Should Prenatal Chromosomal Microarray Analysis Be Offered for Pulmonary Atresia? A Single-Center Retrospective Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Participants
2.2. Chromosome Microarray Analysis
2.2.1. CMA Detection
2.2.2. CMA Results Analysis
2.2.3. CMA Results Validation
2.3. Clinical Follow-Up
2.4. Statistical Analysis
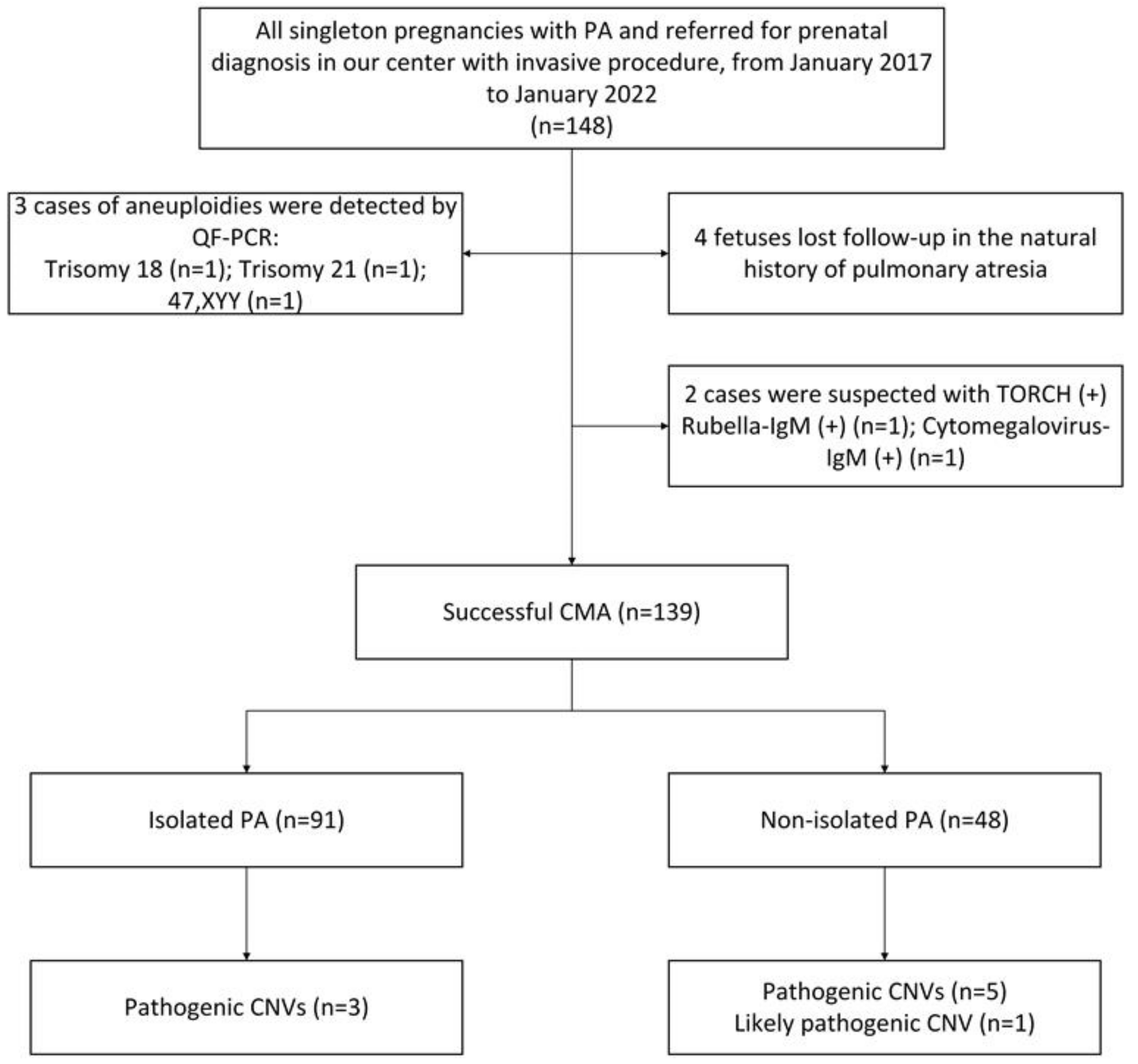
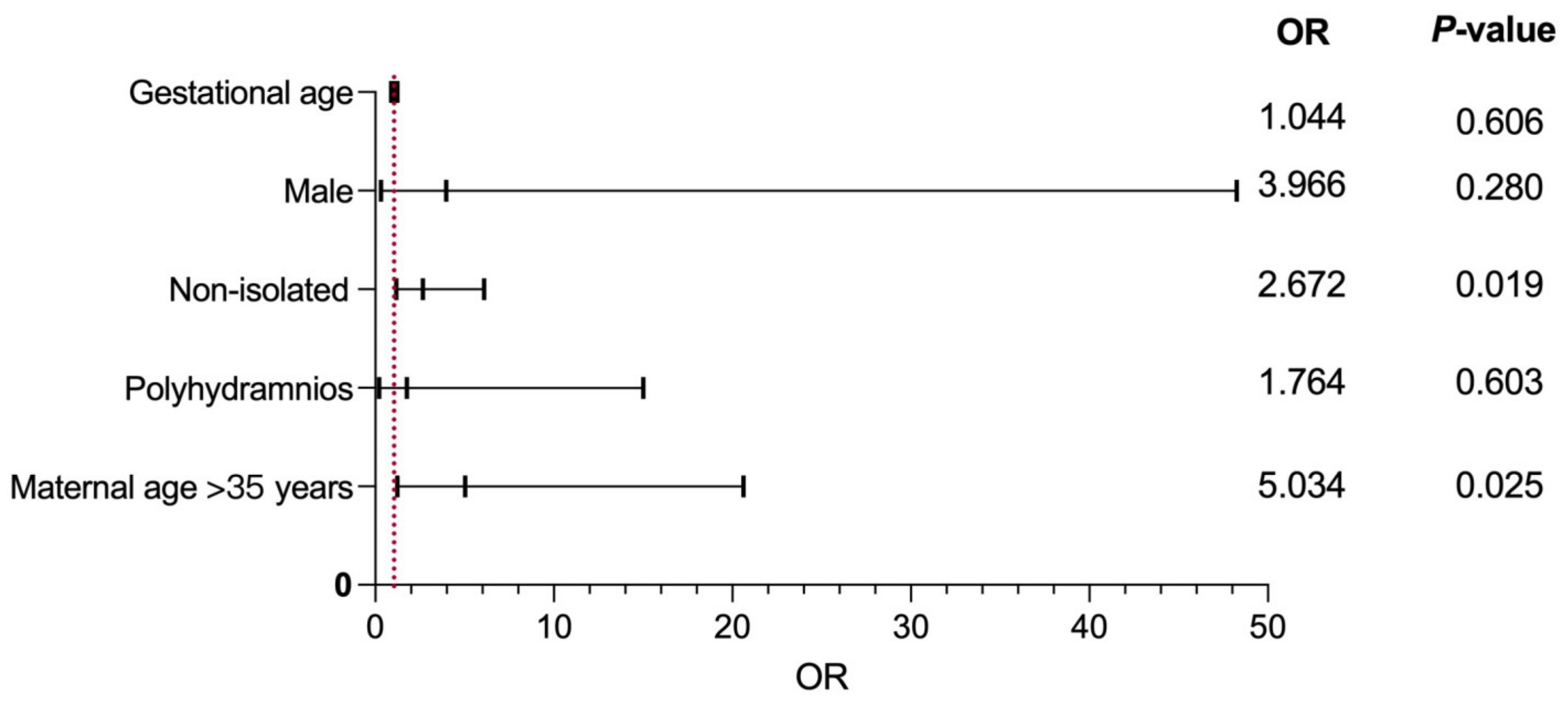
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; He, X.; Zheng, J. Advances in molecular genetics for pulmonary atresia. Cardiol. Young 2016, 27, 207–216. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, Q.; Jiang, Y.; Li, N.; Tang, X.; Xia, H. Echocardiographic and pathomorphological features in fetuses with ductal-dependent congenital heart diseases. Echocardiography 2019, 36, 1736–1743. [Google Scholar] [CrossRef]
- Hopkins, M.K.; Dugoff, L.; Kuller, J.A. Congenital Heart Disease: Prenatal Diagnosis and Genetic Associations. Obstet. Gynecol. Surv. 2019, 74, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Carotti, A.; Albanese, S.B.; Filippelli, S.; Ravà, L.; Guccione, P.; Pongiglione, G.; Di Donato, R.M. Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J. Thorac. Cardiovasc. Surg. 2010, 140, 1092–1103. [Google Scholar] [CrossRef]
- Elias, P.; Poh, C.L.; du Plessis, K.; Zannino, D.; Rice, K.; Radford, D.J.; Bullock, A.; Wheaton, G.R.; Celermajer, D.S.; D’Udekem, Y. Long-term outcomes of single-ventricle palliation for pulmonary atresia with intact ventricular septum: Fontan survivors remain at risk of late myocardial ischaemia and death. Eur. J. Cardio-Thoracic Surg. 2018, 53, 1230–1236. [Google Scholar] [CrossRef]
- Thienpont, B.; Mertens, L.; de Ravel, T.; Eyskens, B.; Boshoff, D.; Maas, N.; Fryns, J.-P.; Gewillig, M.; Vermeesch, J.R.; Devriendt, K. Submicroscopic chromosomal imbalances detected by array-CGH are a frequent cause of congenital heart defects in selected patients. Eur. Heart J. 2007, 28, 2778–2784. [Google Scholar] [CrossRef]
- Richards, A.A.; Santos, L.J.; Nichols, H.A.; Crider, B.P.; Elder, F.F.; Hauser, N.S.; Zinn, A.; Garg, V. Cryptic Chromosomal Abnormalities Identified in Children With Congenital Heart Disease. Pediatr. Res. 2008, 64, 358–363. [Google Scholar] [CrossRef]
