RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and RNA Extraction
2.2. Transcriptome Profiling of the Wounded and Healthy Xylem Tissues from A. sinensis
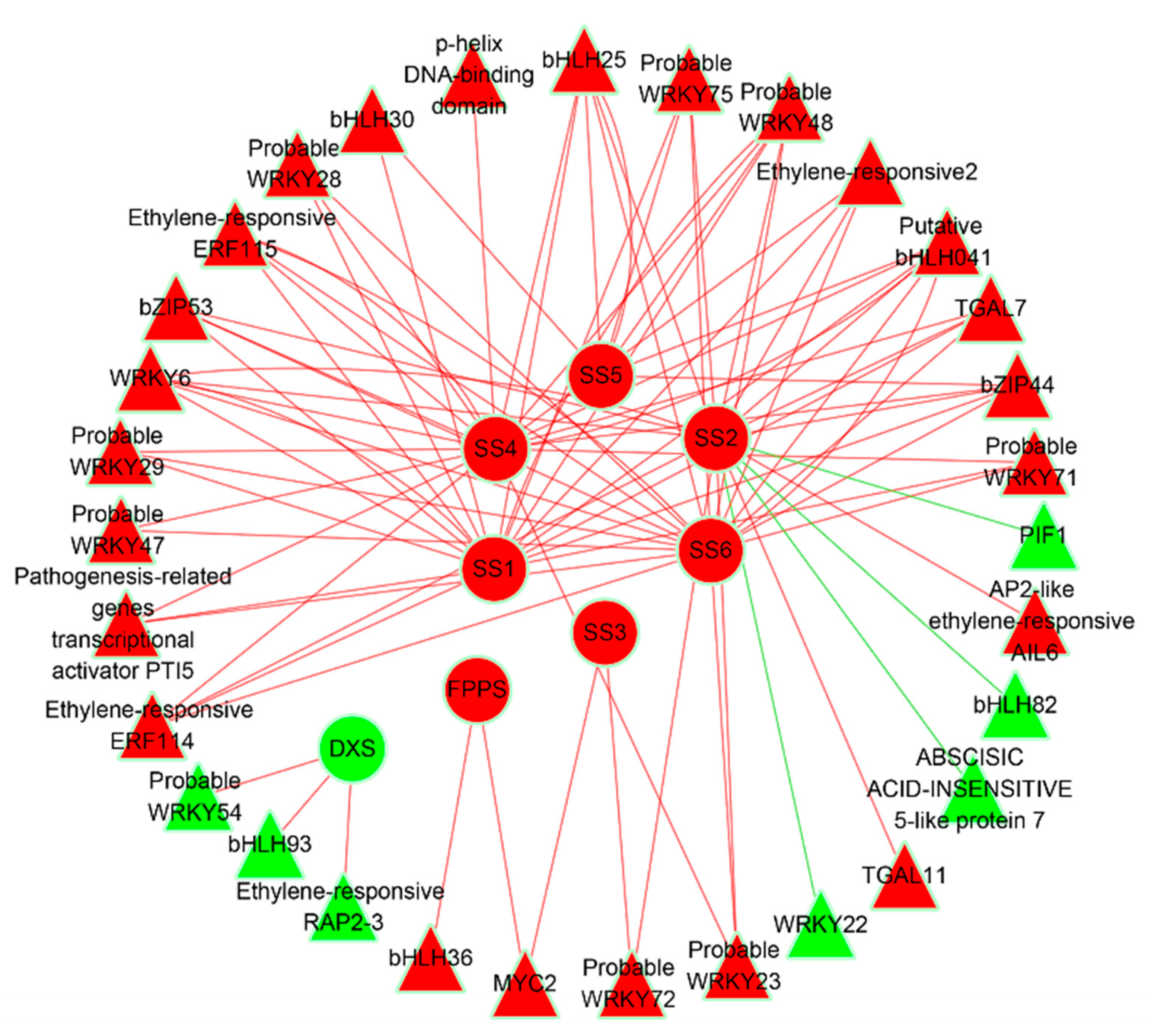
2.3. Correlation Networks
2.4. qRT-PCR Analysis
3. Results
3.1. Global Analysis of Transcriptome of A. sinensis
3.2. Functional Enrichment Analysis of DEGs
3.3. DEGs Involved in Hormone Signal Transduction
3.4. Potential Genes Involved in 2-(2-Phenylethyl)chromone Biosynthesis
3.5. DEGs Involved in Sesquiterpene Biosynthesis
3.6. Transcription Factors Mediated Regulatory Networks Involved in Sesquiterpene Biosynthesis
3.7. RNA-Seq Verification by qRT-PCR
4. Discussion
4.1. A. sinensis Transcriptome Sequencing
4.2. Jasmonic Acid and Salicylic Acid Have Potential Regulatory Roles in Agarwood Formation
4.3. Key Genes Associated with 2-(2-Phenylethyl)chromone Biosynthesis in A. sinensis
4.4. Key Genes Associated with Sesquiterpene Biosynthesis in A. sinensis
4.5. A Transcriptomic Network Underlying the Regulation of Sesquiterpene Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Davies, K.M.; Deroles, S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Kamziah, A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. Forestry Res. 2014, 25, 201–204. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical Constituents and Pharmacological Activity of Agarwood and Aquilaria Plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree Agarwood-Inducing Technique: An Efficient Novel Technique for Producing High-Quality Agarwood in Cultivated Aquilaria sinensis Trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef]
- Peng, C.S.; Osman, M.F.; Bahari, N.; Zakaria, R.; Rahim, K.A. Agarwood inducement technology: A method for producing oil grade agarwood in cultivated Aquilaria malaccensis Lamk. J. Agrobiotechnol. 2015, 6, 1–16. [Google Scholar]
