Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/mTOR Signaling Pathways—Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Presentation
2.2. Laboratory Investigations
2.3. Molecular Investigations
3. Results
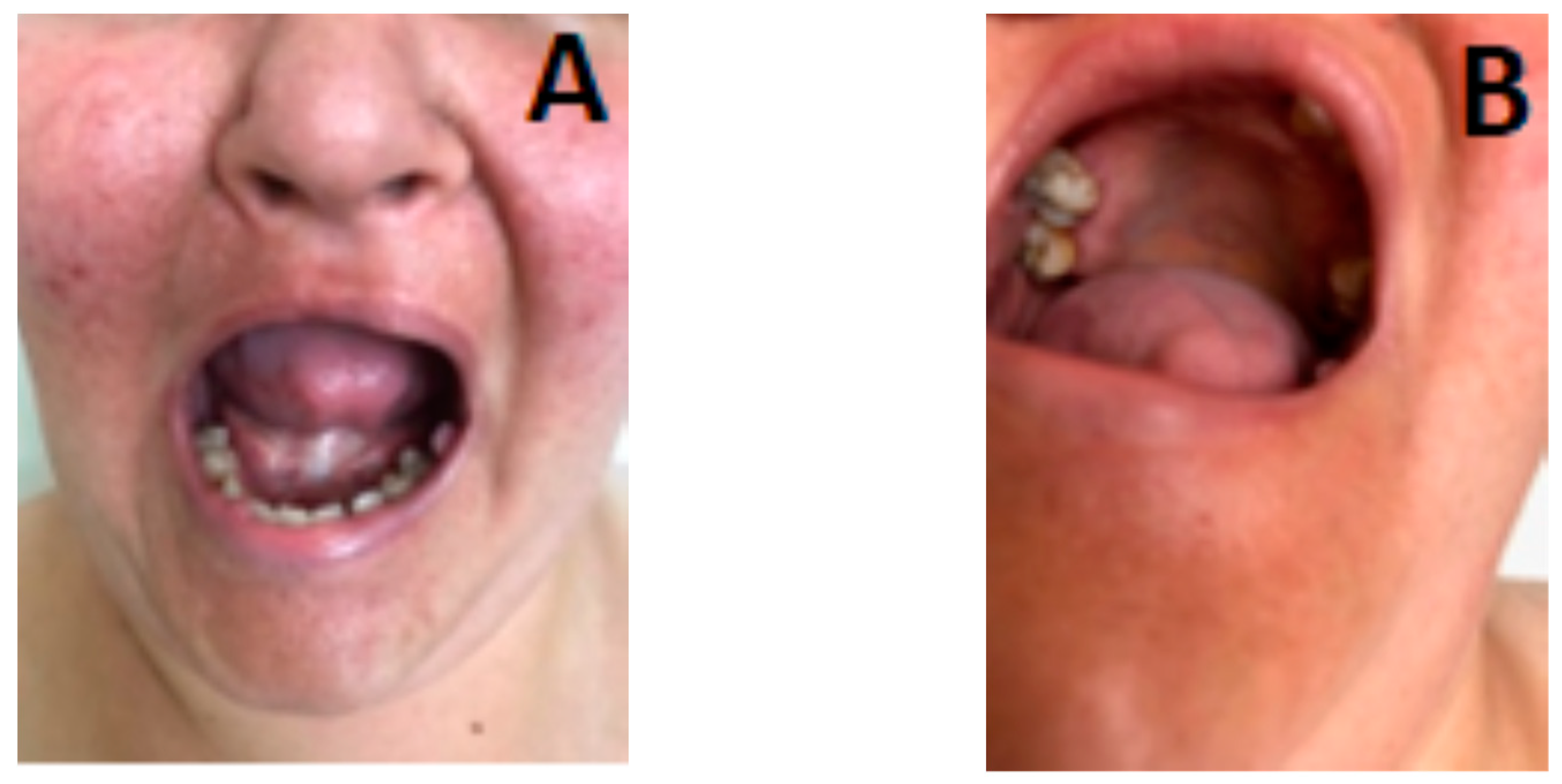
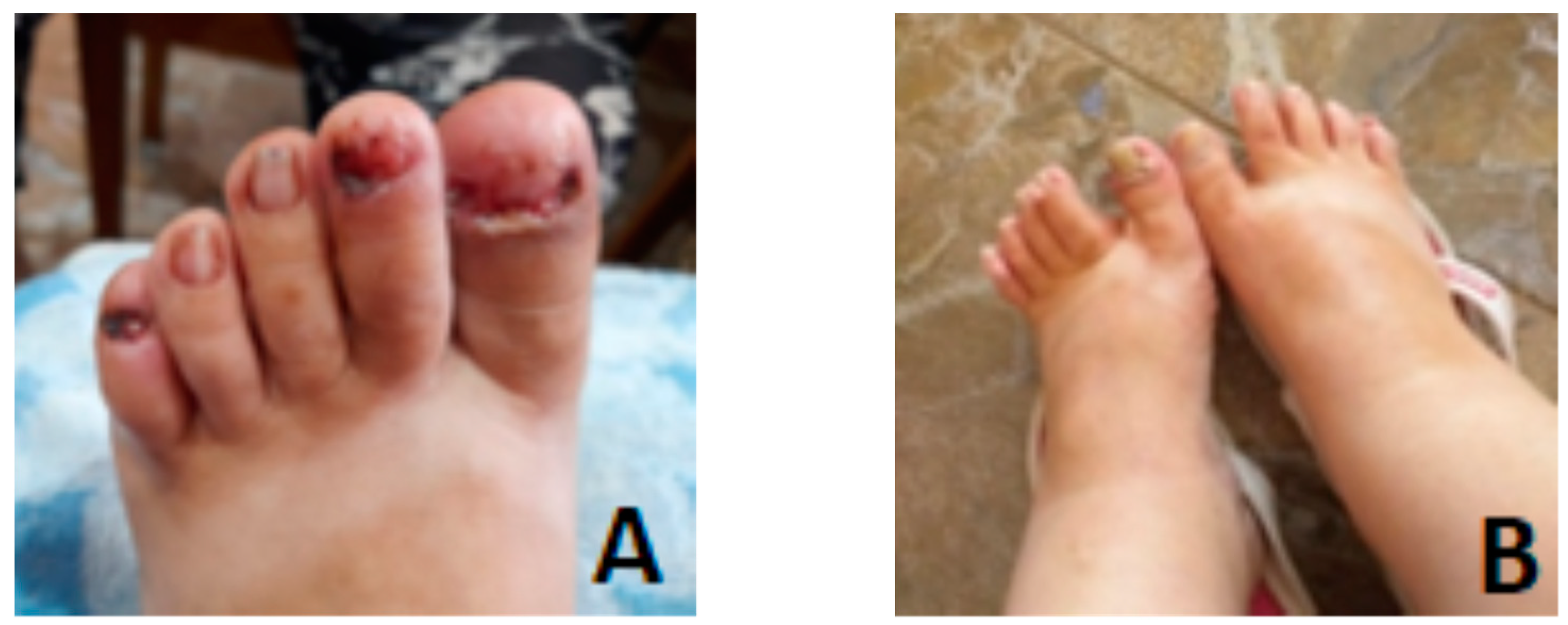
3.1. Clinical Evaluation of the Patient
3.2. Laboratory Investigations
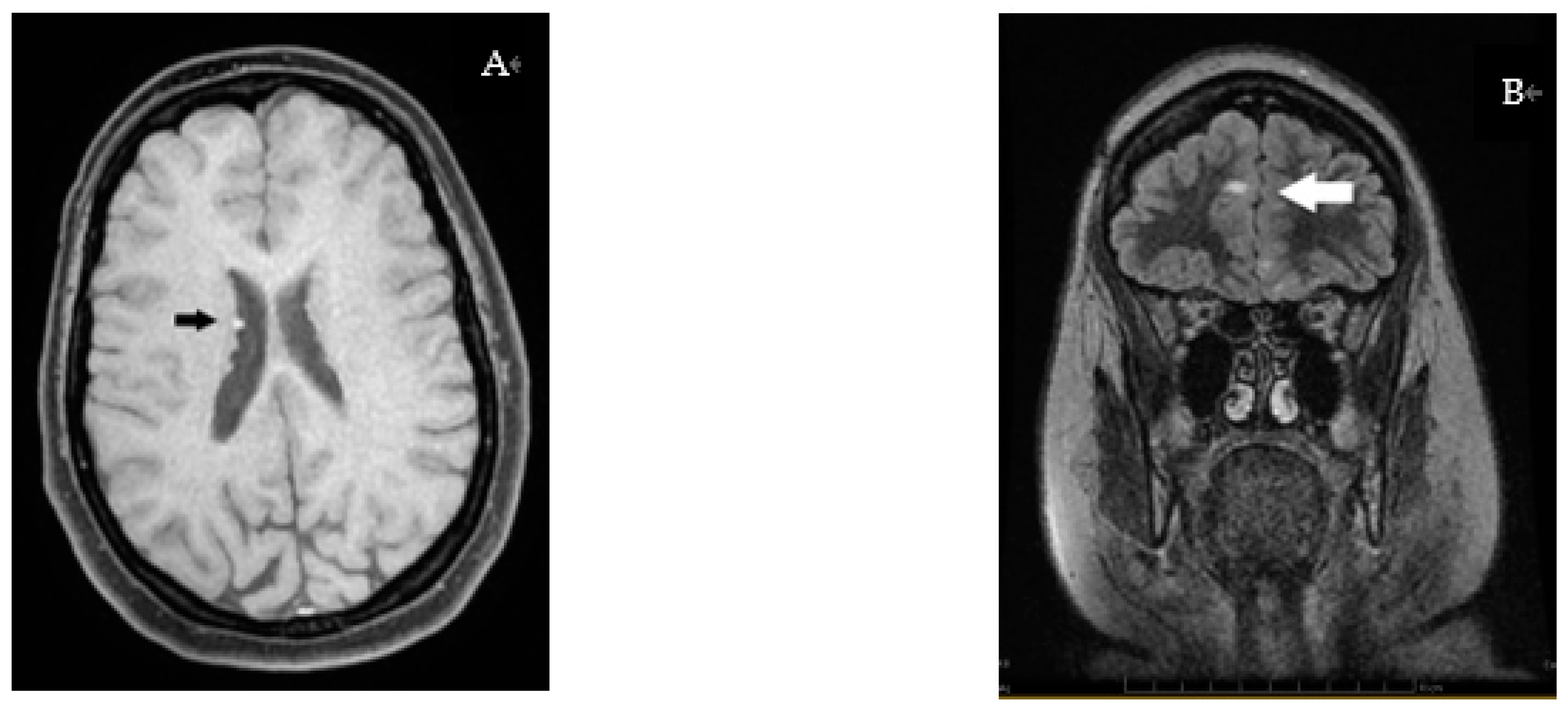
3.3. Radiology Investigations
3.4. Molecular Investigations
