Feasibility of Community Pharmacist-Initiated and Point-of-Care CYP2C19 Genotype-Guided De-Escalation of Oral P2Y12 Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
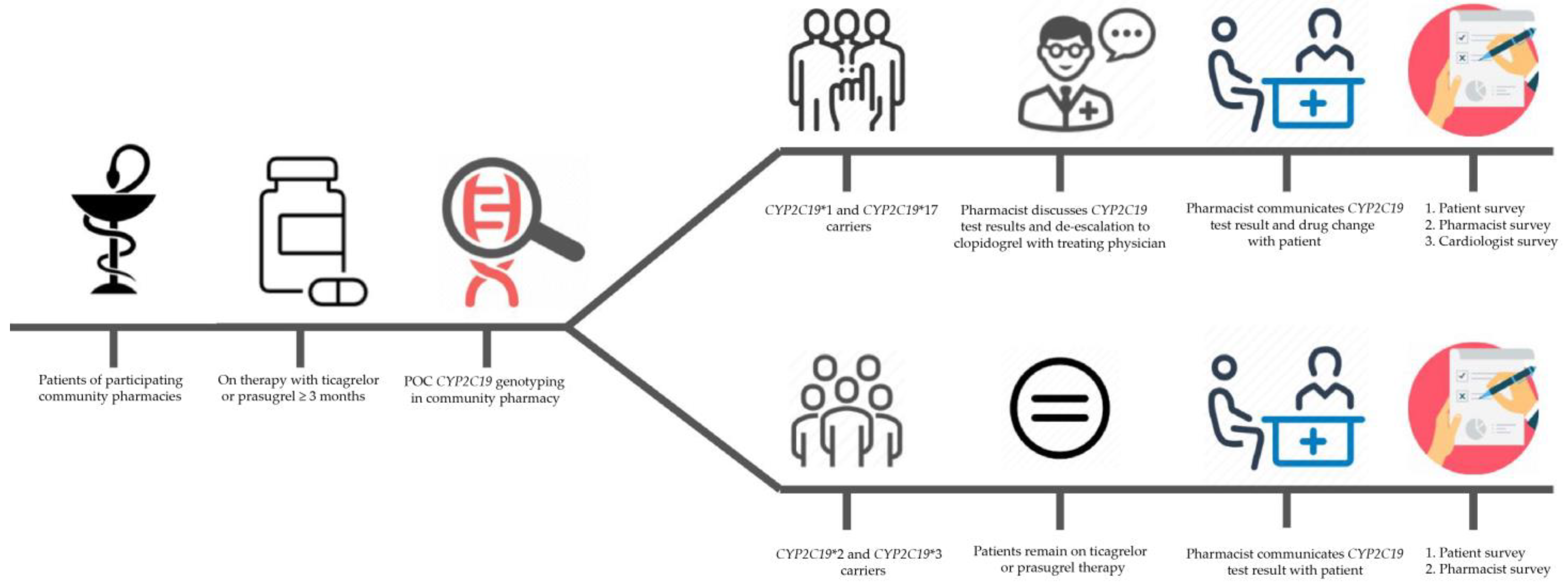
2.2. Study Design
2.3. Ethical Approval
2.4. Study Population
2.5. Data Collection: POC CYP2C19 PGx Testing
2.6. Data Collection: Patient, Pharmacist and Cardiologist Survey
2.7. Cost Analysis
2.8. Data Analysis
3. Results
3.1. Participant Demographics
3.2. POC Genotyping Results
3.3. Patient Survey
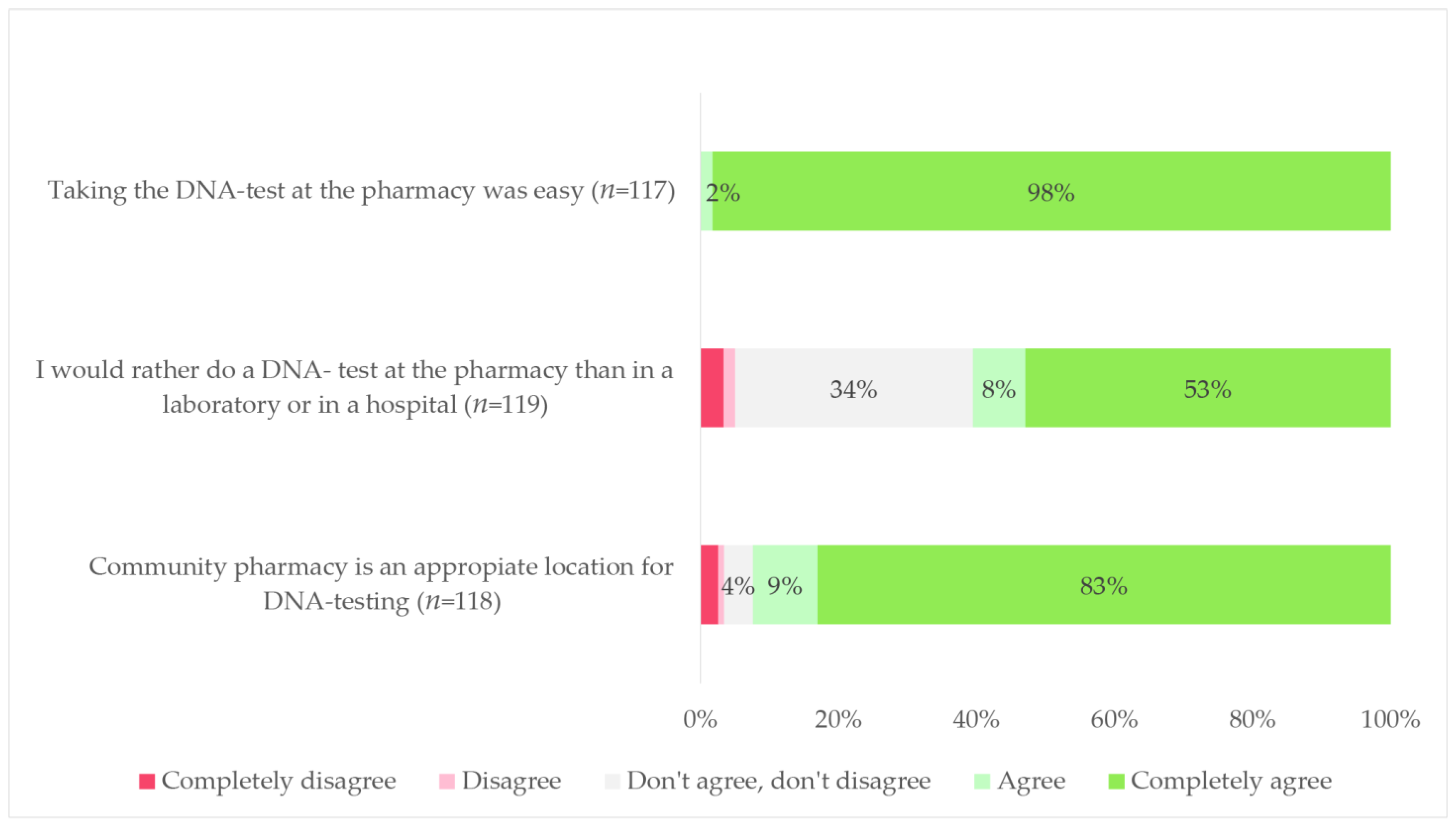
3.3.1. PGx in the Community Pharmacy
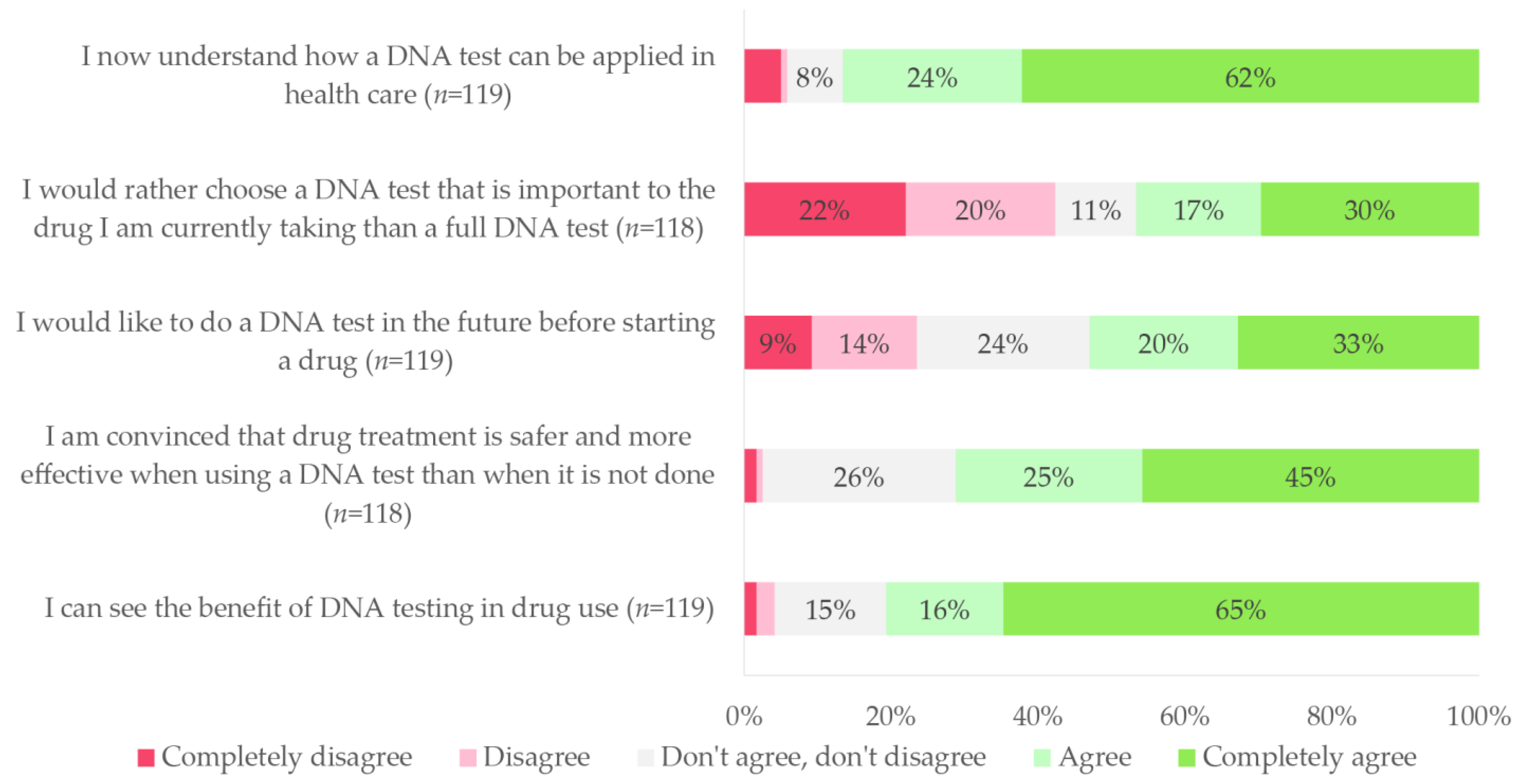
3.3.2. Added Value of PGx
3.3.3. Pharmacist-Patient Communication
3.3.4. Sharing PGx Results
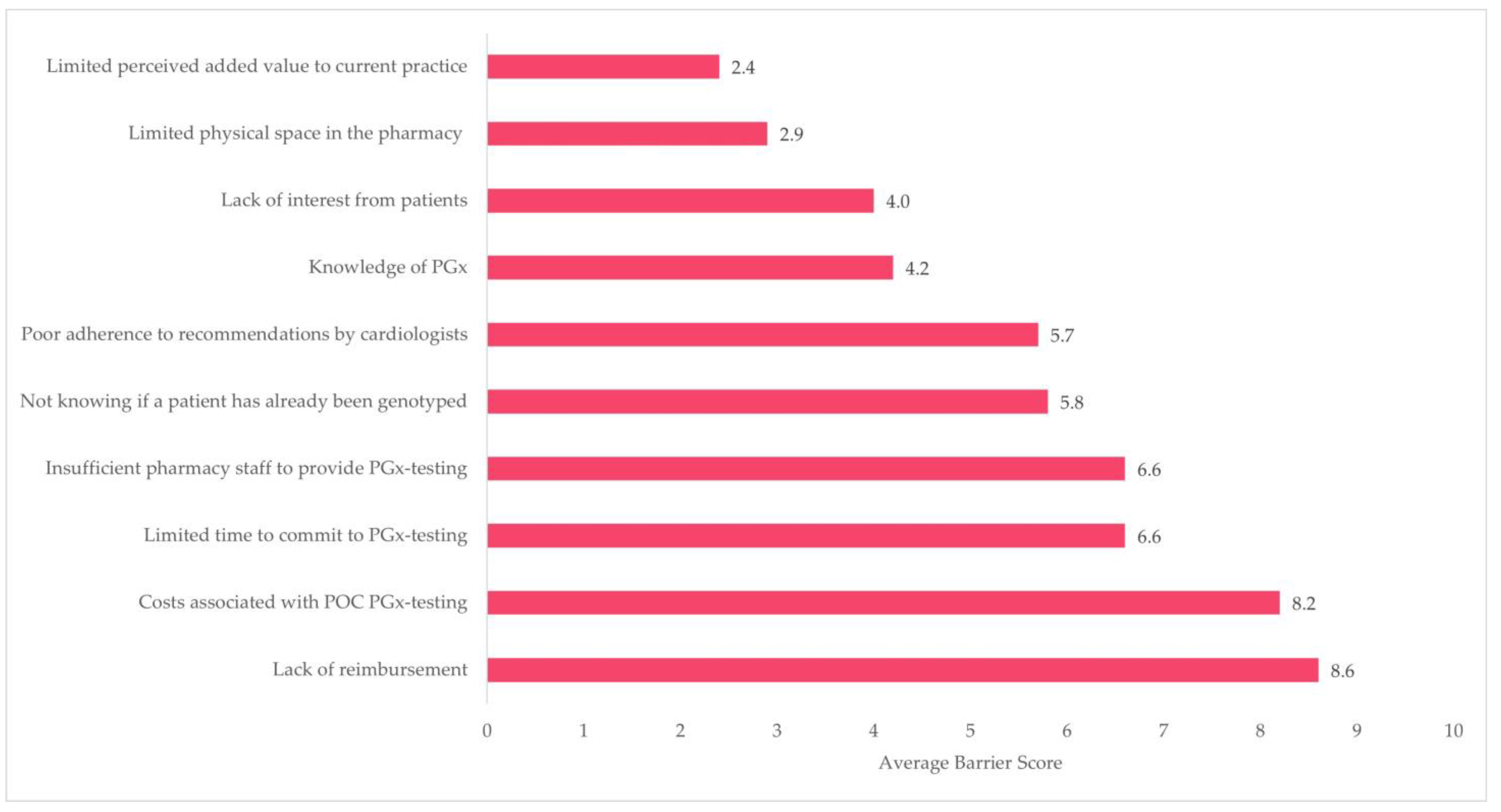
3.4. Pharmacist Survey
3.5. Cardiologist Survey
3.6. Cost Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capodanno, D.; Alfonso, F.; Levine, G.N.; Valgimigli, M.; Angiolillo, D.J. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2018, 72, 2915–2931. [Google Scholar] [CrossRef]