- Ye, W.; Wu, H.; He, X.; Wang, L.; Zhang, W.; Li, H.; Fan, Y.; Tan, G.; Liu, T.; Gao, X. Transcriptome Sequencing of Chemically Induced Aquilaria sinensis to Identify Genes Related to Agarwood Formation. PLoS ONE 2016, 11, e0155505. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, Z.; Wei, J. Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Funct. Plant Biol. 2015, 42, 337–346. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Yang, Y.; Lv, F.; Wei, J. Programmed cell death might involve in progress of wounding induced agarwood formation in stems of Aquilaria sinensis. Microsc. Res. Tech. 2022, 85, 2904–2912. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Yang, Y.; Sui, C.; Xu, Y.; Wei, J. Interxylary phloem and xylem rays are the structural foundation of agarwood resin formation in the stems of Aquilaria sinensis. Trees 2019, 33, 533–542. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Chen, T.; Gao, J.; Zhang, X.; Jiang, C.; Yang, J.; Zhou, J.; Wang, T.; Chi, X. Integrating Multiple Omics Identifies Phaeoacremonium rubrigenum Acting as Aquilaria sinensis Marker Fungus to Promote Agarwood Sesquiterpene Accumulation by Inducing Plant Host Phosphorylation. Microbiol. Spectr. 2022, 10, e02722-21. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Bohlmann, J. An extended model of heartwood secondary metabolism informed by functional genomics. Tree Physiol. 2017, 38, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bergström, B. Chemical and structural changes during heartwood formation in Pinus sylvestris. Forestry 2003, 76, 45–53. [Google Scholar] [CrossRef]
- Lim, K.-J.; Paasela, T.; Harju, A.; Venäläinen, M.; Paulin, L.; Auvinen, P.; Kärkkäinen, K.; Teeri, T.H. Developmental Changes in Scots Pine Transcriptome during Heartwood Formation. Plant Physiol. 2016, 172, 1403–1417. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yen, P.; Chang, T.; Chang, S.; Huang, S.; Yeh, T. Distribution of living ray parenchyma cells and major bioactive compounds during the heartwood formation of Taiwania cryptomerioides Hayata. J. Wood Chem. Technol. 2018, 38, 84–95. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Liu, X.; Dong, X.; Zhang, Z.; Fan, H.; Zhang, L.; Wang, J.; Shi, S.; Tu, P. Salinity stress induces the production of 2-(2-phenylethyl)chromones and regulates novel classes of responsive genes involved in signal transduction in Aquilaria sinensis calli. BMC Plant Biol. 2016, 16, 119. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, M.; Wei, J.; Chen, H.; Gao, Z.; Sui, C.; Luo, H.; Zhang, X.; Yang, Y. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013, 14, 227. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, P.; Tang, X.; Gao, Z.; Zhang, Z.; Wei, J. Genome-wide analysis of WRKY transcription factors in Aquilaria sinensis (Lour.) Gilg. Sci. Rep. 2020, 10, 3018. [Google Scholar] [CrossRef]
- Li, R.; Zhu, J.; Guo, D.; Li, H.; Wang, Y.; Ding, X.; Mei, W.; Chen, Z.; Dai, H.; Peng, S. Genome-wide identification and expression analysis of terpene synthase gene family in Aquilaria sinensis. Plant Physiol. Biochem. 2021, 164, 185–194. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, Y.; Sun, P.; Xu, Y.; Rong, M.; Liu, Y.; Jiang, J.; Yu, C.; Gao, Z.; Wei, J. Genome-wide investigation and expression analysis of the AP2/ERF family for selection of agarwood-related genes in Aquilaria sinensis (Lour.) Gilg. Genome 2022, 65, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liao, Y.; Lv, F.; Zhang, Z.; Sun, P.; Gao, Z.; Hu, K.; Sui, C.; Jin, Y.; Wei, J. Transcription Factor AsMYC2 Controls the Jasmonate-Responsive Expression of ASS1 Regulating Sesquiterpene Biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant Cell Physiol. 