4. Discussion
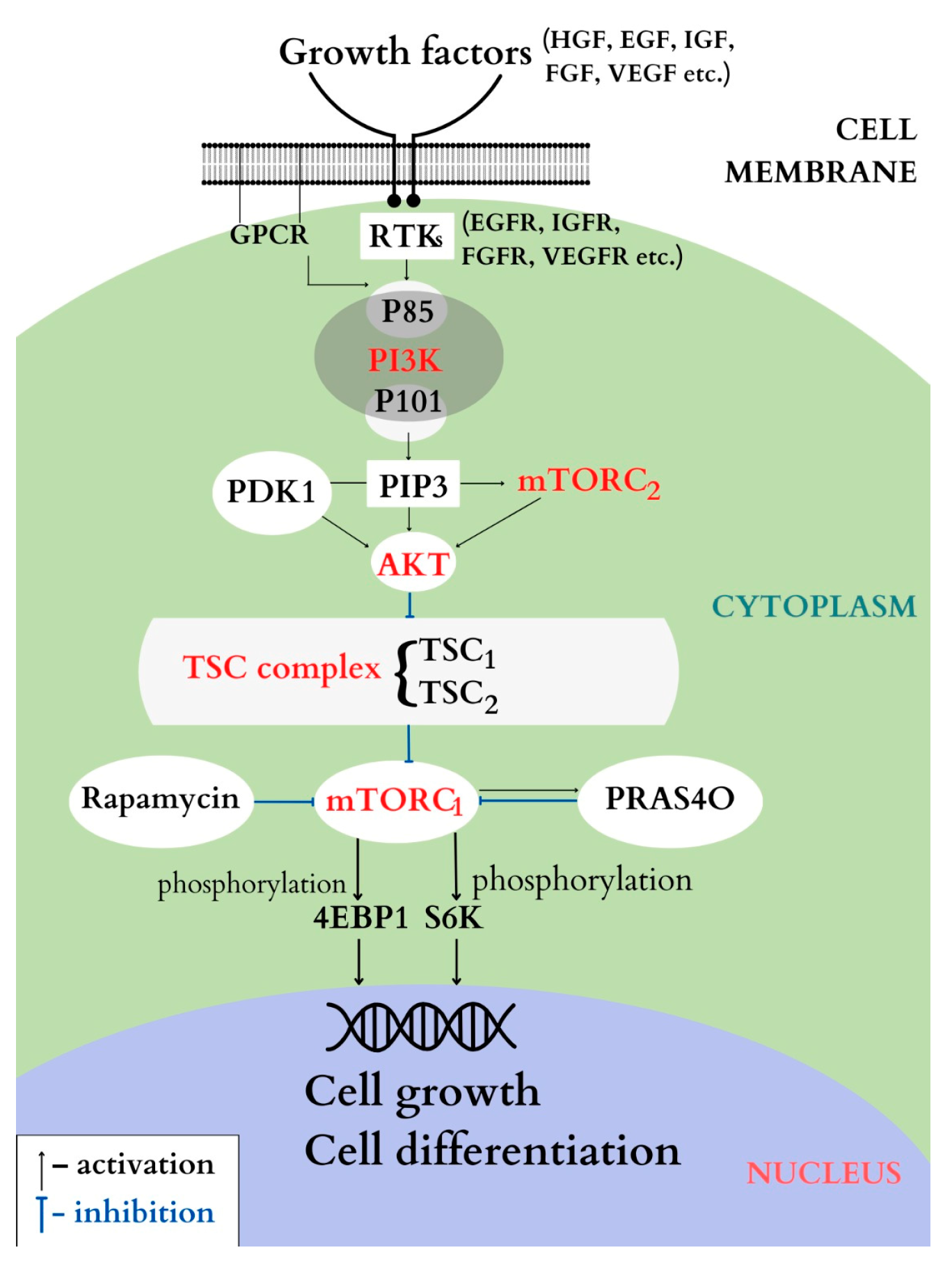
4.1. PI3K/AKT/ TSC1/TSC2/mTOR Pathway
4.1.1. PI3K
4.1.2. AKT
4.1.3. The TSC Complex
4.1.4. mTOR
4.2. Clinical Aspects
4.3. Genetics
4.4. Treatment
4.4.1. Treatment with mTOR Inhibitors
Treatment with Rapamycin
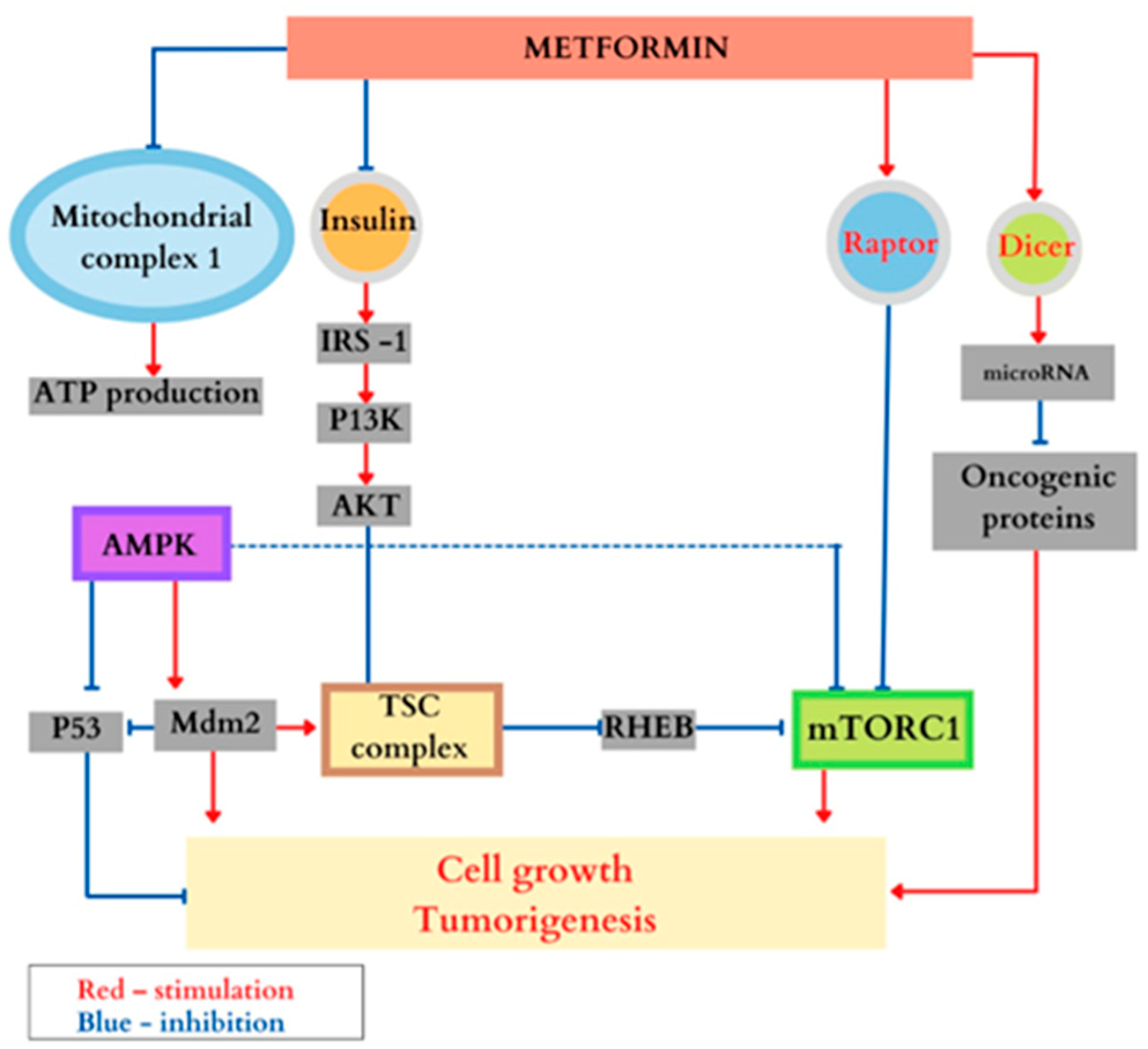
Treatment with Metformin
4.4.2. Treatment of Diabetes Mellitus and Obesity
4.4.3. Treatment for Epilepsy
4.5. Evolution and Monitoring
4.6. Genetic Counseling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randle, S.C. Tuberous Sclerosis Complex: A Review. Pediatr. Ann. 2017, 46, e166–e171. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Zuniga, R.D.; Alas-Pineda, C.; Pineda-Vijil, H.O.; Gaitán-Zambrano, K.; Flores-Reyes, D.L.; Pineda-Villeda, R.H.; Quiñonez-Sánchez, M.A. Diagnosis of Tuberous Sclerosis Complex in Adulthood: A Case Report. Clin. Case Rep. 2022, 10, e6555. [Google Scholar] [CrossRef]
- Roach, E.S. Applying the Lessons of Tuberous Sclerosis: The 2015 Hower Award Lecture. Pediatr. Neurol. 2016, 63, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Bourneville, D. Sclerose Tubereuse Des Circonvolutions Cerebrales: Idioties et Epilepsie Hemiplegique. Arch. Neurol. 1880, 1, 81–91. [Google Scholar]
- Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes/Edited by Paolo Curatolo. Wellcome Collection. Available online: https://wellcomecollection.org/works/r49upmjm (accessed on 8 December 2022).
