TNC Accelerates Hypoxia-Induced Cardiac Injury in a METTL3-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedure and Construction of Overexpression METTL3 Model
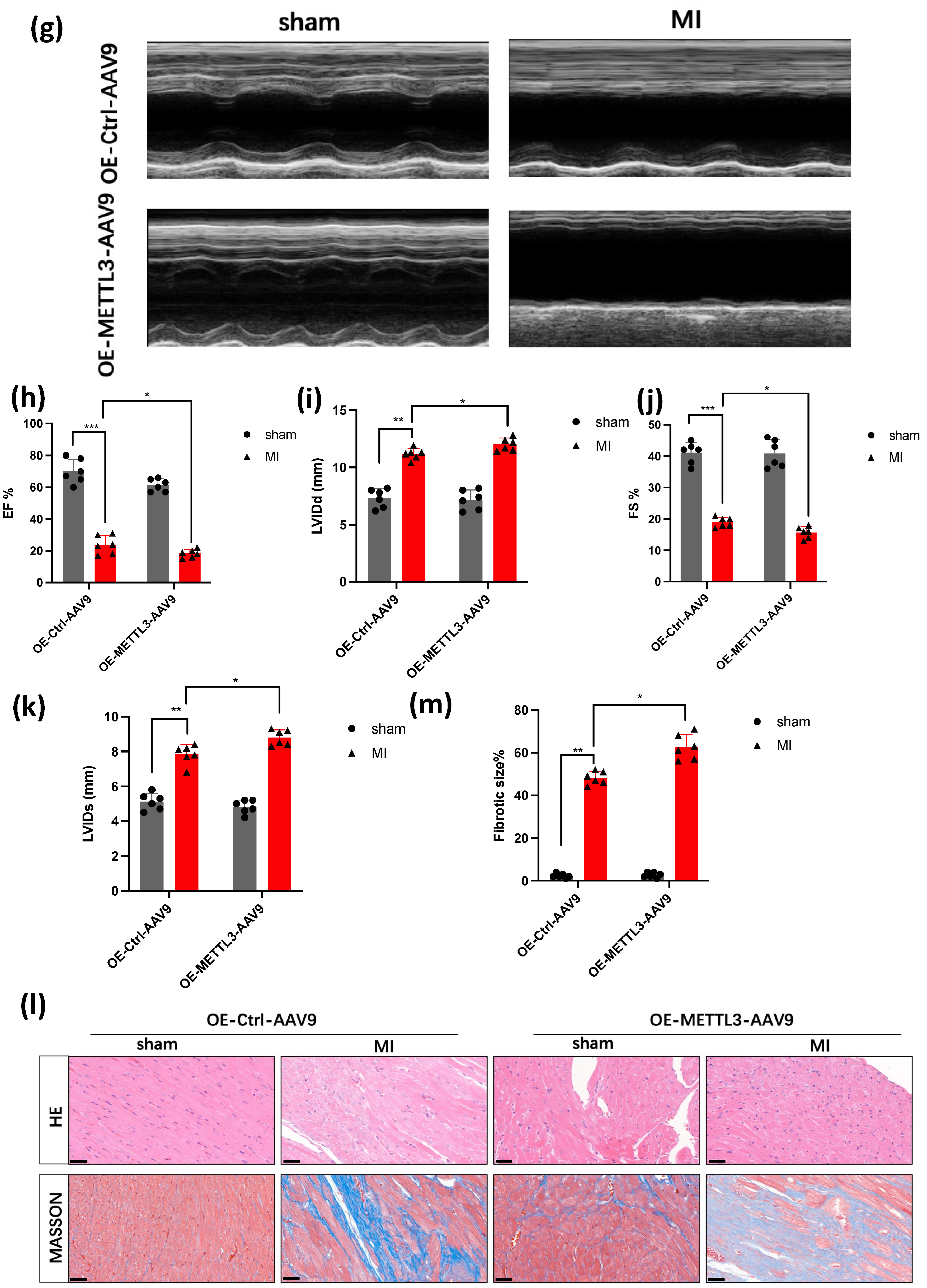
2.2. Echocardiography
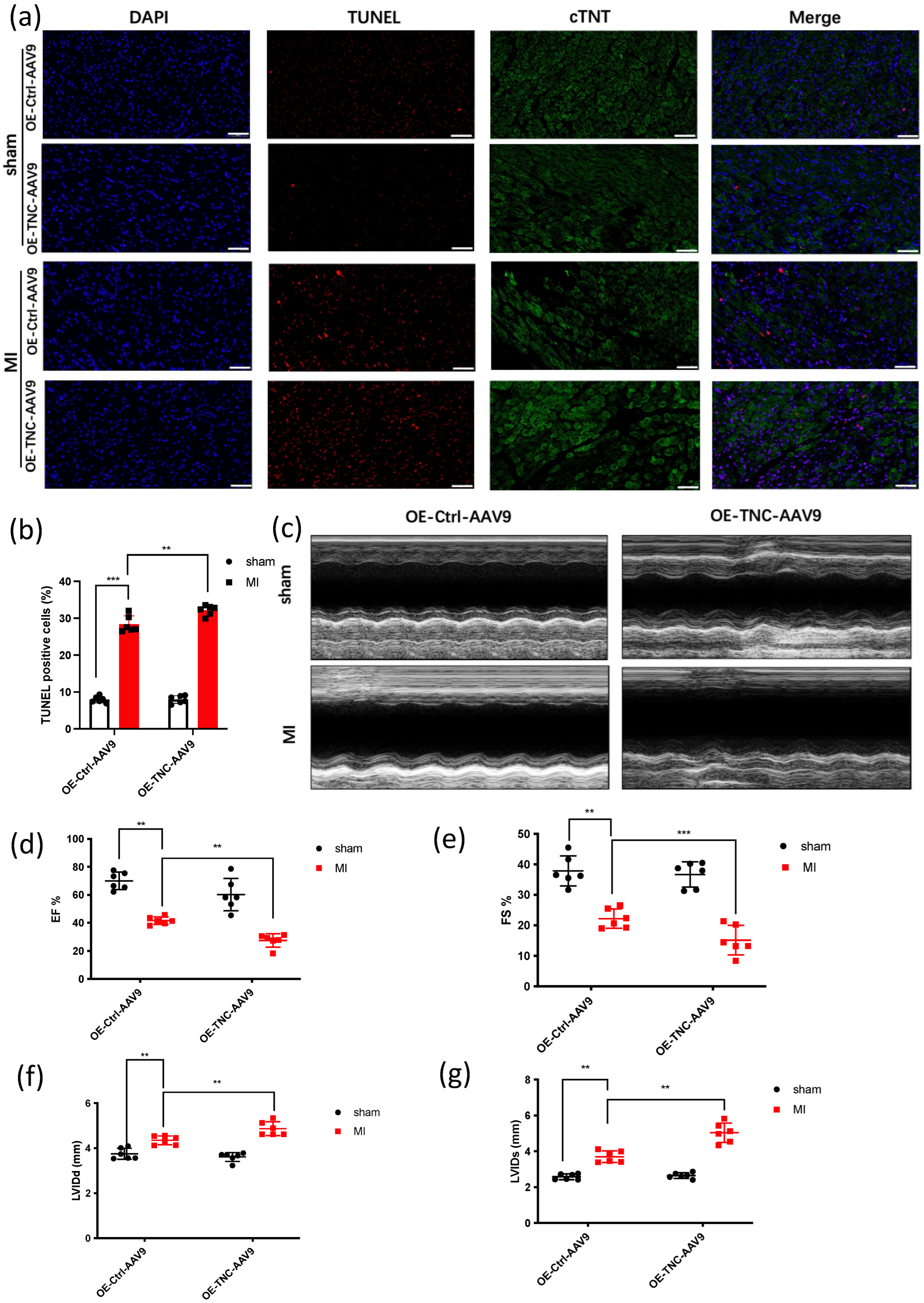
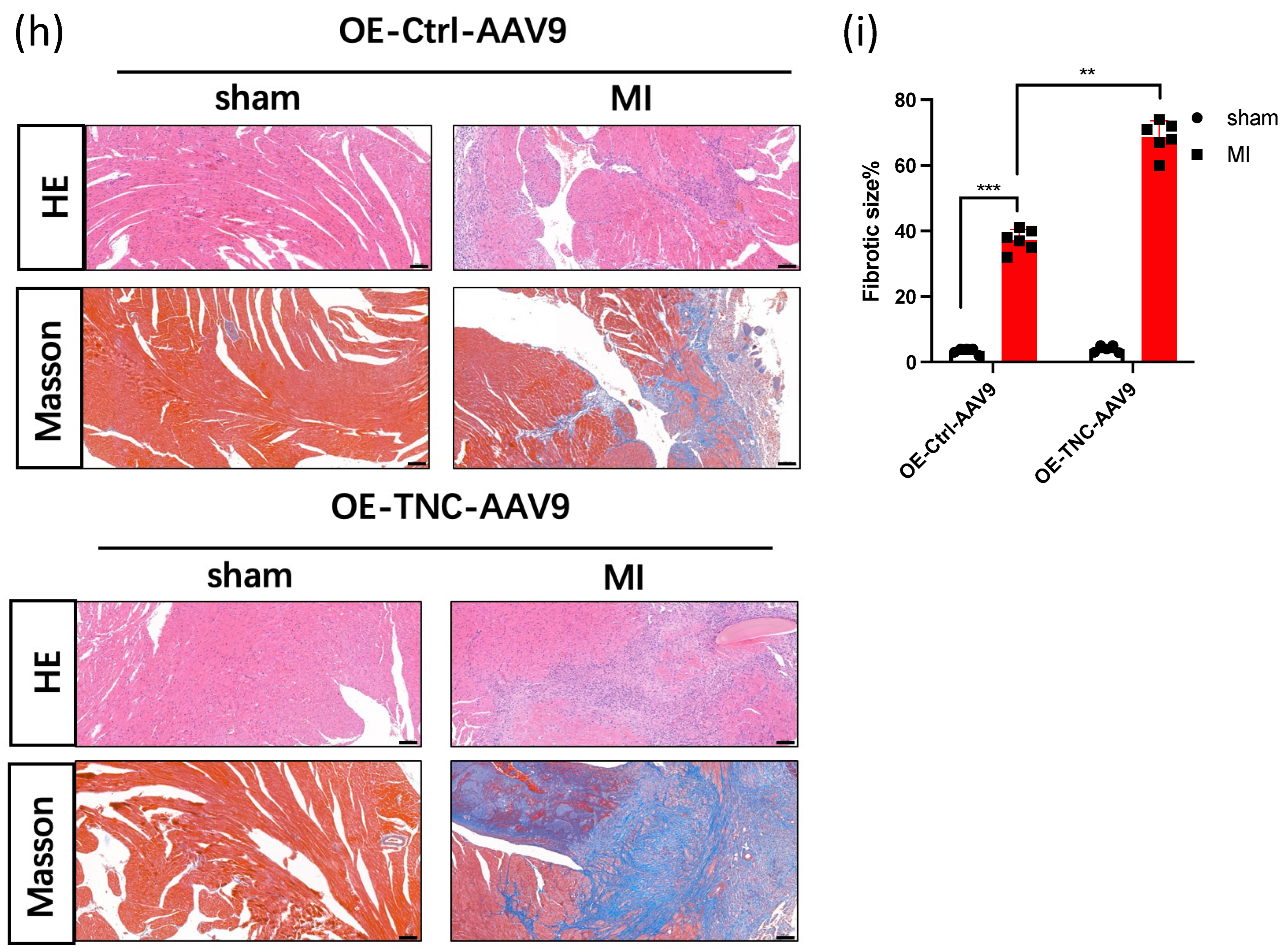
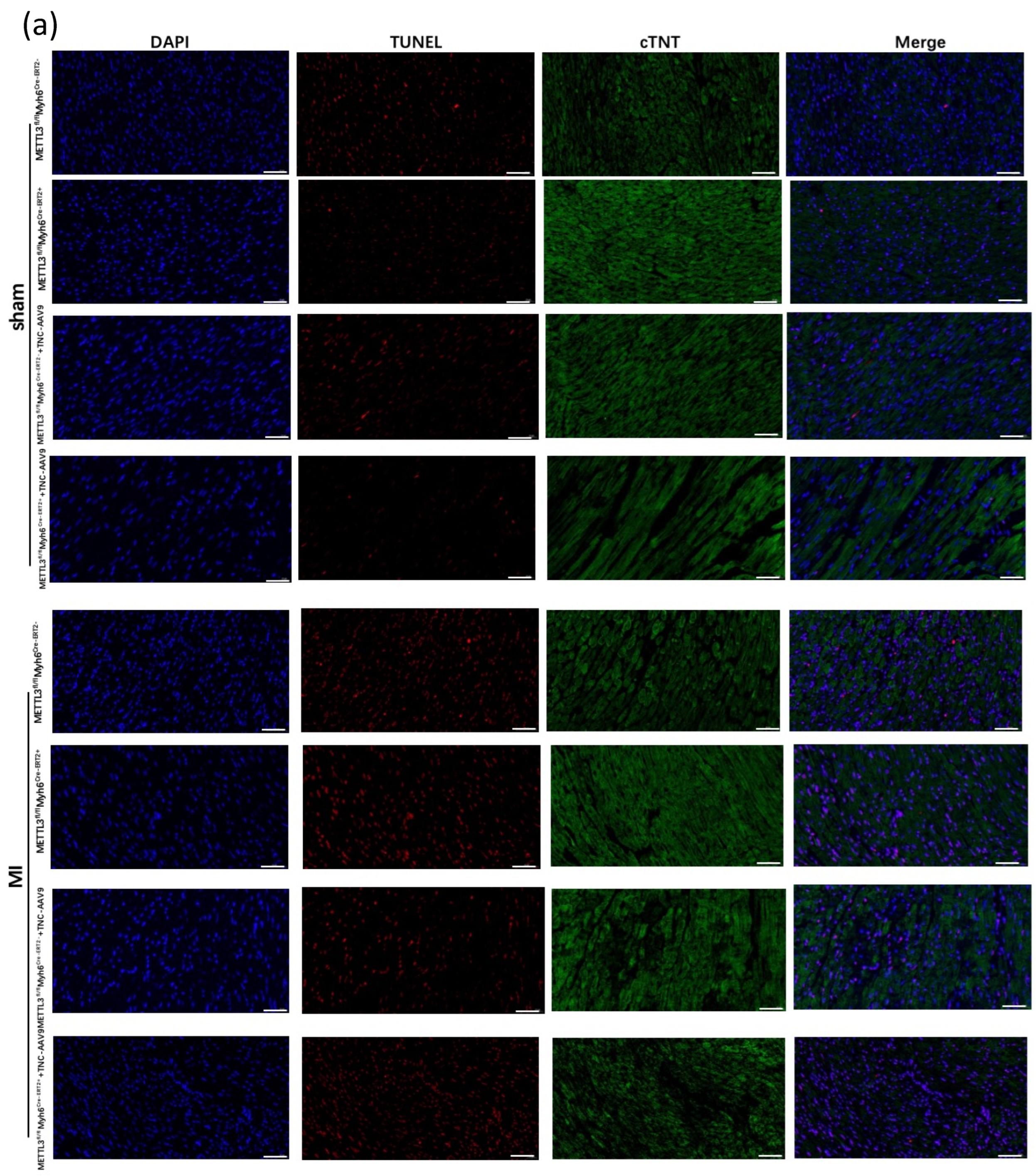
2.3. Histological and Immunohistochemistry Analysis
2.4. TTC Staining
2.5. TUNEL Staining
2.6. Cell Culture and Treatment
2.7. Identification and Analysis of Methylation Sites
2.8. Total RNA Extraction
2.9. PolyA RNA Concentration
2.10. LC-MS/MS for Determination of the m6A/A Ratio
2.11. SELECT for Determination of the m6A%
2.12. Luciferase Reporter Assay
2.13. Statistical Analysis
3. Results
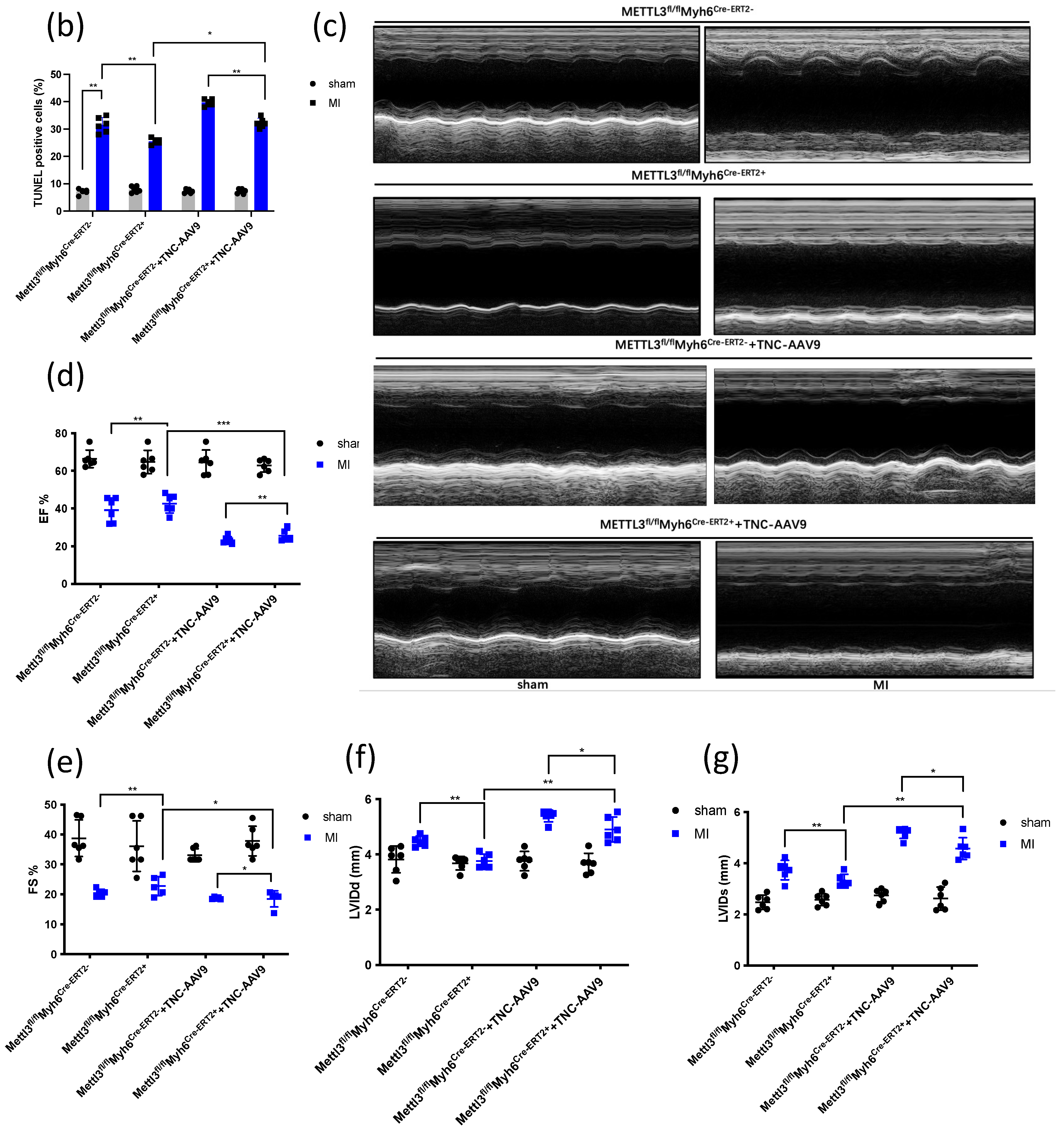
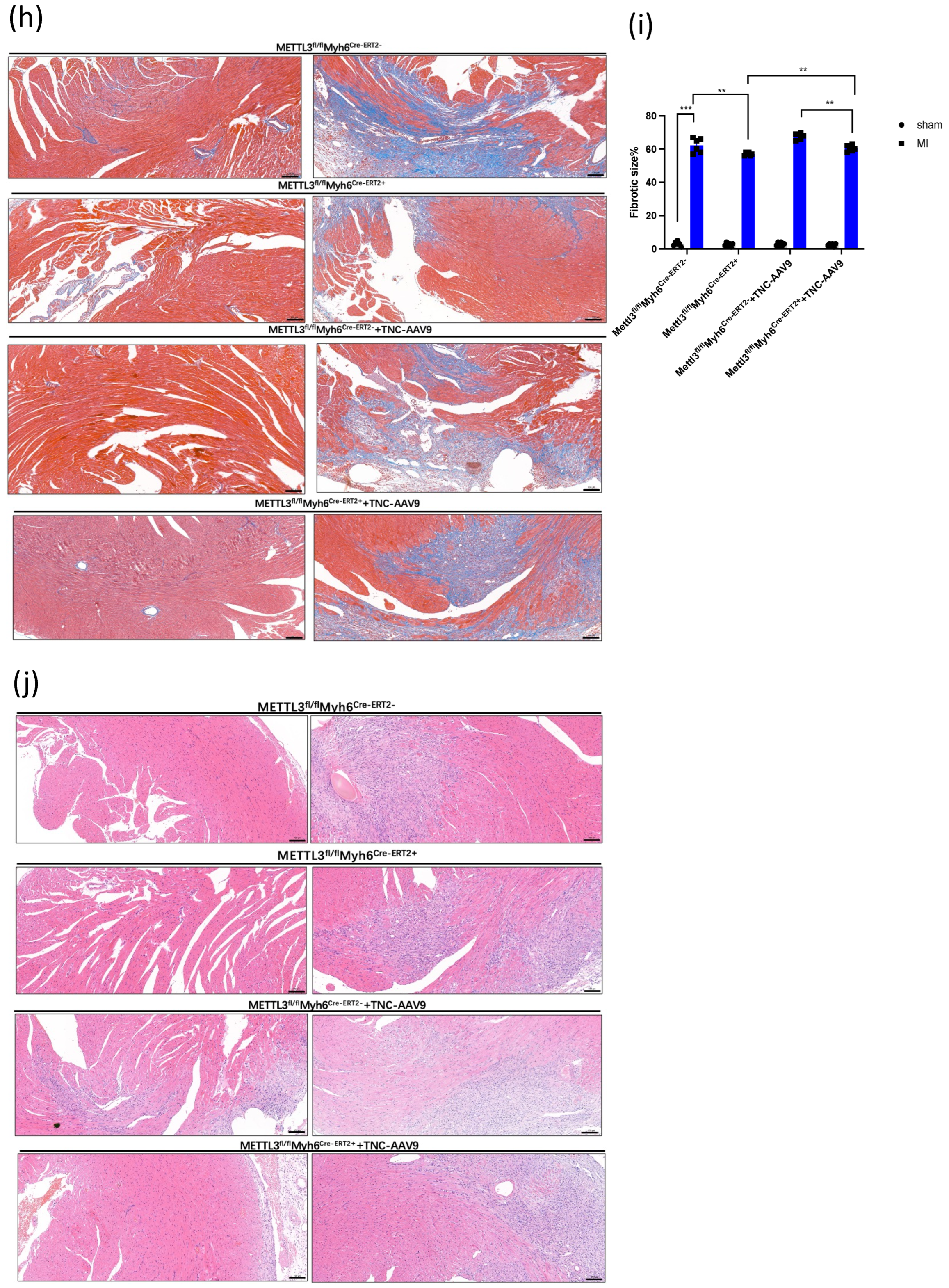
3.1. TNC Promotes Cardiac Dysfunction and Cardiac Fibrosis after Myocardial Infarction
3.2. METTL3 Overexpression Promotes the Deterioration of Cardiac Function
3.3. METTL3 Deficiency Attenuates Cardiac Dysfunction and Cardiac Fibrosis after Myocardial Infarction
3.4. TNC Overexpression in CKO-METTL3 Mice greatly Contributes to the Cardiac Dysfunction and Myocardial Infarction
3.5. METTL3 Regulates the m6A Process of TNC mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MI | myocardial infarction |
| TNC | Tenascin-C |
| OE | overexpression |
| NC | normal control |
| METTL3 | Methyltransferase-like protein 3 |
| LVEF | left ventricular ejection fraction |
| LVFS | left ventricular shortening fraction |
| HE | Hematoxylin-eosin |
| TTC | triphenyl-2,3,5—tetrazolium-chloride |
| FBS | fetal bovine serum |
| NRVM | neonatal rat left ventricle myocytes |
| SRAMP | sequence-based N6-methyladenosine modification site predicator |
| SELECT | single-base elongation- and ligation-based qPCR amplification method |
References