2017, 58, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Nurul Irdayu, I. Succession patterns of fungi associated to wound-induced agarwood in wild Aquilaria malaccensis revealed from quantitative PCR assay. World J. Microbiol. Biotechnol. 2014, 30, 2427–2436. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Cui, Z.; Xu, D. Morphological, physiological, biochemical and molecular analyses reveal wounding-induced agarwood formation mechanism in two types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Ding, X.; Mei, W.; Lin, Q.; Wang, H.; Wang, J.; Peng, S.; Li, H.; Zhu, J.; Li, W.; Wang, P.; et al. Genome sequence of the agarwood tree Aquilaria sinensis (Lour.) Spreng: The first chromosome-level draft genome in the Thymelaeceae family. GigaScience 2020, 9, giaa013. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodological) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.T.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, X. Application of the Gini Correlation Coefficient to Infer Regulatory Relationships in Transcriptome Analysis. Plant Physiol. 2012, 160, 192–203. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Božunović, J.; Skorić, M.; Matekalo, D.; Živković, S.; Dragićević, M.; Aničić, N.; Filipović, B.; Banjanac, T.; Šiler, B.; Mišić, D. Secoiridoids Metabolism Response to Wounding in Common Centaury (Centaurium erythraea Rafn) Leaves. Plants 2019, 8, 589. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Li, J.; Yu, L.; Lin, L. Dynamic analysis of gene expression and determination of chemicals in agarwood in Aquilaria sinensis. J. Forestry Res. 2020, 31, 1833–1841. [Google Scholar] [CrossRef]
- Kang, J.-N.; Lee, W.-H.; Won, S.Y.; Chang, S.; Hong, J.-P.; Oh, T.-J.; Lee, S.M.; Kang, S.-H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites To Modulate Plant Development and Stress Responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and Genetic Factors Involved in Plant Protection-Associated Secondary Metabolite Biosynthesis Pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant, Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Huang, G.