- Srinivasan, A.; Naga Shashi Kiran, B.; Koduvath, A.; Pal, A.; Suresh, A.; Kannan, A.; Ravichandran, M. Metformin for the Management of Tuberous Sclerosis: What Does the Evidence Tell Us? EXCLI J. 2021, 20, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Nasykhova, Y.A.; Tonyan, Z.N.; Mikhailova, A.A.; Danilova, M.M.; Glotov, A.S. Pharmacogenetics of Type 2 Diabetes-Progress and Prospects. Int. J. Mol. Sci. 2020, 21, 6842. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global Estimates of the Prevalence of Diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Rich, S.S. Mapping Genes in Diabetes: Genetic Epidemiological Perspective. Diabetes 1990, 39, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Lotta, L.A. Genomic Insights into the Causes of Type 2 Diabetes. Lancet 2018, 391, 2463–2474. [Google Scholar] [CrossRef]
- Joy, E. Diabetes in Individuals with Tuberous Sclerosis Complex Treated with MTOR Inhibitors. J. Mult. Scler. 2021, 9, 244. [Google Scholar]
- Amin, S.; Kingswood, J.C.; Bolton, P.F.; Elmslie, F.; Gale, D.P.; Harland, C.; Johnson, S.R.; Parker, A.; Sampson, J.R.; Smeaton, M.; et al. The UK Guidelines for Management and Surveillance of Tuberous Sclerosis Complex. QJM 2019, 112, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt Signaling Transduction Pathway, Erythropoiesis and Glycolysis in Hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/MTOR Signalling Pathways in Neurodegenerative Diseases and Tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Martelli, A.M.; Zauli, G.; Vitale, M.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Targeting the Phosphatidylinositol 3-kinase/Akt/Mechanistic Target of Rapamycin Signaling Pathway in B-lineage Acute Lymphoblastic Leukemia: An Update. J. Cell. Physiol. 2018, 233, 6440–6454. [Google Scholar] [CrossRef]
- Tuncel, G.; Kalkan, R. Receptor Tyrosine Kinase-Ras-PI 3 Kinase-Akt Signaling Network in Glioblastoma Multiforme. Med. Oncol. 2018, 35, 122. [Google Scholar] [CrossRef]
- Yang, Q.; Guan, K.-L. Expanding MTOR Signaling. Cell Res. 2007, 17, 666–681. [Google Scholar] [CrossRef]
- Yu, X.; Long, Y.C.; Shen, H.M. Differential Regulatory Functions of Three Classes of Phosphatidylinositol and Phosphoinositide 3-Kinases in Tophagy. Autophagy 2015, 11, 1711–1728. [Google Scholar] [CrossRef]
- Ghigo, A.; Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-Kinases in Health and Disease. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; pp. 183–213. [Google Scholar]
- Zhang, M.; Zhang, X. The Role of PI3K/AKT/FOXO Signaling in Psoriasis. Arch. Derm. Res. 2019, 311, 83–91. [Google Scholar] [CrossRef]
- Szymonowicz, K.; Oeck, S.; Malewicz, N.; Jendrossek, V. New Insights into Protein Kinase B/Akt Signaling: Role of Localized Akt Activation and Compartment-Specific Target Proteins for the Cellular Radiation Response. Cancers 2018, 10, 78. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in Cancer: Mediator and More. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. AKT Kinase Pathway: A Leading Target in Cancer Research. Sci. World J. 2013, 2013, 756134. [Google Scholar] [CrossRef] [PubMed]
- Dibble, C.C.; Manning, B.D. Signal Integration by MTORC1 Coordinates Nutrient Input with Biosynthetic Output. Nat. Cell Biol. 2013, 15, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, A.; Hartkamp, J.; Saitoh, M.; Roworth, W.; Nobukuni, T.; Hodges, A.; Sampson, J.; Thomas, G.; Lamb, R. Tuberous Sclerosis Complex Tumor Suppressor-Mediated S6 Kinase Inhibition by Phosphatidylinositide-3-OH Kinase Is MTOR Independent. J. Cell Biol. 2002, 159, 217–224. [Google Scholar] [CrossRef]
- Kwiatkowski, D.J.; Choueiri, T.K.; Fay, A.P.; Rini, B.I.; Thorner, A.R.; de Velasco, G.; Tyburczy, M.E.; Hamieh, L.; Albiges, L.; Agarwal, N.; et al. Mutations in TSC1, TSC2, and MTOR Are Associated with Response to Rapalogs in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2016, 22, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Plank, T.L.; Yeung, R.S.; Henske, E.P. Hamartin, the Product of the Tuberous Sclerosis 1 (TSC1) Gene, Interacts with Tuberin and Appears to Be Localized to Cytoplasmic Vesicles. Cancer Res. 1998, 58, 4766–4770. [Google Scholar]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/MTOR Pathway Inhibitors in Triple-Negative Breast Cancer: A Review on Drug Discovery and Future Challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Rivera-Calderon, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Vasconcelos, R.O.; Laufer-Amorim, R. p-mTOR, p-4EBP-1 and EIF4E Expression in Canine Prostatic Carcinoma. Res. Vet. Sci. 2019, 122, 86–92. [Google Scholar] [CrossRef]
- Kezic, A.; Popovic, L.; Lalic, K. MTOR Inhibitor Therapy and Metabolic Consequences: Where Do We Stand? Oxid. Med. Cell. Longev. 2018, 2018, 2640342. [Google Scholar] [CrossRef]