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.M.; Xu, W.; Cao, J.; Zhao, J.Y. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct. Target. Ther. 2021, 6, 54. [Google Scholar] [CrossRef]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Jeong, D.; Oh, J.G. Tenascin-C in Cardiac Hypertrophy and Fibrosis: Friend or Foe? J. Am. Coll. Cardiol. 2017, 70, 1616–1617. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases—The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Tawara, I.; Yoshida, T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol. Cell Physiol. 2020, 319, C781–C796. [Google Scholar] [CrossRef]
- Tajiri, K.; Yonebayashi, S.; Li, S.; Ieda, M. Immunomodulatory Role of Tenascin-C in Myocarditis and Inflammatory Cardiomyopathy. Front. Immunol. 2021, 12, 624703. [Google Scholar] [CrossRef]
- Kimura, T.; Tajiri, K.; Sato, A.; Sakai, S.; Wang, Z.; Yoshida, T.; Uede, T.; Hiroe, M.; Aonuma, K.; Ieda, M.; et al. Tenascin-C accelerates adverse ventricular remodelling after myocardial infarction by modulating macrophage polarization. Cardiovasc. Res. 2019, 115, 614–624. [Google Scholar] [CrossRef]
- Podesser, B.K.; Kreibich, M.; Dzilic, E.; Santer, D.; Förster, L.; Trojanek, S.; Abraham, D.; Krššák, M.; Klein, K.U.; Tretter, E.V.; et al. Tenascin-C promotes chronic pressure overload-induced cardiac dysfunction, hypertrophy and myocardial fibrosis. J. Hypertens. 2018, 36, 847–856. [Google Scholar] [CrossRef]
- Hamza, O.; Kiss, A.; Kramer, A.M.; Trojanek, S.; Abraham, D.; Acar, E.; Nagel, F.; Tretter, V.E.; Kitzwögerer, M.; Podesser, B.K. Tenascin C promotes valvular remodeling in two large animal models of ischemic mitral regurgitation. Basic Res. Cardiol. 2020, 115, 76. [Google Scholar] [CrossRef]
- Abbadi, D.; Laroumanie, F.; Bizou, M.; Pozzo, J.; Daviaud, D.; Delage, C.; Calise, D.; Gaits-Iacovoni, F.; Dutaur, M.; Tortosa, F.; et al. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc. Res. 2018, 114, 123–137. [Google Scholar] [CrossRef]