-Q.; Li, Y.; Zheng, Y.; Li, X.-B. Cotton photosynthesis-related PSAK1 protein is involved in plant response to aphid attack. Mol. Biol. Rep. 2014, 41, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Fang, S.; Liu, Y.; Shang, X. Metabolome and Transcriptome Analyses Unravel the Molecular Regulatory Mechanisms Involved in Photosynthesis of Cyclocarya paliurus under Salt Stress. Int. J. Mol. Sci. 2022, 23, 1161. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Huang, J.; Reichelt, M.; Chowdhury, S.; Hammerbacher, A.; Hartmann, H. Increasing carbon availability stimulates growth and secondary metabolites via modulation of phytohormones in winter wheat. J. Exp. Bot. 2017, 68, 1251–1263. [Google Scholar] [CrossRef]
- Lv, F.; Li, S.; Feng, J.; Liu, P.; Gao, Z.; Yang, Y.; Xu, Y.; Wei, J. Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J. Plant Physiol. 2019, 234-235, 167–175. [Google Scholar] [CrossRef]
- Sun, P.-W.; Xu, Y.-H.; Yu, C.-C.; Lv, F.-F.; Tang, X.-L.; Gao, Z.-H.; Zhang, Z.; Wang, H.; Liu, Y.; Wei, J.-H. WRKY44 represses expression of the wound-induced sesquiterpene biosynthetic gene ASS1 in Aquilaria sinensis. J. Exp. Bot. 2019, 71, 1128–1138. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wei, J.; Xu, Y.; Zhang, Z. Cloning, expression and characterization of COI1 gene (AsCOI1) from Aquilaria sinensis (Lour.) Gilg. Acta Pharm. Sin. B 2015, 5, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.; Zhao, Y.; Huang, L. Volatile organic compound and endogenous phytohormone characteristics during callus browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113605. [Google Scholar] [CrossRef]
- Bisht, R.; Bhattacharyya, A.; Shrivastava, A.; Saxena, P. An Overview of the Medicinally Important Plant Type III PKS Derived Polyketides. Front. Plant Sci. 2021, 12, 2155. [Google Scholar] [CrossRef]