- Shah, O.J.; Wang, Z.; Hunter, T. Inappropriate Activation of the TSC/Rheb/MTOR/S6K Cassette Induces IRS1/2 Depletion, Insulin Resistance, and Cell Survival Deficiencies. Curr. Biol. 2004, 14, 1650–1656. [Google Scholar] [CrossRef]
- Ueno, M.; Carvalheira, J.B.C.; Tambascia, R.C.; Bezerra, R.M.N.; Amaral, M.E.; Carneiro, E.M.; Folli, F.; Franchini, K.G.; Saad, M.J.A. Regulation of Insulin Signalling by Hyperinsulinaemia: Role of IRS-1/2 Serine Phosphorylation and the MTOR/P70 S6K Pathway. Diabetologia 2005, 48, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K. MTOR: Role in Cancer, Metastasis and Drug Resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.-L. MTOR as a Central Hub of Nutrient Signalling and Cell Growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zinzalla, V.; Stracka, D.; Oppliger, W.; Hall, M.N. Activation of MTORC2 by Association with the Ribosome. Cell 2011, 144, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. The Role of Mammalian Target of Rapamycin (MTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Hall, M.N.; Zhang, H.; Bandura, J.L.; Heiberger, K.M.; Glogauer, M.; El-Hashemite, N.; Onda, H. A Mouse Model of TSC1 Reveals Sex-Dependent Lethality from Liver Hemangiomas, and up-Regulation of P70S6 Kinase Activity in Tsc1 Null Cells. Nat. Rev. Mol. Cell Biol. 2002, 15, 525–534. [Google Scholar]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 Protects against Age- and Diet-Induced Obesity While Enhancing Insulin Sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef]
- Chakrabarti, P.; English, T.; Shi, J.; Smas, C.M.; Kandror, K.V. Mammalian Target of Rapamycin Complex 1 Suppresses Lipolysis, Stimulates Lipogenesis, and Promotes Fat Storage. Diabetes 2010, 59, 775–781. [Google Scholar] [CrossRef]
- Kumar, A.; Lawrence, J.C., Jr.; Jung, D.Y.; Ko, H.J.; Keller, S.R.; Kim, J.K.; Magnuson, M.A.; Harris, T.E. Fat Cell–Specific Ablation of Rictor in Mice Impairs Insulin-Regulated Fat Cell and Whole-Body Glucose and Lipid Metabolism. Diabetes 2010, 59, 1397–1406. [Google Scholar] [CrossRef]
- Kohn, A.D.; Summers, S.A.; Birnbaum, M.J.; Roth, R.A. Expression of a Constitutively Active Akt Ser/Thr Kinase in 3T3-L1 Adipocytes Stimulates Glucose Uptake and Glucose Transporter 4 Translocation. J. Biol. Chem. 1996, 271, 31372–31378. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; Tullet, J.M.A.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Jurca, C.; Bembea, M.; Pallag, A.; Mureșan, M.; Szilagyi, A.; Balmoș, A.; Pop, O.; Jurca, A.; Dobjanschi, L. Pharmacotherapeutical considerations in the treatment and management of neonatal hyperammonaemia. Farmacia 2018, 66, 216–222. Available online: https://farmaciajournal.com/ (accessed on 1 October 2022).
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. International Tuberous Sclerosis Complex Consensus Group. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Islam, M.P. Tuberous Sclerosis Complex. Semin. Pediatr. Neurol. 2021, 37, 100875. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.P.; Steve Roach, E. Tuberous Sclerosis Complex. In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 935–943. [Google Scholar]
- Bolton, P.F.; Clifford, M.; Tye, C.; Maclean, C.; Humphrey, A.; le Maréchal, K.; Higgins, J.N.P.; Neville, B.G.R.; Rijsdjik, F.; Tuberous Sclerosis 2000 Study Group; et al. Intellectual Abilities in Tuberous Sclerosis Complex: Risk Factors and Correlates from the Tuberous Sclerosis 2000 Study. Psychol. Med. 2015, 45, 2321–2331. [Google Scholar] [CrossRef]
- Jansen, F.E.; Vincken, K.L.; Algra, A.; Anbeek, P.; Braams, O.; Nellist, M.; Zonnenberg, B.A.; Jennekens-Schinkel, A.; van den Ouweland, A.; Halley, D.; et al. Cognitive Impairment in Tuberous Sclerosis Complex Is a Multifactorial Condition. Neurology 2008, 70, 916–923. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y. DM MTORC1 Senses Lysosomal Amino Acids through an Inside-out Mechanism That Requires the Vacuolar H(+)-ATPase. Science 2011, 34, 678–683. [Google Scholar] [CrossRef]
- Pfirmann, P.; Combe, C.; Rigothier, C. Sclérose tubéreuse de Bourneville: Mise au point. Rev. Med. Interne 2021, 42, 714–721. [Google Scholar] [CrossRef]
- Tyburczy, M.E.; Dies, K.A.; Glass, J.; Camposano, S.; Chekaluk, Y.; Thorner, A.R.; Lin, L.; Krueger, D.; Franz, D.N.; Thiele, E.A.; et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genet. 2015, 11, e1005637. [Google Scholar]
- Uysal, S.P.; Şahin, M. Tuberous Sclerosis: A Review of the Past, Present, and Future. Turk. J. Med. Sci. 2020, 50, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Snell, R.G.; Tachataki, M. SA Molecular Genetic and Phenotypic Analysis Reveals Differences between TSC1 and TSC2 Associated Familial and Sporadic Tuberous Sclerosis. Hum. Mol. Genet. 1997, 6, 2155–2161. [Google Scholar]
