Association of Functional Polymorphism in TPH2 Gene with Alcohol Dependence and Personality Traits: Study in Cloninger’s Type I and Type II Alcohol-Dependent Inpatients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. DNA Extraction and Genotyping
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Association of rs4290270 Polymorphism with Type I and Type II AD
3.3. Association of rs4290270 Polymorphism with Personality Traits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGue, M. The Behavioral Genetics of Alcoholism. Curr. Dir. Psychol. Sci. 1999, 8, 109–115. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.-C.; Chen, S.-J.; Lee, C.-H.; Cheng, C.-M. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Charlson, F.; Ferrari, A.; Santomauro, D.; Erskine, H.; Mantilla-Herrara, A.; Whiteford, H.; Leung, J.; Neghavi, M.; Griswold, M.; et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018, 5, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Pascale, E.; Lucarelli, M. Alcohol Addiction: A Molecular Biology Perspective. Curr. Med. Chem. 2015, 22, 670–684. [Google Scholar] [CrossRef]
- Matošić, A.; Marušić, S.; Vidrih, B.; Kovak-Mufić, A.; Čičin-Šain, L. Neurobiological Bases of Alcohol Addiction. Acta Clin. Croat. 2016, 55, 134–150. [Google Scholar] [CrossRef]
- Yang, W.; Singla, R.; Maheshwari, O.; Fontaine, C.J.; Gil-Mohapel, J. Alcohol Use Disorder: Neurobiology and Therapeutics. Biomedicines 2022, 10, 1192. [Google Scholar] [CrossRef]
- Babor, T.F.; Hofmann, M.; Zelig, S.; Hesselbrock, V.; Rounsaville, B. Types of Alcoholics, I. Evidence for an Empirically Derived Typology Based on Indicators of Vulnerability and Severity. Arch. Gen. Psychiatry. 1992, 49, 599–608. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Sigvardsson, S.; Bohman, M. Type I and Type II Alcoholism: An Update. Alcohol Health Res. World 1996, 20, 18–23. [Google Scholar]
- Lesch, O.M.; Walter, H. Subtypes of alcoholism and their role in therapy. Alcohol Alcohol. Suppl. 1996, 31, 63–67. [Google Scholar] [CrossRef]
- Cardoso, J.M.N.; Barbosa, A.; Ismail, F.; Pombo, S. Neter Alcoholic Typology (Nat)A. Alcohol Alcohol. 2006, 41, 133–139. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Bohman, M.; Sigvardsson, S. Inheritance of Alcohol Abuse. Cross-fostering analysis of adopted men. Arch. Gen. Psychiatry 1981, 38, 861–868. [Google Scholar] [CrossRef]
- Mantere, T.; Tupala, E.; Hall, H.; Särkioja, T.; Räsänen, P.; Bergström, K.; Callaway, J.; Tiihonen, J. Serotonin Transporter Distribution and Density in the Cerebral Cortex of Alcoholic and Nonalcoholic Comparison Subjects: A Whole-Hemisphere Autoradiography Study. Am. J. Psychiatry 2002, 159, 599–606. [Google Scholar] [CrossRef]
- Leggio, L.; Addolorato, G. Serotonin transporter (SERT) brain density and neurobiological cloninger subtypes model: A lesson by human autoradiography studies. Alcohol Alcohol. 2008, 43, 148–150. [Google Scholar] [CrossRef]