- Li, T.; Hu, P.S.; Zuo, Z.; Lin, J.F.; Li, X.; Wu, Q.N.; Chen, Z.H.; Zeng, Z.L.; Wang, F.; Zheng, J.; et al. METTL3 facilitates tumor progression via an m6A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer 2019, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, J.Z.; Yang, X.; Yu, H.; Zhou, R.; Lu, H.C.; Yuan, W.B.; Lu, J.C.; Zhou, Z.J.; Lu, Q.; et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol. Cancer 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Song, C.; Yang, L.; Cui, R.; Cheng, X.; Zhang, Z.; Zhao, G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol. Cancer 2019, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Huang, W.; Li, Y.; Weng, H. Roles of METTL3 in cancer: Mechanisms and therapeutic targeting. J. Hematol. Oncol. 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hui, H.; Bray, B.; Gonzalez, G.M.; Zeller, M.; Anderson, K.G.; Knight, R.; Smith, D.; Wang, Y.; Carlin, A.F.; et al. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021, 35, 109091. [Google Scholar] [CrossRef]
- Wang, J.N.; Wang, F.; Ke, J.; Li, Z.; Xu, C.H.; Yang, Q.; Chen, X.; He, X.Y.; He, Y.; Suo, X.G.; et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci. Transl. Med. 2022, 14, eabk2709. [Google Scholar] [CrossRef]
- Song, H.; Feng, X.; Zhang, H.; Luo, Y.; Huang, J.; Lin, M.; Jin, J.; Ding, X.; Wu, S.; Huang, H.; et al. METTL3 and ALKBH5 oppositely regulate m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy 2019, 15, 1419–1437. [Google Scholar] [CrossRef]
- He, J.; Zhou, M.; Yin, J.; Wan, J.; Chu, J.; Jia, J.; Sheng, J.; Wang, C.; Yin, H.; He, F. METTL3 restrains papillary thyroid cancer progression via m6A/c-Rel/IL-8-mediated neutrophil infiltration. Mol. Ther. 2021, 29, 1821–1837. [Google Scholar] [CrossRef]
- Dorn, L.E.; Lasman, L.; Chen, J.; Xu, X.; Hund, T.J.; Medvedovic, M.; Hanna, J.H.; Berlo, J.H.v.; Accornero, F. The N6-Methyladenosine mRNA Methylase METTL3 Controls Cardiac Homeostasis and Hypertrophy. Circulation 2019, 139, 533–545. [Google Scholar] [CrossRef]