- Mayer, K.; Ballhausen, W.; Rott, H.D. Mutation Screening of the Entire Coding Regions of the TSC1 and the TSC2 Gene with the Protein Truncation Test (PTT) Identifies Frequent Splicing Defects. Hum. Mutat. 1999, 14, 401–411. [Google Scholar] [CrossRef]
- Dabora, S.L.; Jozwiak, S.; Franz, D.N.; Roberts, P.S.; Nieto, A.; Chung, J.; Choy, Y.S.; Reeve, M.P.; Thiele, E.; Egelhoff, J.C.; et al. Mutational Analysis in a Cohort of 224 Tuberous Sclerosis Patients Indicates Increased Severity of TSC2, Compared with TSC1, Disease in Multiple Organs. Am. J. Hum. Genet. 2001, 68, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Padrão, E.; Irion, K. Multifocal Micronodular Pneumocyte Hyperplasia Associated with Tuberous Sclerosis Complex: A Case Report without Lymphangioleiomyomatosis Association. Rev. Port. Pneumol. (2006) 2017, 23, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Portocarrero, L.; Quental, K.N.; Samorano, L.P.; Oliveira, Z.; Rivitti-Machado, M. Tuberous Sclerosis Complex: Review Based on New Diagnostic Criteria. An. Bras. Dermatol. 2018, 93, 323–331. [Google Scholar] [CrossRef]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Tuberous Sclerosis Complex: From Molecular Biology to Novel Therapeutic Approaches: Tuberous Sclerosis Complex. IUBMB Life 2016, 68, 955–962. [Google Scholar] [CrossRef]
- Adriaensen, M.E.A.P.M.; Schaefer-Prokop, C.M.; Duyndam, D.A.C.; Zonnenberg, B.A.; Prokop, M. Fatty Foci in the Myocardium in Patients with Tuberous Sclerosis Complex: Common Finding at CT. Radiology 2009, 253, 359–363. [Google Scholar] [CrossRef]
- Koenig, M.K.; Hebert, A.A.; Roberson, J.; Samuels, J.; Slopis, J.; Woerner, A.; Northrup, H. Topical Rapamycin Therapy to Alleviate the Cutaneous Manifestations of Tuberous Sclerosis Complex: A Double-Blind, Randomized, Controlled Trial to Evaluate the Safety and Efficacy of Topically Applied Rapamycin: A Double-Blind, Randomized, Controlled Trial to Evaluate the Safety and Efficacy of Topically Applied Rapamycin. Drugs R&D 2012, 12, 121–126. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, A.K.; Punj, V.; Priya, P. Recent Nanotechnological Interventions Targeting PI3K/Akt/MTOR Pathway: A Focus on Breast Cancer. Semin. Cancer Biol. 2019, 59, 133–146. [Google Scholar] [CrossRef]
- Ram, G.; Sharma, V.; Sheikh, I. Anti-Cancer Potential of Natural Products: Recent Trends, Scope and Relevance. Lett. Appl. NanoBioSci 2020, 9, 902–907. [Google Scholar]
- MacKeigan, J.P.; Krueger, D.A. Differentiating the MTOR Inhibitors Everolimus and Sirolimus in the Treatment of Tuberous Sclerosis Complex. Neuro. Oncol. 2015, 17, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Kingswood, J.C.; d’Augères, G.B.; Belousova, E.; Ferreira, J.C.; Carter, T.; Castellana, R.; Cottin, V.; Curatolo, P.; Dahlin, M.; TOSCA consortium and TOSCA investigators; et al. TuberOus SClerosis Registry to Increase Disease Awareness (TOSCA)—Baseline Data on 2093 Patients. Orphanet J. Rare Dis. 2017, 12, 2. [Google Scholar] [CrossRef]
- Gov.br. Available online: http://anvisa.gov.br/datavisa/fila_bula/ (accessed on 8 December 2022).
- Gov.br. Available online: http://www.anvisa.gov.br/datavisa/fila_bula/frmVisualizarBula (accessed on 8 December 2022).
- Nathan, N.; Wang, J.-A.; Li, S.; Cowen, E.W.; Haughey, M.; Moss, J.; Darling, T.N. Improvement of Tuberous Sclerosis Complex (TSC) Skin Tumors during Long-Term Treatment with Oral Sirolimus. J. Am. Acad. Dermatol. 2015, 73, 802–808. [Google Scholar] [CrossRef] [PubMed]
- DiMario, F.J., Jr.; Sahin, M.; Ebrahimi-Fakhari, D. Tuberous Sclerosis Complex. Pediatr. Clin. North Am. 2015, 62, 633–648. [Google Scholar] [CrossRef]
- Krebs, M.; Brunmair, B.; Brehm, A.; Artwohl, M.; Szendroedi, J.; Nowotny, P.; Roth, E.; Fürnsinn, C.; Promintzer, M.; Anderwald, C.; et al. The Mammalian Target of Rapamycin Pathway Regulates Nutrient-Sensitive Glucose Uptake in Man. Diabetes 2007, 56, 1600–1607. [Google Scholar] [CrossRef]
- Chakraborty, S. Molecular Mechanism of Rapamycin Resistance in Cancer Cells. Doctoral Dissertation, City University of New York (CUNY), New York, NY, USA, 2022. [Google Scholar]
- Van Nostrand, J.L.; Hellberg, K.; Luo, E.-C.; Van Nostrand, E.L.; Dayn, A.; Yu, J.; Shokhirev, M.N.; Dayn, Y.; Yeo, G.W.; Shaw, R.J. AMPK Regulation of Raptor and TSC2 Mediate Metformin Effects on Transcriptional Control of Anabolism and Inflammation. Genes Dev. 2020, 34, 1330–1344. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Amin, S.; Lux, A.; O’Callaghan, F. The Journey of Metformin from Glycaemic Control to MTOR Inhibition and the Suppression of Tumour Growth: Metformin and Suppression of Tumour Growth. Br. J. Clin. Pharmacol. 2019, 85, 37–46. [Google Scholar] [CrossRef]