- Liao, G.; Dong, W.-H.; Yang, J.-L.; Li, W.; Wang, J.; Mei, W.-L.; Dai, H.-F. Monitoring the Chemical Profile in Agarwood Formation within One Year and Speculating on the Biosynthesis of 2-(2-Phenylethyl)Chromones. Molecules 2018, 23, 1261. [Google Scholar] [CrossRef]
- Wang, X.-H.; Gao, B.-W.; Nakashima, Y.; Mori, T.; Zhang, Z.-X.; Kodama, T.; Lee, Y.-E.; Zhang, Z.-K.; Wong, C.-P.; Liu, Q.-Q.; et al. Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood. Nat. Commun. 2022, 13, 348. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef]
- Bartram, S.; Jux, A.; Gleixner, G.; Boland, W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 2006, 67, 1661–1672. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Eisenreich, W.; Arrillaga, I. Metabolic cross-talk between pathways of terpenoid backbone biosynthesis in spike lavender. Plant Physiol. Biochem. 2015, 95, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wei, Q.; Liu, Y.; Wei, X.; Chen, X.; Yin, X.; Xie, T. Transcriptome sequencing and functional characterization of new sesquiterpene synthases from Curcuma wenyujin. Arch. Biochem. Biophys. 2021, 709, 108986. [Google Scholar] [CrossRef]
- Meng, F.; Chu, T.; Tang, Q.; Chen, W. A tetraploidization event shaped the Aquilaria sinensis genome and contributed to the ability of sesquiterpenes synthesis. BMC Genom. 2021, 22, 647. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses Through Mediating Secondary Metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, X.; Lv, Z.; Lu, X.; Shen, Q.; Zhang, L.; Zhu, M.; Wang, G.; Sun, X.; Liao, Z.; et al. A Basic Leucine Zipper Transcription Factor, AabZIP1, Connects Abscisic Acid Signaling with Artemisinin Biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 163–175. [Google Scholar] [CrossRef]