- Ramalingam, H.; Kashyap, S.; Cobo-Stark, P.; Flaten, A.; Chang, C.M.; Hajarnis, S.; Hein, K.Z.; Lika, J.; Warner, G.M.; Espindola-Netto, J.M.; et al. A methionine-Mettl3-N6-methyladenosine axis promotes polycystic kidney disease. Cell Metab. 2021, 33, 1234–1247.e7. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, W.; Guo, H.; Wang, Z.; Xu, K.; Chen, C.; Wang, S. Emerging role of RNA methyltransferase METTL3 in gastrointestinal cancer. J. Hematol. Oncol. 2020, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Huang, H.; Yu, Y.; Ding, J.; Yu, Y.; Li, K.; Wei, D.; Ye, Q.; Wang, F.; et al. YTHDF1 Regulates Pulmonary Hypertension through Translational Control of MAGED1. Am. J. Respir. Crit. Care Med. 2021, 203, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.S.; Li, J.Y.S.; Chien, Y.; Wang, M.L.; Yarmishyn, A.A.; Tsai, P.H.; Juan, C.C.; Nguyen, P.; Cheng, H.M.; Huo, T.I.; et al. METTL3-dependent N6-methyladenosine RNA modification mediates the atherogenic inflammatory cascades in vascular endothelium. Proc. Natl. Acad. Sci. USA 2021, 118, e2025070118. [Google Scholar] [CrossRef]
- Gong, R.; Wang, X.; Li, H.; Liu, S.; Jiang, Z.; Zhao, Y.; Yu, Y.; Han, Z.; Yu, Y.; Dong, C.; et al. Loss of m6A methyltransferase METTL3 promotes heart regeneration and repair after myocardial injury. Pharmacol. Res. 2021, 174, 105845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, C.; Zhang, J.; Sun, W.; Zhou, B.; Kong, X.; Shi, J. METTL3 improves cardiomyocyte proliferation upon myocardial infarction via upregulating miR-17-3p in a DGCR8-dependent manner. Cell Death Discov. 2021, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Longhini, A.P.; Zhang, X.; Hoang, V.; Wilson, M.Z.; Kosik, K.S. Dynamic assembly of the mRNA m6A methyltransferase complex is regulated by METTL3 phase separation. PLoS Biol. 2022, 20, e3001535. [Google Scholar] [CrossRef]