- Ning, J.; Clemmons, D.R. AMP-Activated Protein Kinase Inhibits IGF-I Signaling and Protein Synthesis in Vascular Smooth Muscle Cells via Stimulation of Insulin Receptor Substrate 1 S794 and Tuberous Sclerosis 2 S1345 Phosphorylation. Mol. Endocrinol. 2010, 24, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Karuman, P.; Gozani, O.; Odze, R.D.; Zhou, X.C.; Zhu, H.; Shaw, R.; Brien, T.P.; Bozzuto, C.D.; Ooi, D.; Cantley, L.C.; et al. The Peutz-Jegher Gene Product LKB1 Is a Mediator of P53-Dependent Cell Death. Mol. Cell 2001, 7, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S.; et al. Metformin Elicits Anticancer Effects through the Sequential Modulation of DICER and C-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef] [PubMed]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, Independent of AMPK, Inhibits MTORC1 in a Rag GTPase-Dependent Manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Algire, C.; Amrein, L.; Zakikhani, M.; Panasci, L.; Pollak, M. Metformin Blocks the Stimulative Effect of a High-Energy Diet on Colon Carcinoma Growth in Vivo and Is Associated with Reduced Expression of Fatty Acid Synthase. Endocr. Relat. Cancer 2010, 17, 351–360. [Google Scholar] [CrossRef]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef]
- Amin, S.; Mallick, A.A.; Edwards, H.; Cortina-Borja, M.; Laugharne, M.; Likeman, M.; O’Callaghan, F.J.K. The Metformin in Tuberous Sclerosis (MiTS) Study: A Randomised Double-Blind Placebo-Controlled Trial. EClinicalMedicine 2021, 32, 100715. [Google Scholar] [CrossRef]
- Blonde, L. Management of Type 2 Diabetes: Update on New Pharmacological Options. Manag. Care 2000, 9, 11–17; discussion 24–28. [Google Scholar]
- Chu-Shore, C.J.; Major, P.; Camposano, S.; Muzykewicz, D.; Thiele, E.A. The Natural History of Epilepsy in Tuberous Sclerosis Complex: Epilepsy in TSC. Epilepsia 2010, 51, 1236–1241. [Google Scholar] [CrossRef]

| Investigations | Jan 2021 | July 2021 | March 2022 | June 2022 | August 2022 |
|---|---|---|---|---|---|
| Glycemia (RV 74–106 mg/dL) | 264.7 | 160 | 190 | 178 | 141 |
| Glycated hemoglobin (RV 4–6%) | 8.7 | 7.6 | 8.1 | 7.4 | 6.5 |
| Cholesterol (RV sub < 200 mg/dL) | 212.2 | 187.6 | 193.1 | 178 | 190 |
| HDL cholesterol (RV 29.8–84.75 mg/dL) | 36.45 | 57.25 | 65.7 | 54.6 | 50 |
| LDL cholesterol (RV 74–106 mg/dL) | 142 | 100 | 112 | 99.8 | 102 |
| Triglycerides (RV < 150 mg/dL) | 168 | 152 | 158 | 161 | 213 |
| Urea (RV 16.8–43.2 mg/dL) | 48.5 | 17.4 | 22.1 | 24.5 | 32.48 |
| Creatinine (RV 0.6–1.2 mg/dL) | 1.14 | 0.67 | 0.78 | 0.83 | 0.58 |
| Glomerular filtration rate * RV > 90; between 60–89 moderate decrease | 64.20 | 117.13 | 131.62 | 124.86 | 122.01 |
| Major Criteria (11) | Observation |
|---|---|
| Depigmented macules with a 3–5 mm diameter Facial angiofibromas (over 3) Ungual fibromas (over 2) Shagreen patch Retinal hamartomas Cortical dysplasia Astrocytomas and subependymal nodules Cardiac rhabdomyomas Lymphangioleiomyomatosis renal angiomyolipomas | Genetic diagnosis: for a positive diagnosis it is sufficient to identify a pathogenic variant in one of the two genes |
| Minor criteria (7) | |
| “Confetti” skin lesions Intraoral fibromas Changes in tooth enamel White patches on the retina Renal cysts Other hamartomas Sclerotic bone changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurca, C.M.; Kozma, K.; Petchesi, C.D.; Zaha, D.C.; Magyar, I.; Munteanu, M.; Faur, L.; Jurca, A.; Bembea, D.; Severin, E.; et al. Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/mTOR Signaling Pathways—Case Report and Literature Review. Genes 2023, 14, 433. https://doi.org/10.3390/genes14020433
Jurca CM, Kozma K, Petchesi CD, Zaha DC, Magyar I, Munteanu M, Faur L, Jurca A, Bembea D, Severin E, et al. Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/mTOR Signaling Pathways—Case Report and Literature Review. Genes. 2023; 14(2):433. https://doi.org/10.3390/genes14020433
Chicago/Turabian StyleJurca, Claudia Maria, Kinga Kozma, Codruta Diana Petchesi, Dana Carmen Zaha, Ioan Magyar, Mihai Munteanu, Lucian Faur, Aurora Jurca, Dan Bembea, Emilia Severin, and et al. 2023. "Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/mTOR Signaling Pathways—Case Report and Literature Review" Genes 14, no. 2: 433. https://doi.org/10.3390/genes14020433
APA StyleJurca, C. M., Kozma, K., Petchesi, C. D., Zaha, D. C., Magyar, I., Munteanu, M., Faur, L., Jurca, A., Bembea, D., Severin, E., & Jurca, A. D. (2023). Tuberous Sclerosis, Type II Diabetes Mellitus and the PI3K/AKT/mTOR Signaling Pathways—Case Report and Literature Review. Genes, 14(2), 433. https://doi.org/10.3390/genes14020433