- Shen, S.-l.; Yin, X.-r.; Zhang, B.; Xie, X.-l.; Jiang, Q.; Grierson, D.; Chen, K.-s. CitAP2.10 activation of the terpene synthase CsTPS1 is associated with the synthesis of (+)-valencene in ‘Newhall’ orange. J. Exp. Bot. 2016, 67, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of GaWRKY1, a Cotton Transcription Factor That Regulates the Sesquiterpene Synthase Gene (+)-δ-Cadinene Synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Hong, G.-J.; Xue, X.-Y.; Mao, Y.-B.; Wang, L.-J.; Chen, X.-Y. Arabidopsis MYC2 Interacts with DELLA Proteins in Regulating Sesquiterpene Synthase Gene Expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Memelink, J. ORCA3, a Jasmonate-Responsive Transcriptional Regulator of Plant Primary and Secondary Metabolism. Science 2000, 289, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-X.; Li, J.-X.; Yang, C.-Q.; Hu, W.-L.; Wang, L.-J.; Chen, X.-Y. The Jasmonate-Responsive AP2/ERF Transcription Factors AaERF1 and AaERF2 Positively Regulate Artemisinin Biosynthesis in Artemisia annua L. Mol. Plant 2012, 5, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gou, J.; Chen, F.; Li, C.; Zhang, Y. Comparative Transcriptome Analysis Identifies Putative Genes Involved in the Biosynthesis of Xanthanolides in Xanthium strumarium L. Front. Plant Sci. 2016, 7, 1317. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.-R.; Xu, Y.-H.; Ge, Z.-Y.; Sui, C.; Wei, J.-H. Overexpression of BcbZIP134 negatively regulates the biosynthesis of saikosaponins. Plant Cell Tiss. Org. Cult. 2019, 137, 297–308. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Huang, Y.; An, W.; Liu, S.; Huang, S.; Zheng, X. Metabolism and transcriptome profiling provides insight into the genes and transcription factors involved in monoterpene biosynthesis of borneol chemotype of Cinnamomum camphora induced by mechanical damage. PeerJ 2021, 9, e11465. [Google Scholar] [CrossRef] [PubMed]
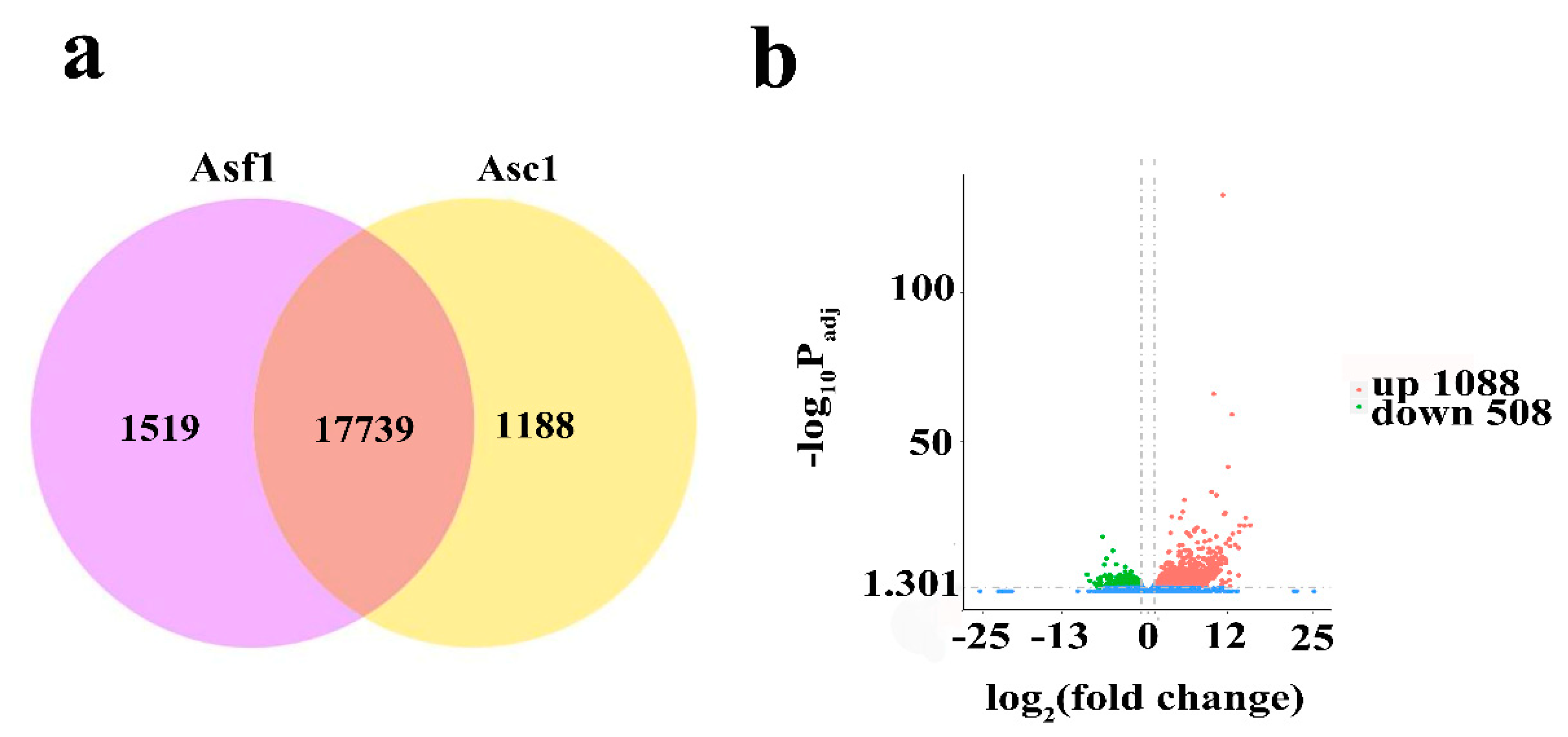