- Gao, X.Q.; Zhang, Y.H.; Liu, F.; Ponnusamy, M.; Zhao, X.M.; Zhou, L.Y.; Zhai, M.; Liu, C.Y.; Li, X.M.; Wang, M.; et al. The piRNA CHAPIR regulates cardiac hypertrophy by controlling METTL3-dependent N6-methyladenosine methylation of Parp10 mRNA. Nat. Cell Biol. 2020, 22, 1319–1331. [Google Scholar] [CrossRef]
- Kumari, R.; Ranjan, P.; Suleiman, Z.G.; Goswami, S.K.; Li, J.; Prasad, R.; Verma, S.K. mRNA modifications in cardiovascular biology and disease: With a focus on m6A modification. Cardiovasc. Res. 2022, 118, 1680–1692. [Google Scholar] [CrossRef]
- Cheng, H.; Chang, S.; Xu, R.; Chen, L.; Song, X.; Wu, J.; Qian, J.; Zou, Y.; Ma, J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res. Ther. 2020, 11, 224. [Google Scholar] [CrossRef]
- Bai, W.W.; Wang, H.; Gao, C.H.; Liu, K.Y.; Guo, B.X.; Jiang, F.; Zhang, M.X.; Li, C.; Qin, W.D. Continuous Infusion of Angiotensin IV Protects against Acute Myocardial Infarction via the Inhibition of Inflammation and Autophagy. Oxid. Med. Cell Longev. 2021, 2021, 2860488. [Google Scholar] [CrossRef]
- Lee, S.Y.; An, H.-J.; Kim, J.M.; Sung, M.-J.; Kim, D.K.; Kim, H.K.; Oh, J.; Jeong, H.Y.; Lee, Y.H.; Yang, T.; et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res. Ther. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, P.; Li, Y.-H.; Zhang, Z.; Cui, Q. SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016, 44, e91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, Y.; Hu, W.; Zhang, Y.; Wu, X.; Lu, J.; Li, M.; Li, W.; Wu, W.; Wang, J.; et al. m6A RNA modification modulates PI3K/Akt/mTOR signal pathway in Gastrointestinal Cancer. Theranostics 2020, 10, 9528–9543. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Lloyd, M.M.; Devine, J.M. Characterization and transcription analysis of a cloned sequence derived from a major developmentally regulated mRNA of D. discoideum. Cell 1979, 17, 903–913. [Google Scholar] [CrossRef]