- Koski, R.; Kennedy, B. Comparative Review of Oral P2Y(12) Inhibitors. Pharm. Ther. 2018, 43, 352–357. [Google Scholar] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Medicijnkosten.nl. Available online: https://www.medicijnkosten.nl (accessed on 11 November 2021).
- Goodman, S.G.; Nicolau, J.C.; Requena, G.; Maguire, A.; Blankenberg, S.; Chen, J.Y.; Granger, C.B.; Grieve, R.; Pocock, S.J.; Simon, T.; et al. Longer-term oral antiplatelet use in stable post-myocardial infarction patients: Insights from the long Term rIsk, clinical manaGement and healthcare Resource utilization of stable coronary artery dISease (TIGRIS) observational study. Int. J. Cardiol. 2017, 236, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Rihal, C.S.; So, D.Y.F.; Rosenberg, Y.; Lennon, R.J.; Mathew, V.; Goodman, S.G.; Weinshilboum, R.M.; Wang, L.; Baudhuin, L.M.; et al. Clopidogrel Pharmacogenetics. Circ. Cardiovasc. Interv. 2019, 12, e007811. [Google Scholar] [CrossRef]
- Bueno, H.; Sinnaeve, P.; Annemans, L.; Danchin, N.; Licour, M.; Medina, J.; Pocock, S.; Sánchez-Covisa, J.; Storey, R.F.; Jukema, J.W.; et al. Opportunities for improvement in anti-thrombotic therapy and other strategies for the management of acute coronary syndromes: Insights from EPICOR, an international study of current practice patterns. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 3–12. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication: Reduced Effectiveness of Plavix (Clopidogrel) in Patients Who Are Poor Metabolizers of the Drug. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-reduced-effectiveness-plavix-clopidogrel-patients-who-are-poor (accessed on 4 December 2022).
- Mega, J.L.; Simon, T.; Collet, J.P.; Anderson, J.L.; Antman, E.M.; Bliden, K.; Cannon, C.P.; Danchin, N.; Giusti, B.; Gurbel, P.; et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA 2010, 304, 1821–1830. [Google Scholar] [CrossRef]
- Harmsze, A.M.; van Werkum, J.W.; Ten Berg, J.M.; Zwart, B.; Bouman, H.J.; Breet, N.J.; van ‘t Hof, A.W.; Ruven, H.J.; Hackeng, C.M.; Klungel, O.H.; et al. CYP2C19*2 and CYP2C9*3 alleles are associated with stent thrombosis: A case-control study. Eur. Heart J. 2010, 31, 3046–3053. [Google Scholar] [CrossRef]
- Sorich, M.J.; Rowland, A.; McKinnon, R.A.; Wiese, M.D. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: A meta-analysis. Circ. Cardiovasc. Genet. 2014, 7, 895–902. [Google Scholar] [CrossRef]
- Cavallari, L.H.; Lee, C.R.; Beitelshees, A.L.; Cooper-DeHoff, R.M.; Duarte, J.D.; Voora, D.; Kimmel, S.E.; McDonough, C.W.; Gong, Y.; Dave, C.V.; et al. Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van ‘t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y(12) Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Annotation of DPWG Guideline for Clopidogrel and CYP2C19. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166104956 (accessed on 12 January 2022).