- Southard, A.E.; Edelmann, L.J.; Gelb, B.D. Role of Copy Number Variants in Structural Birth Defects. Pediatrics 2012, 129, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal Microarray versus Karyotyping for Prenatal Diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef]
- South, S.T.; Lee, C.; Lamb, A.N.; Higgins, A.W.; Kearney, H.M. Acmg Standards and Guidelines for Constitutional Cytogenomic Microarray Analysis, Including Postnatal and Prenatal Applications: Revision 2013. Genet Med. 2013, 15, 901–909. [Google Scholar] [CrossRef]
- Armour, C.M.; Dougan, S.D.; Brock, J.-A.; Chari, R.; Chodirker, B.N.; DeBie, I.; Evans, J.A.; Gibson, W.T.; Kolomietz, E.; Nelson, T.N.; et al. Practice guideline: Joint CCMG-SOGC recommendations for the use of chromosomal microarray analysis for prenatal diagnosis and assessment of fetal loss in Canada. J. Med. Genet. 2018, 55, 215–221. [Google Scholar] [CrossRef]
- Hillman, S.C.; McMullan, D.J.; Hall, G.; Togneri, F.S.; James, N.; Maher, E.J.; Meller, C.H.; Williams, D.; Wapner, R.J.; Kilby, M.D. Use of prenatal chromosomal microarray: Prospective cohort study and systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 610–620. [Google Scholar] [CrossRef]
- Breman, A.; Pursley, A.N.; Hixson, P.; Bi, W.; Ward, P.; Bacino, C.A.; Shaw, C.; Lupski, J.R.; Beaudet, A.L.; Patel, A.; et al. Prenatal chromosomal microarray analysis in a diagnostic laboratory; experience with >1000 cases and review of the literature. Prenat. Diagn. 2012, 32, 351–361. [Google Scholar] [CrossRef]
- Schmid, M.; Stary, S.; Blaicher, W.; Gollinger, M.; Husslein, P.; Streubel, B. Prenatal genetic diagnosis using microarray analysis in fetuses with congenital heart defects. Prenat. Diagn. 2011, 32, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, J.H.; Burt, A.A.; Crosslin, D.R.; Burnham, N.; Kim, C.E.; McDonald-McGinn, D.M.; Zackai, E.H.; Nicolson, S.C.; Spray, T.L.; et al. Burden of potentially pathologic copy number variants is higher in children with isolated congenital heart disease and significantly impairs covariate-adjusted transplant-free survival. J. Thorac. Cardiovasc. Surg. 2015, 151, 1147–1151.e4. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Brueckner, M. Genetics and Genomics of Congenital Heart Disease. Circ. Res. 2017, 120, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Pertl, B.; Kopp, S.; Kroisel, P.M.; Häusler, M.; Sherlock, J.; Winter, R.; Adinolfi, M. Quantitative fluorescence polymerase chain reaction for the rapid prenatal detection of common aneuploidies and fetal sex. Am. J. Obstet. Gynecol. 1997, 177, 899–906. [Google Scholar] [CrossRef]
- Michielon, G.; Marino, B.; Oricchio, G.; Digilio, M.C.; Iorio, F.; Filippelli, S.; Placidi, S.; Di Donato, R.M. Impact of DEL22q11, trisomy 21, and other genetic syndromes on surgical outcome of conotruncal heart defects. J. Thorac. Cardiovasc. Surg. 2009, 138, 565–570.e2. [Google Scholar] [CrossRef]
- Ward, C.; Stadt, H.; Hutson, M.; Kirby, M.L. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev. Biol. 2005, 284, 72–83. [Google Scholar] [CrossRef]
- Anderson, R.H.; Chaudhry, B.; Mohun, T.J.; Bamforth, S.D.; Hoyland, D.; Phillips, H.M.; Webb, S.; Moorman, A.F.; Brown, N.A.; Henderson, D.J. Normal and abnormal development of the intrapericardial arterial trunks in humans and mice. Cardiovasc. Res. 2012, 95, 108–115. [Google Scholar] [CrossRef]
- Bassett, A.S.; McDonald-McGinn, D.M.; Devriendt, K.; Digilio, M.C.; Goldenberg, P.; Habel, A.; Marino, B.; Oskarsdottir, S.; Philip, N.; Sullivan, K.; et al. Practical Guidelines for Managing Patients with 22q11.2 Deletion Syndrome. J. Pediatr. 2011, 159, 332–339.e1. [Google Scholar] [CrossRef] [PubMed]
- Hofbeck, M.; Rauch, A.; Buheitel, G.; Leipold, G.; von der Emde, J.; Pfeiffer, R.; Singer, H. Monosomy 22q11 in Patients with Pulmonary Atresia, Ventricular Septal Defect, and Major Aortopulmonary Collateral Arteries. Heart 1998, 79, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Elmagrpy, Z.; Rayani, A.; Shah, A.; Habas, E.; Aburawi, E.H. Down Syndrome and Congenital Heart Disease: Why the Regional Difference as Observed in the Libyan Experience? Cardiovasc. J. Afr. 2011, 22, 306–309. [Google Scholar] [CrossRef]
- Roper, R.J.; VanHorn, J.F.; Cain, C.C.; Reeves, R.H. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech. Dev. 2009, 126, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Baś-Budecka, E.; Perenc, M.; Sieroszewski, P. The role of fetal nuchal translucency (NT) and ductus venosus blood flow (DV) in the detection of congenital heart defects. Ginekol. Polska 2010, 81, 272–276. [Google Scholar]
