resistance to fungal pathogens—A review of progress and future prospects. Can. J. Plant Pathol. 2001, 23, 216–235. [Google Scholar] [CrossRef]
- Horser, C.; Abbott, D.; Wesley, V.; Smith, N.; Waterhouse, P. Gene silencing–principles and application. Genet. Eng. 2002, 24, 239–256. [Google Scholar] [CrossRef]
- Aslam, U.; Tabassum, B.; Nasir, I.A.; Khan, A.; Husnain, T. A virus-derived short hairpin RNA confers resistance against sugarcane mosaic virus in transgenic sugarcane. Transgen. Res. 2018, 27, 203–210. [Google Scholar] [CrossRef]
- Duan, C.-G.; Wang, C.-H.; Guo, H.-S. Application of RNA silencing to plant disease resistance. Silence 2012, 3, 5–8. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Tabassum, B.; Toufiq, N.; Bhatti, M.U.; Riaz, S.; Nasir, I.A.; Husnain, T. Antifungal activity of chitinase II against Colletotrichum falcatum Went. causing red rot disease in transgenic sugarcane. Turk. J. Biol. 2018, 42, 45–53. [Google Scholar] [CrossRef]
- Durechova, D.; Jopcik, M.; Rajninec, M.; Moravcikova, J.; Libantova, J. Expression of Drosera rotundifolia chitinase in transgenic tobacco plants enhanced their antifungal potential. Mol. Biotechnol. 2019, 61, 916–928. [Google Scholar] [CrossRef]
- Anwar, W.; Javed, M.A.; Shahid, A.A.; Nawaz, K.; Akhter, A.; Rehman, M.Z.U.; Hameed, U.; Iftikhar, S.; Haider, M.S. Chitinase genes from Metarhizium anisopliae for the control of whitefly in cotton. R. Soc. Open Sci. 2019, 6, 190412. [Google Scholar] [CrossRef]
- Serfling, A.; Templer, S.E.; Winter, P.; Ordon, F. Microscopic and Molecular Characterization of the Prehaustorial Resistance against Wheat Leaf Rust (Puccinia triticina) in Einkorn (Triticum monococcum). Front. Plant Sci. 2016, 7, 1668. [Google Scholar] [CrossRef]
- Mazrou, Y.; Makhlouf, A.; Hassan, M.; Baazeem, A.; Hamad, A.; Farid, M. Influence of chitinase production on the antagonistic activity of Trichoderma against plant-pathogenic fungi. J. Environ. Biol. 2020, 41, 1501–1510. [Google Scholar] [CrossRef]
- Ryder, L.S.; Harris, B.D.; Soanes, D.M.; Kershaw, M.J.; Talbot, N.J.; Thornton, C.R. Saprotrophic competitiveness and biocontrol fitness of a genetically modified strain of the plant-growth-promoting fungus Trichoderma hamatum GD12. Microbiology 2012, 158, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.; Furman, N.; Mencacci, N.; Picca, P.; Toum, L.; Lentz, E.; Bravo-Almonacid, F.; Mentaberry, A. Stacking of antimicrobial genes in potato transgenic plants confers increased resistance to bacterial and fungal pathogens. J. Biotechnol. 2012, 157, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.P.; Forbes, G.A.; Manandhar, H.K.; Shrestha, S.M.; Thapa, R.B. Determination of Resistance to Phytophthora infestans on Potato Plants in Field, Laboratory and Greenhouse Conditions. J. Agric. Sci. 2013, 5, p148. [Google Scholar] [CrossRef]
- Jiang, L.; Du, Z.; Zhang, G.; Wang, T.; Jin, G. Advances in RNA-Silencing-Related Resistance against Viruses in Potato. Genes 2022, 13, 731. [Google Scholar] [CrossRef]
- Kwon, J.; Kasai, A.; Maoka, T.; Masuta, C.; Sano, T.; Nakahara, K.S. RNA silencing-related genes contribute to tolerance of infection with potato virus X and Y in a susceptible tomato plant. Virol. J. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Sun, Z.-N.; Yin, G.-H.; Song, Y.-Z.; An, H.; Zhu, C.-X.; Wen, F.-J. Bacterially Expressed Double-Stranded RNAs against Hot-Spot Sequences of Tobacco Mosaic Virus or Potato Virus Y Genome Have Different Ability to Protect Tobacco from Viral Infection. Appl. Biochem. Biotechnol. 2010, 162, 1901–1914. [Google Scholar] [CrossRef]


| Name of Primers | Sequence of Primers | Amplicon Size |
|---|---|---|
| DCHIF | GTGATCACTCAATCAGTATACGC | 672 bp |
| DCHIR | CCAAGCATACCGCAATACCT | |
| PVX-F | GGCAGCAGCAATTAAAGAGG | 120 bp |
| PVX-R | GAAACCTTGTGCTTGCCAGT | |
| PVY-F | GCCAAATGTCAACGGAGTTT | 100 bp |
| PVY-R | TTGCCTAAGGGTTGGTTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, S.; Khan, A.; Jahan, N.; Aaliya, K.; Muzaffar, A.; Tabassum, B.; Inayatullah, S.; Moeezullah, S.; Tariq, M.; Rehmat, Z.; et al. Expression of Chitinase and shRNA Gene Exhibits Resistance to Fungi and Virus. Genes 2023, 14, 1090. https://doi.org/10.3390/genes14051090
Parveen S, Khan A, Jahan N, Aaliya K, Muzaffar A, Tabassum B, Inayatullah S, Moeezullah S, Tariq M, Rehmat Z, et al. Expression of Chitinase and shRNA Gene Exhibits Resistance to Fungi and Virus. Genes. 2023; 14(5):1090. https://doi.org/10.3390/genes14051090
Chicago/Turabian StyleParveen, Samia, Anwar Khan, Nusrat Jahan, Khadija Aaliya, Adnan Muzaffar, Bushra Tabassum, Syed Inayatullah, Syed Moeezullah, Muhammad Tariq, Zainia Rehmat, and et al. 2023. "Expression of Chitinase and shRNA Gene Exhibits Resistance to Fungi and Virus" Genes 14, no. 5: 1090. https://doi.org/10.3390/genes14051090
APA StyleParveen, S., Khan, A., Jahan, N., Aaliya, K., Muzaffar, A., Tabassum, B., Inayatullah, S., Moeezullah, S., Tariq, M., Rehmat, Z., Ali, N., & Hussain, A. (2023). Expression of Chitinase and shRNA Gene Exhibits Resistance to Fungi and Virus. Genes, 14(5), 1090. https://doi.org/10.3390/genes14051090