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Phamacogenomic Recommendations, Farmacogenetica-Update-Januari 2022. Available online: https://www.knmp.nl/dossiers/farmacogenetica (accessed on 1 July 2022).
- Abdelazeem, B.; Shehata, J.; Abbas, K.S.; El-Shahat, N.A.; Baral, N.; Adhikari, G.; Khan, H.; Hassan, M. De-escalation from Prasugrel or Ticagrelor to Clopidogrel in Patients with Acute Coronary Syndrome Managed with Percutaneous Coronary Intervention: An Updated Meta-analysis of Randomized Clinical Trials. Am. J. Cardiovasc. Drugs 2021, 22, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Williams, A.K.; Klein, M.D.; Sriramoju, V.B.; Madan, S.; Rossi, J.S.; Clarke, M.; Cicci, J.D.; Cavallari, L.H.; Weck, K.E.; et al. Frequency and clinical outcomes of CYP2C19 genotype-guided escalation and de-escalation of antiplatelet therapy in a real-world clinical setting. Genet. Med. 2020, 22, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Park, M.W.; Kim, M.C.; Choo, E.H.; Hwang, B.H.; Lee, K.Y.; Choi, Y.S.; Kim, H.Y.; Yoo, K.D.; Jeon, D.S.; et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): An investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet 2021, 398, 1305–1316. [Google Scholar] [CrossRef]
- Breaux, S.; Desrosiers, F.A.D.; Neira, M.; Sinha, S.; Nislow, C. Pharmacogenomics at the Point of Care: A Community Pharmacy Project in British Columbia. J. Pers. Med. 2020, 11, 11. [Google Scholar] [CrossRef]
- Padgett, L.; O’Connor, S.; Roederer, M.; McLeod, H.; Ferreri, S. Pharmacogenomics in a community pharmacy: ACT now. J. Am. Pharm. Assoc. 2011, 51, 189–193. [Google Scholar] [CrossRef]
- Ferreri, S.P.; Greco, A.J.; Michaels, N.M.; O’Connor, S.K.; Chater, R.W.; Viera, A.J.; Faruki, H.; McLeod, H.L.; Roederer, M.W. Implementation of a pharmacogenomics service in a community pharmacy. J. Am. Pharm. Assoc. 2014, 54, 172–180. [Google Scholar] [CrossRef]
- Bright, D.R.; Kisor, D.F.; Smith, A.; Conaway, M.; Yu, M. Implementation of a pharmacogenetic management service for postmyocardial infarction care in a community pharmacy. Pers. Med. 2015, 12, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Bergmeijer, T.O.; Vos, G.J.; Claassens, D.M.; Janssen, P.W.; Harms, R.; der Heide, R.V.; Asselbergs, F.W.; Ten Berg, J.M.; Deneer, V.H. Feasibility and implementation of CYP2C19 genotyping in patients using antiplatelet therapy. Pharmacogenomics 2018, 19, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Van Mil, J.W. Pharmaceutical care in community pharmacy: Practice and research in the Netherlands. Ann. Pharmacother. 2005, 39, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- KNMP. Algemene Achtergrondtekst Farmacogenetica—CYP2C19; KNMP: Zuid-Holland, The Netherlands, 2021. [Google Scholar]
- KNMP. Handleiding Interpretatie en Vastlegging Farmacogenetica; KNMP: Zuid-Holland, The Netherlands, 2017. [Google Scholar]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Lanting, P.; Drenth, E.; Boven, L.; van Hoek, A.; Hijlkema, A.; Poot, E.; van der Vries, G.; Schoevers, R.; Horwitz, E.; Gans, R.; et al. Practical Barriers and Facilitators Experienced by Patients, Pharmacists and Physicians to the Implementation of Pharmacogenomic Screening in Dutch Outpatient Hospital Care-An Explorative Pilot Study. J. Pers. Med. 2020, 10, 293. [Google Scholar] [CrossRef]
- Alexander, K.M.; Divine, H.S.; Hanna, C.R.; Gokun, Y.; Freeman, P.R. Implementation of personalized medicine services in community pharmacies: Perceptions of independent community pharmacists. J. Am. Pharm. Assoc. 2014, 54, 510–517. [Google Scholar] [CrossRef]
- Van der Wouden, C.H.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.C.; Dávila-Fajardo, C.L.; Deneer, V.H.; Dolžan, V.; Ingelman-Sundberg, M.; Jönsson, S.; Karlsson, M.O.; et al. Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2017, 101, 341–358. [Google Scholar] [CrossRef]
- Formdesk; Innovero Software Solutions B.V.: Wassenaar, The Netherlands, 2022.