- Hutchinson, D.; McBrien, A.; Howley, L.; Yamamoto, Y.; Sekar, P.; Motan, T.; Jain, V.; Savard, W.; Hornberger, L.K. First-Trimester Fetal Echocardiography: Identification of Cardiac Structures for Screening from 6 to 13 Weeks’ Gestational Age. J. Am. Soc. Echocardiogr. 2017, 30, 763–772. [Google Scholar] [CrossRef]
- Zheng, M.M.; Tang, H.R.; Zhang, Y.; Ru, T.; Li, J.; Xu, B.Y.; Xu, Y.; Hu, Y.L. Contribution of the Fetal Cardiac Axis and V-Sign Angle in First-Trimester Screening for Major Cardiac Defects. J. Ultrasound Med. 2018, 38, 1179–1187. [Google Scholar] [CrossRef]
- Wiechec, M.; Knafel, A.; Nocun, A. Prenatal Detection of Congenital Heart Defects at the 11- to 13-Week Scan Using a Simple Color Doppler Protocol including the 4-Chamber and 3-Vessel and Trachea Views. J. Ultrasound Med. 2015, 34, 585–594. [Google Scholar] [CrossRef]
| Maternal Age (Median) | 30.1 (Range 19.3–47.2) Years |
|---|---|
| Gestational weeks (median) A | 23.3 (range 21.7–27.6) weeks |
| Sex of fetuses (M/F) | 68/71 |
| Sample types | |
| Amniotic fluid | 101 |
| Cord blood | 38 |
| Malformation classification | |
| Isolated | 98 |
| Non-isolated | 41 |
| Follow-ups B | 139 |
| Patient | MA | GA at the Suspicion of PA | Associated Anomaly | CMA Results | Length | Type | Classification | Outcome | Parental Study |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33.1 | 32.4 | kidney agenesis, hemivertebrae | arr[hg19] 22q11.21(18916842–21465659) × 1 | 2.55 Mb | Deletion | P | spontaneous labor | de novo |
| 2 | 34.25 | 27.1 | single ventricle | arr[hg19] 22q11.21(18648856–21800471) × 1 | 3.15 Mb | Deletion | P | termination of pregnancy | de novo |
| 3 | 28.2 | 21.71 | hemivertebrae | arr[hg19] 22q11.21(18648856–21800471) × 1 | 3.15 Mb | Deletion | P | cesarean section | de novo |
| 4 | 29.07 | 26.5 | VSD | arr[hg19] 22q11.21(18631365–21800471) × 1 | 3.17 Mb | Deletion | P | termination of pregnancy | de novo |
| 5 | 25.76 | 28.2 | hemivertebrae | arr[hg19] 7q11.23(72557180–74628840) × 1 | 2.07 Mb | Deletion | P | termination of pregnancy | de novo |
| 6 | 19.31 | 33.21 | double outlet right ventricle, VSD | arr[hg19] 21q11.2q22.3(15016487–48093361) × 1~2 mos | 33.08 Mb | Mosaic deletion | P | termination of pregnancy | de novo |