- Virkkunen, M.; Linnoila, M. Serotonin in Early Onset, Male Alcoholics with Violent Behaviour. Ann. Med. 1990, 22, 327–331. [Google Scholar] [CrossRef]
- Bauer, I.E.; Graham, D.P.; Soares, J.C.; Nielsen, D.A. Serotonergic gene variation in substance use pharmacotherapy: A systematic review. Pharmacogenomics 2015, 16, 1307–1314. [Google Scholar] [CrossRef]
- Mokrović, G.; Matošić, A.; Hranilović, D.; Štefulj, J.; Novokmet, M.; Orešković, D.; Balija, M.; Marušić, S.; Čičin-Šain, L. Alcohol Dependence and Polymorphisms of Serotonin-Related Genes: Association Studies. Coll. Antropol. 2008, 32, 127–131. [Google Scholar]
- Parsian, A.; Cloninger, C.R. Serotonergic pathway genes and subtypes of alcoholism: Association studies. Psychiatr. Genet. 2001, 11, 89–94. [Google Scholar] [CrossRef]
- Lesch, K.-P. Alcohol dependence and gene x environment interaction in emotion regulation: Is serotonin the link? Eur. J. Pharmacol. 2005, 526, 113–124. [Google Scholar] [CrossRef]
- LeMarquand, D.; Pihl, R.O.; Benkelfat, C. Serotonin and alcohol intake, abuse, and dependence: Clinical evidence. Biol. Psychiatry 1994, 36, 326–337. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Bektic Hodzic, J.; Repovecki, S.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Tudor, L.; Mimica, N.; Svob Strac, D.; Pivac, N. Alcohol-related phenotypes and platelet serotonin concentration. Alcohol 2021, 97, 41–49. [Google Scholar] [CrossRef]
- Ho, P.-S.; Shih, M.-C.; Ma, K.-H.; Huang, W.-S.; Ho, K.K.-J.; Yen, C.-H.; Lu, R.-B.; Huang, S.-Y. Availability of the serotonin transporter in patients with alcohol dependence. World, J. Biol. Psychiatry 2011, 12, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Mann, K.; Weinberger, D.R.; Goldman, D. Serotonergic Dysfunction, Negative Mood States, and Response to Alcohol. Alcohol. Clin. Exp. Res. 2001, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Plemenitaš, A.; Kores Plesničar, B.; Kastelic, M.; Porcelli, S.; Serretti, A.; Dolžan, V. Genetic variability in tryptophan hydroxylase 2 gene in alcohol dependence and alcohol-related psychopathological symptoms. Neurosci. Lett. 2015, 604, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, X.; Han, S.; Zhang, C.K.; Liu, Z.; Li, D. Association of the HTR2A gene with alcohol and heroin abuse. Hum. Genet. 2014, 133, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Kenna, G.A. Variation in the Serotonin Transporter Gene and Alcoholism: Risk and Response to Pharmacotherapy. Alcohol Alcohol. 2016, 51, 164–171. [Google Scholar] [CrossRef]
- Kapitau, A.; Goloenko, I.; Obyedkov, V.; Pavlov, K.; Dariusz Szajda, S.; Waszkiewicz, N. Serotonin transporter gene linked polymorphism (5-HTTLPR) determines progredience of alcohol dependence in Belarusian young males. Adv. Med Sci. 2019, 64, 169–173. [Google Scholar] [CrossRef]