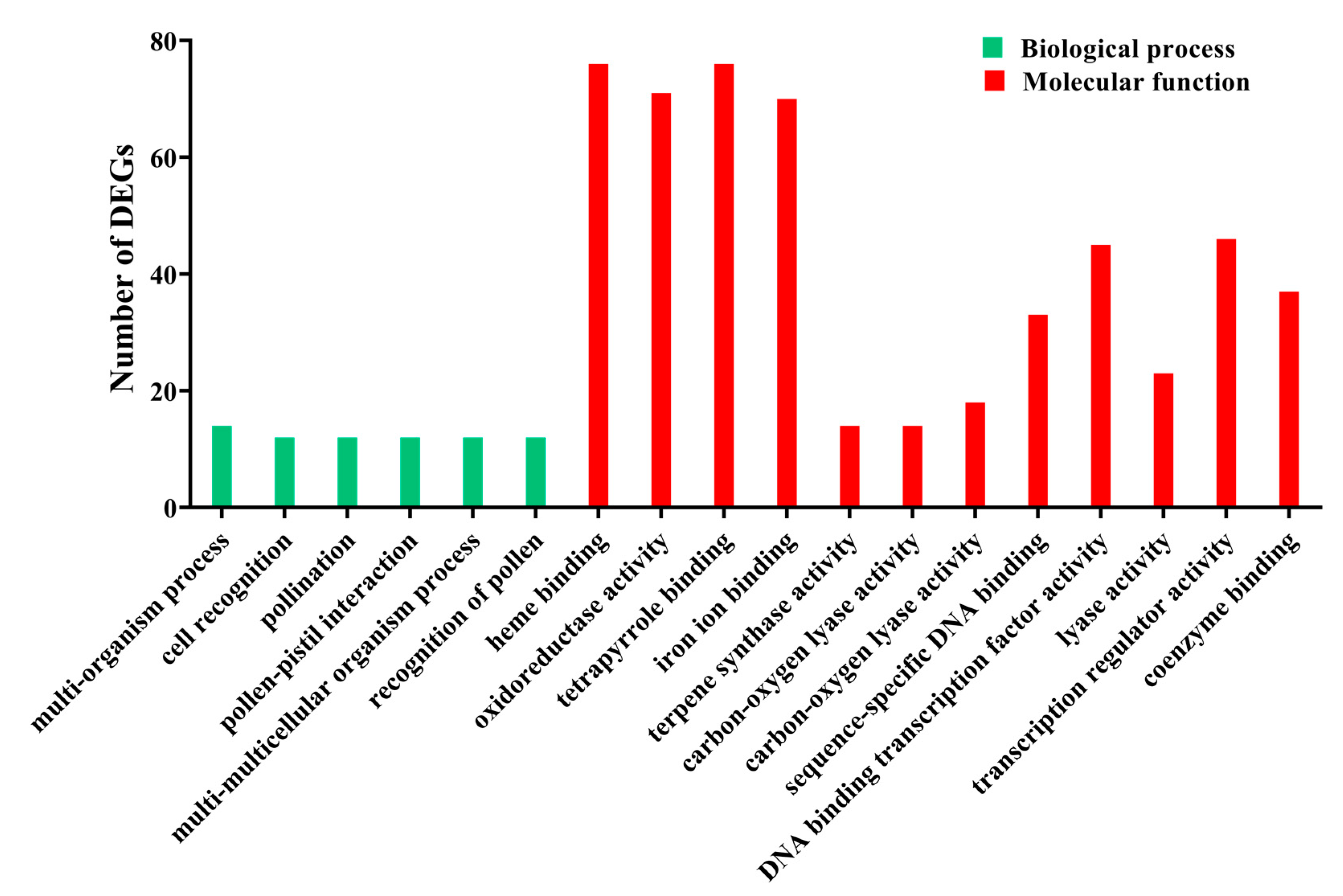
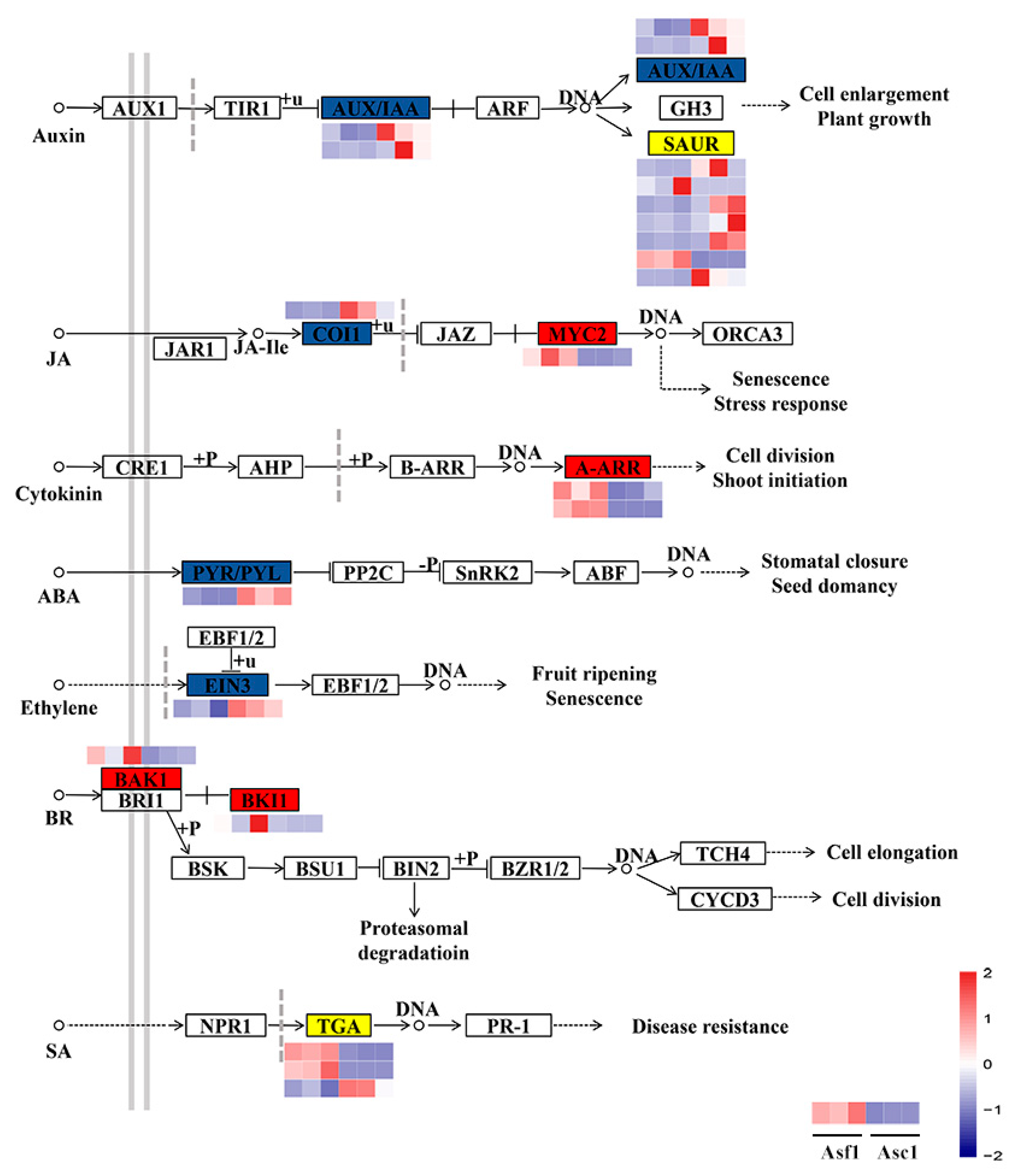
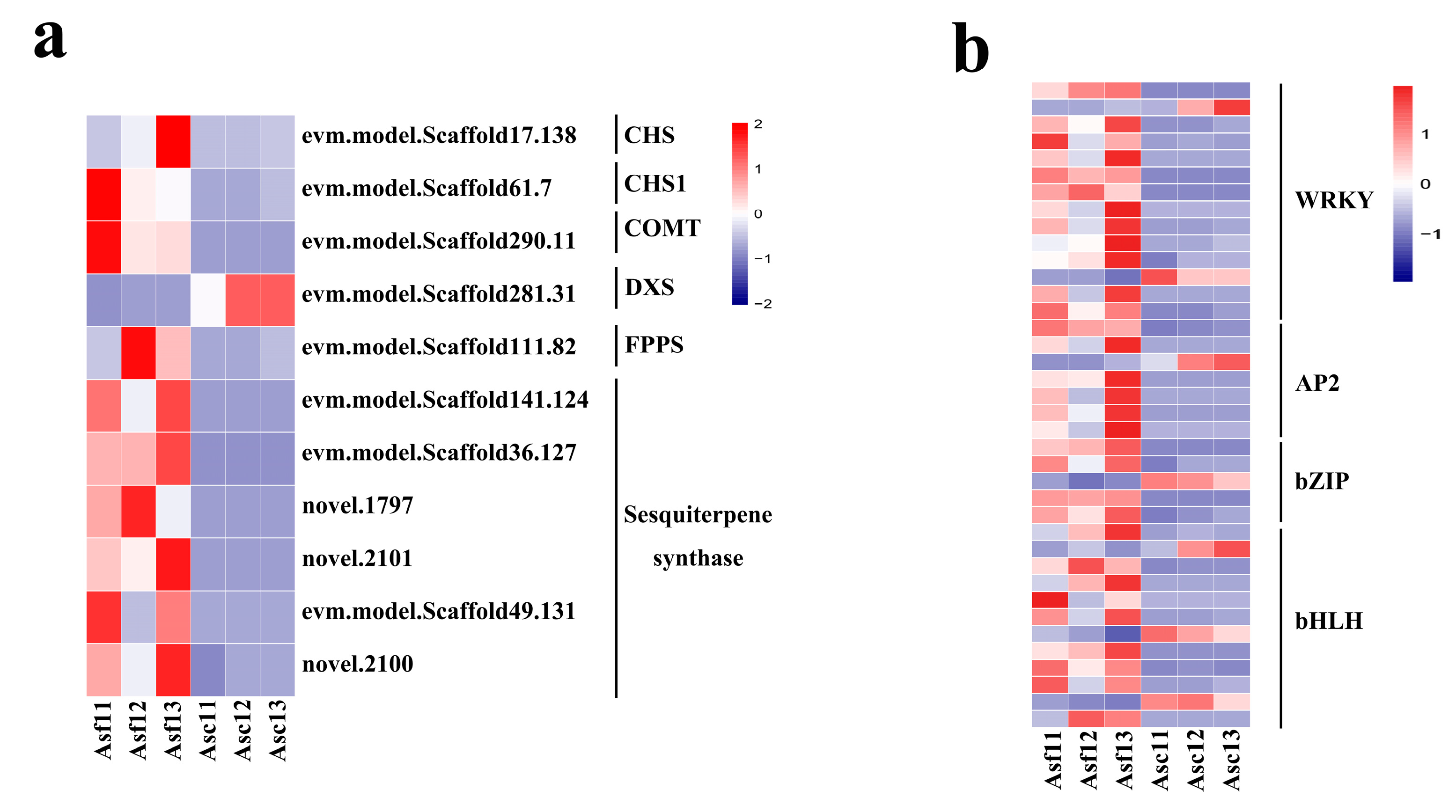
| Sample | Raw Reads | Clean Reads | Mapped to Genome | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| Asc11 | 48,158,840 | 45,845,016 | 41,524,528 (90.58%) | 98.33 | 94.85 | 46.96 |
| Asc12 | 49,229,480 | 48,487,386 | 44,432,205 (91.64%) | 97.59 | 93.02 | 46.49 |
| Asc13 | 55,055,938 | 52,975,166 | 48,697,477 (91.93%) | 98.20 | 94.51 | 46.32 |
| Asf11 | 46,747,414 | 43,317,726 | 38,986,805 (90.00%) | 98.34 | 94.86 | 46.17 |
| Asf12 | 50,747,240 | 47,754,238 | 43,633,605 (91.37%) | 98.10 | 94.27 | 46.50 |
| Asf13 | 45,935,934 | 44,470,980 | 36,366,124(81.77%) | 97.70 | 93.60 | 47.84 |
| KEGG_ID | Pathway Name | Number | Up | Down |
|---|---|---|---|---|
| pop00941 | Flavonoid biosynthesis | 14 | 14 | 0 |
| pop00940 | Phenylpropanoid biosynthesis | 24 | 24 | 0 |
| pop00480 | Glutathione metabolism | 12 | 12 | 0 |
| pop00909 | Sesquiterpenoid and triterpenoid biosynthesis | 6 | 6 | 0 |
| pop00400 | Phenylalanine, tyrosine and tryptophan biosynthesis | 8 | 8 | 0 |
| pop00360 | Phenylalanine metabolism | 7 | 6 | 1 |
| pop00195 | Photosynthesis | 9 | 0 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Du, R.; Wang, Y.; Chen, J. RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes 2023, 14, 464. https://doi.org/10.3390/genes14020464
Xu J, Du R, Wang Y, Chen J. RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes. 2023; 14(2):464. https://doi.org/10.3390/genes14020464
Chicago/Turabian StyleXu, Jieru, Ruyue Du, Yue Wang, and Jinhui Chen. 2023. "RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis" Genes 14, no. 2: 464. https://doi.org/10.3390/genes14020464
APA StyleXu, J., Du, R., Wang, Y., & Chen, J. (2023). RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes, 14(2), 464. https://doi.org/10.3390/genes14020464