| 7 | 27.59 | 29.71 | VSD | arr[hg19] 8q24.3(140131302–146295771) × 3 arr[hg19] 21q22.2q22.3(39737188–48093361) × 1 | 6.16 Mb 8.36 Mb | Duplication Deletion | P P | termination of pregnancy | Paternally inherited |
| 8 | 47.31 | 29.7 | VSD | arr[hg19] 16p13.11(15481921–16388343) × 1 | 906 Kb | Deletion | P | termination of pregnancy | de novo |
| 9 | 25.67 | 27.63 | VSD | arr[hg19] 7q36.1(152092064–152341146) × 1 | 249 Kb | Deletion | LP | termination of pregnancy | de novo |
| Perinatal Outcome | Total (n = 139) | Isolated PA (n = 91) | Non-Isolated PA (n = 48) | p-Value | CMA Testing Results | Isolated PA (n = 91) | Non-Isolated PA (n = 48) | p-Value |
|---|---|---|---|---|---|---|---|---|
| TOP | 52 (37.41%) | 23 (14.28%) | 29 (60.42%) | 0.000 | CNVs (n = 9) | 3 (3.30%) | 6 (12.50%) | 0.036 |
| Live birth | 85 (61.15%) | 67 (73.62%) | 18 (37.50%) | 0.001 | VOUS (n = 18) | 12 (13.19%) | 6 (12.50%) | 0.909 |
| Intrauterine death | 2 (1.44%) | 1 (1.10%) | 1 (2.08%) | 0.643 | negative (n = 112) | 76 (83.52%) | 36 (75.00%) | 0.228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, C.; Fu, F.; Zhou, H.; Cheng, K.; Huang, R.; Li, R.; Li, D.; Liao, C. Should Prenatal Chromosomal Microarray Analysis Be Offered for Pulmonary Atresia? A Single-Center Retrospective Study in China. Genes 2023, 14, 722. https://doi.org/10.3390/genes14030722
Wang Y, Ma C, Fu F, Zhou H, Cheng K, Huang R, Li R, Li D, Liao C. Should Prenatal Chromosomal Microarray Analysis Be Offered for Pulmonary Atresia? A Single-Center Retrospective Study in China. Genes. 2023; 14(3):722. https://doi.org/10.3390/genes14030722
Chicago/Turabian StyleWang, You, Chunling Ma, Fang Fu, Hang Zhou, Ken Cheng, Ruibin Huang, Ru Li, Dongzhi Li, and Can Liao. 2023. "Should Prenatal Chromosomal Microarray Analysis Be Offered for Pulmonary Atresia? A Single-Center Retrospective Study in China" Genes 14, no. 3: 722. https://doi.org/10.3390/genes14030722
APA StyleWang, Y., Ma, C., Fu, F., Zhou, H., Cheng, K., Huang, R., Li, R., Li, D., & Liao, C. (2023). Should Prenatal Chromosomal Microarray Analysis Be Offered for Pulmonary Atresia? A Single-Center Retrospective Study in China. Genes, 14(3), 722. https://doi.org/10.3390/genes14030722