- Chung, I.-W.; Kim, H.; Sribney, W.; Hong, J.-B.; Lee, C.-H.; Lee, K.-Y.; Nan, H.-M.; Kim, Y.-S.; Manowitz, P. Tryptophan hydroxylase polymorphism is associated with age of onset of alcoholism related behaviors. Alcohol 2005, 36, 1–3. [Google Scholar] [CrossRef]
- Drago, A.; Liappas, I.; Petio, C.; Albani, D.; Forloni, G.; Malitas, P.; Piperi, C.; Politis, A.; Tzavellas, E.O.; Zisaki, K.K.; et al. Epistasis between IL1A, IL1B, TNF, HTR2A, 5-HTTLPR and TPH2 Variations Does Not Impact Alcohol Dependence Disorder Features. Int. J. Environ. Res. Public Health 2009, 6, 1980–1990. [Google Scholar] [CrossRef]
- Gacek, P.; Conner, T.S.; Tennen, H.; Kranzler, H.R.; Covault, J. Tryptophan hydroxylase 2 gene and alcohol use among college students. Addict. Biol. 2008, 13, 440–448. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Przybeck, T.R.; Svrakic, D.M. The Tridimensional Personality Questionnaire: U.S. Normative Data. Psychol. Rep. 1991, 69, 1047–1057. [Google Scholar] [CrossRef]
- Cloninger, C.R. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr. Dev. 1986, 4, 167–226. [Google Scholar]
- Cloninger, C.R. A Systematic Method for Clinical Description and Classification of Personality Variants. Arch. Gen. Psychiatry 1987, 44, 573–588. [Google Scholar] [CrossRef]
- Raistrick, D.; Dunbar, G.; Davidson, R. Development of a Questionnaire to Measure Alcohol Dependence. Br. J. Addict. 1983, 78, 89–95. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gaunt, T.R.; Day, I.N.M. Hardy-Weinberg Equilibrium Testing of Biological Ascertainment for Mendelian Randomization Studies. Am. J. Epidemiol. 2009, 169, 505–514. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.-M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan Hydroxylase-2 Controls Brain Serotonin Synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef]
- Bach, H.; Arango, V.; Kassir, S.A.; Tsaava, T.; Dwork, A.J.; Mann, J.J.; Underwood, M.D. Alcoholics have more tryptophan hydroxylase 2 mRNA and protein in the dorsal and median raphe nuclei. Alcohol. Clin. Exp. Res. 2014, 38, 1894–1901. [Google Scholar] [CrossRef]
- Lim, J.-E.; Pinsonneault, J.; Sadee, W.; Saffen, D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Mol. Psychiatry 2007, 12, 491–501. [Google Scholar] [CrossRef]
- Ottenhof, K.W.; Sild, M.; Lévesque, M.L.; Ruhé, H.G.; Booij, L. TPH2 polymorphisms across the spectrum of psychiatric morbidity: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 29–42. [Google Scholar] [CrossRef]
- Grohmann, M.; Hammer, P.; Walther, M.; Paulmann, N.; Büttner, A.; Eisenmenger, W.; Baghai, T.C.; Schüle, C.; Rupprecht, R.; Bader, M.; et al. Alternative Splicing and Extensive RNA Editing of Human TPH2 Transcripts. PLoS ONE 2010, 5, e8956. [Google Scholar] [CrossRef]