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Haga, S.B.; Moaddeb, J.; Mills, R.; Voora, D. Assessing feasibility of delivering pharmacogenetic testing in a community pharmacy setting. Pharmacogenomics 2017, 18, 327–335. [Google Scholar] [CrossRef]
- O’Connor, S.K.; Ferreri, S.P.; Michaels, N.M.; Greco, A.J.; Viera, A.J.; Faruki, H.; McLeod, H.L.; Roederer, M.W. Exploratory planning and implementation of a pilot pharmacogenetic program in a community pharmacy. Pharmacogenomics 2012, 13, 955–962. [Google Scholar] [CrossRef]
- Moaddeb, J.; Mills, R.; Haga, S.B. Community pharmacists’ experience with pharmacogenetic testing. J. Am. Pharm. Assoc. 2015, 55, 587–594. [Google Scholar] [CrossRef]
- Baudhuin, L.M.; Train, L.J.; Goodman, S.G.; Lane, G.E.; Lennon, R.J.; Mathew, V.; Murthy, V.; Nazif, T.M.; So, D.Y.F.; Sweeney, J.P.; et al. Point of care CYP2C19 genotyping after percutaneous coronary intervention. Pharm. J. 2022, 22, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.; Glick, H.; Matthai, W.; Nachamkin, I.; Nathan, A.; Monono, K.; Carcuffe, C.; Maslowski, K.; Chang, G.; Kobayashi, T.; et al. Prospective CYP2C19 Genotyping to Guide Antiplatelet Therapy Following Percutaneous Coronary Intervention: A Pragmatic Randomized Clinical Trial. Circ. Genom. Precis. Med. 2020, 13, e002640. [Google Scholar] [CrossRef]
- Meng, X.; Wang, A.; Zhang, G.; Niu, S.; Li, W.; Han, S.; Fang, F.; Zhao, X.; Dong, K.; Jin, Z.; et al. Analytical validation of GMEX rapid point-of-care CYP2C19 genotyping system for the CHANCE-2 trial. Stroke Vasc. Neurol. 2021, 6, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, L.H.; Franchi, F.; Rollini, F.; Been, L.; Rivas, A.; Agarwal, M.; Smith, D.M.; Newsom, K.; Gong, Y.; Elsey, A.R.; et al. Clinical implementation of rapid CYP2C19 genotyping to guide antiplatelet therapy after percutaneous coronary intervention. J. Transl. Med. 2018, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Hamdy, D.A.; Mahmoud, S.H. Applications for pharmacogenomics in pharmacy practice: A scoping review. Res. Soc. Adm. Pharm. 2022, 18, 3094–3118. [Google Scholar] [CrossRef]
- Morrow, D.A.; Wiviott, S.D.; White, H.D.; Nicolau, J.C.; Bramucci, E.; Murphy, S.A.; Bonaca, M.P.; Ruff, C.T.; Scirica, B.M.; McCabe, C.H.; et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: An application of the classification system from the universal definition of myocardial infarction. Circulation 2009, 119, 2758–2764. [Google Scholar] [CrossRef]
- Velders, M.A.; Abtan, J.; Angiolillo, D.J.; Ardissino, D.; Harrington, R.A.; Hellkamp, A.; Himmelmann, A.; Husted, S.; Katus, H.A.; Meier, B.; et al. Safety and efficacy of ticagrelor and clopidogrel in primary percutaneous coronary intervention. Heart 2016, 102, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Bassand, J.P.; Budaj, A.; Wojdyla, D.M.; James, S.K.; Cornel, J.H.; French, J.; Held, C.; Horrow, J.; Husted, S.; et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J. 2011, 32, 2933–2944. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Wiviott, S.D.; Murphy, S.A.; Voitk, J.; Hasin, Y.; Widimsky, P.; Chandna, H.; Macias, W.; McCabe, C.H.; Braunwald, E. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J. Am. Coll. Cardiol. 2008, 51, 2028–2033. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.L.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y(12) Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Galli, M.; Benenati, S.; Capodanno, D.; Franchi, F.; Rollini, F.; D’Amario, D.; Porto, I.; Angiolillo, D.J. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Lancet 2021, 397, 1470–1483. [Google Scholar] [CrossRef]
- De Luca, L.; D’Ascenzo, F.; Musumeci, G.; Saia, F.; Parodi, G.; Varbella, F.; Marchese, A.; De Servi, S.; Berti, S.; Bolognese, L. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: The SCOPE registry. EuroIntervention 2017, 13, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bagai, A.; Peterson, E.D.; Honeycutt, E.; Effron, M.B.; Cohen, D.J.; Goodman, S.G.; Anstrom, K.J.; Gupta, A.; Messenger, J.C.; Wang, T.Y. In-hospital switching between adenosine diphosphate receptor inhibitors in patients with acute myocardial infarction treated with percutaneous coronary intervention: Insights into contemporary practice from the TRANSLATE-ACS study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 499–508. [Google Scholar] [CrossRef]