- Oomoto, I.; Suzuki-Hirano, A.; Umeshima, H.; Han, Y.-W.; Yanagisawa, H.; Carlton, P.; Harada, Y.; Kengaku, M.; Okamoto, A.; Shimogori, T.; et al. ECHO-liveFISH: In vivo RNA labeling reveals dynamic regulation of nuclear RNA foci in living tissues. Nucleic Acids Res. 2015, 43, e126. [Google Scholar] [CrossRef]
- Li, H.-B.; Huang, G.; Tu, J.; Lv, D.-M.; Jin, Q.-L.; Chen, J.-K.; Zou, Y.-T.; Lee, D.-F.; Shen, J.-N.; Xie, X.-B. METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine 2022, 82, 104142. [Google Scholar] [CrossRef]
- Hou, N.; Li, C.; He, J.; Liu, Y.; Yu, S.; Malnoy, M.; Tahir, M.M.; Xu, L.; Ma, F.; Guan, Q. MdMTA-mediated m6 A modification enhances drought tolerance by promoting mRNA stability and translation efficiency of genes involved in lignin deposition and oxidative stress. New Phytol. 2022, 234, 1294–1314. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Tang, Q.; Wei, L.; Zhang, X.; Jia, G. An Elongation- and Ligation-Based qPCR Amplification Method for the Radiolabeling-Free Detection of Locus-Specific N6-Methyladenosine Modification. Angew. Chem. Int. Ed. 2018, 57, 15995–16000. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Deng, S.; Lu, J.; Huang, H.; Zhang, Y.; Gong, P.; Shen, X.; Ruan, H.; Jin, M.; et al. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol. Ther. Nucleic Acids 2018, 11, 103–115. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Jiang, W.; Li, Y. MiR-484 Protects Rat Myocardial Cells from Ischemia-Reperfusion Injury by Inhibiting Caspase-3 and Caspase-9 during Apoptosis. Korean Circ. J. 2020, 50, 250–263. [Google Scholar] [CrossRef]
- Xiang, Y.; Liang, B.; Zhang, X.; Qiu, X.; Deng, Q.; Yu, L.; Yu, H.; Lu, Z.; Zheng, F. Atheroprotective mechanism by which folic acid regulates monocyte subsets and function through DNA methylation. Clin. Epigenetics 2022, 14, 32. [Google Scholar] [CrossRef]