- Zupanc, T.; Pregelj, P.; Tomori, M.; Komel, R.; Paska, A.V. TPH2 polymorphisms and alcohol-related suicide. Neurosci. Lett. 2011, 490, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.A.; Barral, S.; Proudnikov, D.; Kellogg, S.; Ho, A.; Ott, J.; Kreek, M.J. TPH2 and TPH1: Association of Variants and Interactions with Heroin Addiction. Behav. Genet. 2008, 38, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, M.A.; Hamon, S.C.; Harding, M.J.; Nielsen, E.M.; Newton, T.F.; De La Garza, R.; Nielsen, D. Genetic moderation of cocaine subjective effects by variation in the TPH1, TPH2, and SLC6A4 serotonin genes. Psychiatr. Genet. 2017, 27, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Wu, Y.; Wu, J. The Relationship Between Tryptophan Hydroxylase-2 Gene with Primary Insomnia and Depressive Symptoms in the Han Chinese Population. Balk. Med. J. 2018, 35, 412–416. [Google Scholar] [CrossRef]
- Zill, P.; Preuss, U.W.; Koller, G.; Bondy, B.; Soyka, M. SNP- and Haplotype Analysis of the Tryptophan Hydroxylase 2 Gene in Alcohol-Dependent Patients and Alcohol-Related Suicide. Neuropsychopharmacology 2007, 32, 1687–1694. [Google Scholar] [CrossRef]
- Gutknecht, L.; Jacob, C.; Strobel, A.; Kriegebaum, C.; Müller, J.; Zeng, Y.; Markert, C.; Escher, A.; Wendland, J.; Reif, A.; et al. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int. J. Neuropsychopharmacol. 2007, 10, 309–320. [Google Scholar] [CrossRef]
- Reuter, M.; Kuepper, Y.; Hennig, J. Association between a polymorphism in the promoter region of the TPH2 gene and the personality trait of harm avoidance. Int. J. Neuropsychopharmacol. 2007, 10, 401–404. [Google Scholar] [CrossRef]
- Zwir, I.; Arnedo, J.; Del-Val, C.; Pulkki-Råback, L.; Konte, B.; Yang, S.S.; Romero-Zaliz, R.; Hintsanen, M.; Cloninger, K.M.; Garcia, D.; et al. Uncovering the complex genetics of human temperament. Mol. Psychiatry 2020, 25, 2275–2294. [Google Scholar] [CrossRef]

| Characteristics | Type I | Type II | p-Value | |
|---|---|---|---|---|
| Age (years) | 50 (9) | 31 (7) | <0.0001 a | |
| NS score | 14 (7) | 18 (6) | <0.0001 a | |
| HA score | 19 (8) | 17.5 (9) | 0.043 a | |
| RD score | 20 (4) | 20 (4) | 0.695 a | |
| SADD score | 22 (3) | 21 (4) | <0.0001 a | |
| Age at AD onset (years) | ≤25 | 22 (11.1) | 86 (81.1) | <0.0001 c |
| >25 | 176 (88.9) | 20 (18.9) | ||
| Education | Elementary school | 31 (15.1) | 21 (19.1) | 0.003 b |
| High school | 140 (68.0) | 85 (77.3) | ||
| University | 35 (17.0) | 4 (3.6) | ||
| Marital status | Married | 132 (64.1) | 46 (41.8) | <0.0001 b |
| Unmarried | 34 (16.5) | 55 (50.0) | ||
| Divorced | 30 (14.6) | 9 (8.2) | ||
| Widower | 10 (4.9) | 0 (0.0) | ||
| Family history of PD | Yes | 12 (5.8) | 14 (12.7) | 0.051 c |
| No | 194 (94.2) | 96 (87.3) | ||
| Family history of AD | Yes | 135 (65.5) | 85 (77.3) | 0.040 c |
| No | 71 (34.5) | 25 (22.7) | ||
| Smoking | Yes | 140 (68.0) | 97 (88.2) | <0.0001 c |
| No | 66 (32.0) | 13 (11.8) | ||
| Childhood abuse | Yes | 94 (45.6) | 62 (56.4) | 0.077 c |
| No | 112 (54.4) | 48 (43.6) | ||
| Mood disorders | Yes | 64 (31.1) | 21(19.1) | 0.024 c |
| No | 142 (68.9) | 89 (80.9) | ||