- Motovska, Z.; Hlinomaz, O.; Kala, P.; Hromadka, M.; Knot, J.; Varvarovsky, I.; Dusek, J.; Jarkovsky, J.; Miklik, R.; Rokyta, R.; et al. 1-Year Outcomes of Patients Undergoing Primary Angioplasty for Myocardial Infarction Treated With Prasugrel Versus Ticagrelor. J. Am. Coll. Cardiol. 2018, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Jansen, M.; Leyens, L.; Lal, J.A.; Sudbrak, R.; Hackenitz, E.; Bußhoff, U.; Ballensiefen, W.; Brand, A. An index of barriers for the implementation of personalised medicine and pharmacogenomics in Europe. Public Health Genom. 2014, 17, 287–298. [Google Scholar] [CrossRef]
- Nickola, T.J.; Green, J.S.; Harralson, A.F.; O’Brien, T.J. The current and future state of pharmacogenomics medical education in the USA. Pharmacogenomics 2012, 13, 1419–1425. [Google Scholar] [CrossRef]
- Stanek, E.J.; Sanders, C.L.; Taber, K.A.; Khalid, M.; Patel, A.; Verbrugge, R.R.; Agatep, B.C.; Aubert, R.E.; Epstein, R.S.; Frueh, F.W. Adoption of pharmacogenomic testing by US physicians: Results of a nationwide survey. Clin. Pharmacol. Ther. 2012, 91, 450–458. [Google Scholar] [CrossRef]
- Luzum, J.A.; Luzum, M.J. Physicians’ attitudes toward pharmacogenetic testing before and after pharmacogenetic education. Pers. Med. 2016, 13, 119–127. [Google Scholar] [CrossRef]
- Valgimigli, M.; Borghesi, M.; Tebaldi, M.; Vranckx, P.; Parrinello, G.; Ferrari, R. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur. Heart J. 2013, 34, 909–919. [Google Scholar] [CrossRef]
- Van der Wouden, C.H.; Paasman, E.; Teichert, M.; Crone, M.R.; Guchelaar, H.J.; Swen, J.J. Assessing the Implementation of Pharmacogenomic Panel-Testing in Primary Care in the Netherlands Utilizing a Theoretical Framework. J. Clin. Med. 2020, 9, 814. [Google Scholar] [CrossRef]
- Bright, D.; Worley, M.; Porter, B.L. Patient perceptions of pharmacogenomic testing in the community pharmacy setting. Res. Soc. Adm. Pharm. 2021, 17, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; So, D.; Bae, J.H.; Chavez, I.; Jeong, M.H.; Kim, S.W.; Madan, M.; Graham, J.; O’Cochlain, F.; Pauley, N.; et al. International survey of patients undergoing percutaneous coronary intervention and their attitudes toward pharmacogenetic testing. Pharm. Genom. 2019, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ayorinde, A.A.; Porteous, T.; Sharma, P. Screening for major diseases in community pharmacies: A systematic review. Int. J. Pharm. Pract. 2013, 21, 349–361. [Google Scholar] [CrossRef]
- El-Den, S.; Lee, Y.L.E.; Gide, D.N.; O’Reilly, C.L. Stakeholders’ Acceptability of Pharmacist-Led Screening in Community Pharmacies: A Systematic Review. Am. J. Prev. Med. 2022, 63, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; van Dorst, P.W.M.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van ‘t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; et al. Cost Effectiveness of a CYP2C19 Genotype-Guided Strategy in Patients with Acute Myocardial Infarction: Results from the POPular Genetics Trial. Am. J. Cardiovasc. Drugs 2022, 22, 195–206. [Google Scholar] [CrossRef]
- Lala, A.; Berger, J.S.; Sharma, G.; Hochman, J.S.; Scott Braithwaite, R.; Ladapo, J.A. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: A cost-effectiveness analysis. J. Thromb. Haemost. 2013, 11, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.P.; Liew, D.; Lee, V.W.Y. Cost-effectiveness of cytochrome P450 2C19 *2 genotype-guided selection of clopidogrel or ticagrelor in Chinese patients with acute coronary syndrome. Pharm. J. 2018, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Limdi, N.A.; Cavallari, L.H.; Lee, C.R.; Hillegass, W.B.; Holmes, A.M.; Skaar, T.C.; Pisu, M.; Dillon, C.; Beitelshees, A.L.; Empey, P.E.; et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharm. J. 2020, 20, 724–735. [Google Scholar] [CrossRef]
- FDA. The CLIA Framework; FDA: Silver Spring, MD, USA, 2014.
- CPIC® Guideline for Clopidogrel and CYP2C19. Available online: https://cpicpgx.org/guidelines/guideline-for-clopidogrel-and-cyp2c19 (accessed on 11 November 2022).