- Cai, W.; Xu, D.; Zeng, C.; Liao, F.; Li, R.; Lin, Y.; Zhao, Y.; Dong, W.; Wang, Q.; Yang, H.; et al. Modulating Lysine Crotonylation in Cardiomyocytes Improves Myocardial Outcomes. Circ. Res. 2022, 131, 456–472. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Huang, T.; Zhao, X.; Chen, W.; Gu, N.; Zhang, R. Dynamic landscape and evolution of m6A methylation in human. Nucleic Acids Res. 2020, 48, 6251–6264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, J.; Xu, F.; Zhu, Q.; Chen, Y.; Ge, D.; Lu, C. Molecular characterization, biological function, tumor microenvironment association and clinical significance of m6A regulators in lung adenocarcinoma. Brief. Bioinform. 2021, 22, bbaa225. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, T.; Sugano, Y.; Nakayama, T.; Nagai, T.; Matsuyama, T.; Ohta-Ogo, K.; Ikeda, Y.; Ishibashi-Ueda, H.; Nakatani, T.; Yasuda, S.; et al. Significance of myocardial tenascin-C expression in left ventricular remodelling and long-term outcome in patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Chelmicki, T.; Roger, E.; Teissandier, A.; Dura, M.; Bonneville, L.; Rucli, S.; Dossin, F.; Fouassier, C.; Lameiras, S.; Bourc’his, D. m6A RNA methylation regulates the fate of endogenous retroviruses. Nature 2021, 591, 312–316. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Dong, S.; Yu, Q.; Jia, G. Antibody-free enzyme-assisted chemical approach for detection of N6-methyladenosine. Nat. Chem. Biol. 2020, 16, 896–903. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Li, J.; Xi, H.; Ying, Y.; Chen, H.; Yan, H.; He, L.; Xu, M.; Xu, X.; Liang, Z.; Liu, B.; et al. YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol. Cancer 2020, 19, 152. [Google Scholar] [CrossRef]




| Position | Sequence Context | Score (Binary) | Score (Knn) | Score (Spectrum) | Score (Combined) | |
|---|---|---|---|---|---|---|
| 1 | 1611 | GACGA GGGCU AUACC GGAGA AGACU * GUAGC CAGCG GCGAU GCCCC | 0.811 | 0.619 | 0.807 | 0.838 |
| 2 | 1797 | GAUGA CGACU ACACU GGGGA AGACU * GCAGA GACCG GCGCU GUCCC | 0.836 | 0.612 | 0.860 | 0.834 |
| 3 | 1806 | UACAC UGGGG AAGAC UGCAG AGACC * GGCGC UGUCC CCGGG ACUGU | 0.798 | 0.263 | 0.858 | 0.795 |