| Personality disorders | Yes | 7 (3.4) | 40 (36.4) | <0.0001 c |
| No | 199 (96.6) | 70 (63.6) | ||
| Suicidal ideation/attempt | Yes | 31 (15.1) | 26 (23.6) | 0.066 c |
| No | 175 (85.0) | 84 (76.4) | ||
| Liver and GI tract lesions | Yes | 94 (45.6) | 23 (20.9) | <0.0001 c |
| No | 112 (54.4) | 87 (79.1) | ||
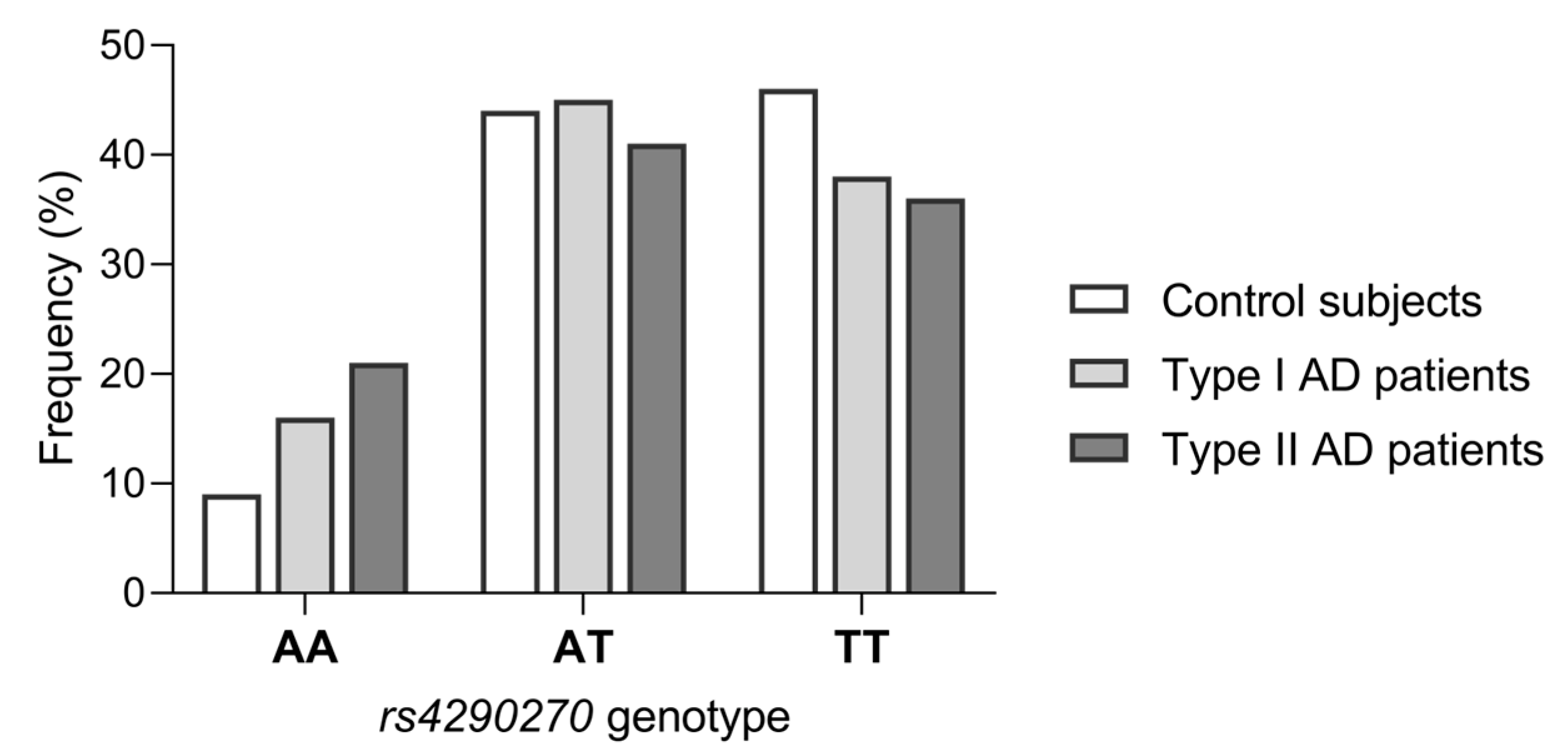
| TPH2 rs4290270 | Type I AD (n = 206) | Type II AD (n = 110) | Controls (n = 373) | Type I AD vs. Controls | Type II AD vs. Controls | |
|---|---|---|---|---|---|---|
| Genotypes | AA | 33 (16.0) | 24 (21.8) | 37 (9.9) | 0.057 a | 0.003 a |
| AT | 93 (45.2) | 46 (41.8) | 164 (44.0) | |||
| TT | 80 (38.8) | 40 (36.4) | 172 (46.1) | |||
| Minor allele homozygotes | AA | 33 (16.0) | 24 (21.8) | 37 (9.9) | 0.034 b | 0.002 b |
| AT/TT | 173 (84.0) | 86 (78.2) | 336 (90.1) | |||
| Heterozygotes | AT | 93 (45.2) | 46 (41.8) | 164 (44.0) | 0.794 b | 0.743 b |
| AA/TT | 113 (54.9) | 64 (58.2) | 209 (56.0) | |||
| Major allele homozygotes | TT | 80 (38.8) | 40 (36.4) | 172 (46.1) | 0.097 b | 0.083 b |
| AT/AA | 126 (61.2) | 70 (63.6) | 201 (53.9) | |||
| Alleles | A | 159 (38.6) | 94 (42.7) | 238 (31.9) | 0.024 b | 0.004 b |
| T | 253 (61.4) | 126 (57.3) | 508 (68.1) |
| Type I AD (n = 206) | Type II AD (n = 110) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | n | NS | HA | RD | n | NS | HA | RD |
| AA | 33 | 15 (7) | 22 (9) | 20 (3) | 24 | 21 (11) | 14 (9) | 20 (4) |
| AT | 93 | 14 (7) | 19 (7) | 20 (5) | 46 | 18 (7) | 17 (9) | 21 (4) |
| TT | 80 | 15 (7) | 19 (8) | 20 (5) | 40 | 19 (6) | 20 (8) | 19 (5) |
| p-value | 0.143 a | 0.302 b | 0.403 a | 0.757 a | 0.006 b | 0.194 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konjevod, M.; Rešetar, M.; Matošić, A.; Čičin-Šain, L.; Štefulj, J. Association of Functional Polymorphism in TPH2 Gene with Alcohol Dependence and Personality Traits: Study in Cloninger’s Type I and Type II Alcohol-Dependent Inpatients. Genes 2023, 14, 413. https://doi.org/10.3390/genes14020413
Konjevod M, Rešetar M, Matošić A, Čičin-Šain L, Štefulj J. Association of Functional Polymorphism in TPH2 Gene with Alcohol Dependence and Personality Traits: Study in Cloninger’s Type I and Type II Alcohol-Dependent Inpatients. Genes. 2023; 14(2):413. https://doi.org/10.3390/genes14020413
Chicago/Turabian StyleKonjevod, Marcela, Mirta Rešetar, Ana Matošić, Lipa Čičin-Šain, and Jasminka Štefulj. 2023. "Association of Functional Polymorphism in TPH2 Gene with Alcohol Dependence and Personality Traits: Study in Cloninger’s Type I and Type II Alcohol-Dependent Inpatients" Genes 14, no. 2: 413. https://doi.org/10.3390/genes14020413
APA StyleKonjevod, M., Rešetar, M., Matošić, A., Čičin-Šain, L., & Štefulj, J. (2023). Association of Functional Polymorphism in TPH2 Gene with Alcohol Dependence and Personality Traits: Study in Cloninger’s Type I and Type II Alcohol-Dependent Inpatients. Genes, 14(2), 413. https://doi.org/10.3390/genes14020413