- Van der Wouden, C.H.; Bank, P.C.D.; Özokcu, K.; Swen, J.J.; Guchelaar, H.J. Pharmacist-Initiated Pre-Emptive Pharmacogenetic Panel Testing with Clinical Decision Support in Primary Care: Record of PGx Results and Real-World Impact. Genes 2019, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Track Your Own Healthcare with ‘Volgjezorg’. Available online: https://www.volgjezorg.nl/en (accessed on 26 January 2023).
| Category | Value |
|---|---|
| Number of patients | 144 |
| Age (mean, range) | 64, 34–87 years |
| Gender | |
| Male | 110 (76%) |
| Female | 34 (24%) |
| P2Y12 inhibitor regimen | |
| Ticagrelor | 127 (88%) |
| Prasugrel | 17 (12%) |
| P2Y12 therapy duration at study inclusion | |
| All users (median, range) | 5.9 (0.4–127) months |
| 12-month users (mean, range) | 4.8 (0.4–11.2) months |
| Chronic users (mean, range) | 38 (13–127) months |
| Patients on chronic treatment (>12 months) | 29 (20%) |
| CYP2C19 Genotype | CYP2C19 Phenotype | Study Sample (%) | Expected Percentages from Population Demographics (%) |
|---|---|---|---|
| *1/*1, 1/*17 | Extensive metabolizer | 87/142 (61%) | 61–71 |
| *1/*2, *1/*3, *2/*17, *3/*17 | Intermediate metabolizer | 41/142 (29%) | 23–30 |
| *17/*17 | Ultrarapid metabolizer | 8/142 (5.6%) | 4.8–5.8 |
| *2/*2, *2/*3, *3/*3 | Poor metabolizer | 6/142 (4.2%) | 1.7–3.3 |
| Key Cost Drivers | Outcomes | ||||
|---|---|---|---|---|---|
| De-Escalation Rate | Average Duration of Treatment Impact (in Months) | Use of Ticagrelor versus Prasugrel | Costs per Patient (in €) | Costs per Prevented PLATO Major or Minor Bleeding | |
| Base-case scenario | 20% | 10.8 | 88% | 54 | €17,000 |
| Alternative scenarios | |||||
| 1. Improved de-escalation | 40% | * | * | −43 | Cost saving |
| 2. Full de-escalation | 100% | * | * | −332 | Cost saving |
| 3. Early testing in standard 12-month users | * | 14.7 | * | 19 | €4000 |
| 4. Five-year impact in chronic users | * | 17.7 | * | −7 | Cost saving |
| 5. Testing only in standard 12-month users | * | 7.2 | * | 86 | €40,000 |
| 6. Testing only and early in standard 12-month users | * | 12.0 | * | 43 | €12,000 |
| 7. Testing only in chronic users | * | 25.6 | * | −77 | Cost saving |
| 8. Testing only in prasugrel users | * | * | 0% | 99 | €31,000 |
| 9. Testing only in ticagrelor users | * | * | 100% | 48 | €15,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levens, A.D.; den Haan, M.C.; Jukema, J.W.; Heringa, M.; van den Hout, W.B.; Moes, D.J.A.R.; Swen, J.J. Feasibility of Community Pharmacist-Initiated and Point-of-Care CYP2C19 Genotype-Guided De-Escalation of Oral P2Y12 Inhibitors. Genes 2023, 14, 578. https://doi.org/10.3390/genes14030578
Levens AD, den Haan MC, Jukema JW, Heringa M, van den Hout WB, Moes DJAR, Swen JJ. Feasibility of Community Pharmacist-Initiated and Point-of-Care CYP2C19 Genotype-Guided De-Escalation of Oral P2Y12 Inhibitors. Genes. 2023; 14(3):578. https://doi.org/10.3390/genes14030578
Chicago/Turabian StyleLevens, Amar D., Melina C. den Haan, J. Wouter Jukema, Mette Heringa, Wilbert B. van den Hout, Dirk Jan A. R. Moes, and Jesse J. Swen. 2023. "Feasibility of Community Pharmacist-Initiated and Point-of-Care CYP2C19 Genotype-Guided De-Escalation of Oral P2Y12 Inhibitors" Genes 14, no. 3: 578. https://doi.org/10.3390/genes14030578
APA StyleLevens, A. D., den Haan, M. C., Jukema, J. W., Heringa, M., van den Hout, W. B., Moes, D. J. A. R., & Swen, J. J. (2023). Feasibility of Community Pharmacist-Initiated and Point-of-Care CYP2C19 Genotype-Guided De-Escalation of Oral P2Y12 Inhibitors. Genes, 14(3), 578. https://doi.org/10.3390/genes14030578