| 4 | 1983 | CACGA GGGCU UCACU GGCAA AGACU * GCAAA GAGCA AAGGU GCCCC | 0.882 | 0.614 | 0.840 | 0.852 |
| 5 | 2076 | CAUGA GGGCU UUACG GGCCU GGACU * GUGGG CAGCG CUCCU GUCCC | 0.893 | 0.858 | 0.822 | 0.862 |
| 6 | 3596 | GGAUG GCCUC AGACU CAACU GGACU * GCAGA UGACC UGGCC UAUGA | 0.725 | 0.717 | 0.755 | 0.736 |
| 7 | 3869 | GGAUG CCCUC ACGCU CAACU GGACU * GCUCC AGAAG GAGCC UAUAA | 0.739 | 0.600 | 0.663 | 0.701 |
| 8 | 6211 | GCAGA GAAGA AUUUU GGCUU GGACU * GGAUA ACCUG AGCAA AAUCA | 0.762 | 0.824 | 0.622 | 0.709 |
| Position | Sequence Context | Score (Binary) | Score (Knn) | Score (Spectrum) | Score (Combined) | |
|---|---|---|---|---|---|---|
| 1 | 1555 | AGCGA GAAGA GGUGU CCUGC UGACU * GUCAC AAUCG UGGCC GCUGU | 0.846 | 0.540 | 0.803 | 0.814 |
| 2 | 2113 | AAGGA GCAAA GAUGU CCCAG UGACU * GUCAU GGCCA GGGCC GCUGC | 0.877 | 0.697 | 0.818 | 0.844 |
| 3 | 2179 | CACGA GGGCU UCACA GGCCU GGACU * GUGGC CAGCA CUCCU GCCCC | 0.754 | 0.773 | 0.568 | 0.681 |
| 4 | 3972 | GGAUG CCCUC AAACU CAACU GGACU * GCUCC AGAAG GGGCC UAUGA | 0.741 | 0.662 | 0.609 | 0.684 |
| 5 | 4673 | AUUUU AUUGU CUACC UCUCU GGACU * UGCUC CCAGC AUCCG GACCA | 0.730 | 0.775 | 0.601 | 0.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Li, L.; Xue, J.; Ma, J.; Ge, J. TNC Accelerates Hypoxia-Induced Cardiac Injury in a METTL3-Dependent Manner. Genes 2023, 14, 591. https://doi.org/10.3390/genes14030591
Cheng H, Li L, Xue J, Ma J, Ge J. TNC Accelerates Hypoxia-Induced Cardiac Injury in a METTL3-Dependent Manner. Genes. 2023; 14(3):591. https://doi.org/10.3390/genes14030591
Chicago/Turabian StyleCheng, Hao, Linnan Li, Junqiang Xue, Jianying Ma, and Junbo Ge. 2023. "TNC Accelerates Hypoxia-Induced Cardiac Injury in a METTL3-Dependent Manner" Genes 14, no. 3: 591. https://doi.org/10.3390/genes14030591
APA StyleCheng, H., Li, L., Xue, J., Ma, J., & Ge, J. (2023). TNC Accelerates Hypoxia-Induced Cardiac Injury in a METTL3-Dependent Manner. Genes, 14(3), 591. https://doi.org/10.3390/genes14030591








