Abstract
Paenibacillus mucilaginosus has widely been reported as a plant growth-promoting rhizobacteria (PGPR). However, the important genomic insights into plant growth promotion in this species remain undescribed. In this study, the genome of P. mucilaginosus G78 was sequenced using Illumina NovaSeq PE150. It contains 8,576,872 bp with a GC content of 58.5%, and was taxonomically characterized. Additionally, a total of 7337 genes with 143 tRNAs, 41 rRNAs, and 5 ncRNAs were identified. This strain can prohibit the growth of the plant pathogen, but also has the capability to form biofilm, solubilize phosphate, and produce IAA. Twenty-six gene clusters encoding secondary metabolites were identified, and the genotypic characterization indirectly proved its resistant ability to ampicillin, bacitracin, polymyxin and chloramphenicol. The putative exopolysaccharide biosynthesis and biofilm formation gene clusters were explored. According to the genetic features, the potential monosaccharides of its exopolysaccharides for P. mucilaginosus G78 may include glucose, mannose, galactose, fucose, that can probably be acetylated and pyruvated. Conservation of the pelADEFG compared with other 40 Paenibacillus species suggests that Pel may be specific biofilm matrix component in P. mucilaginosus. Several genes relevant to plant growth-promoting traits, i.e., IAA production and phosphate solubilization are well conserved compared with other 40 other Paenibacillus strains. The current study can benefit for understanding the plant growth-promoting traits of P. mucilaginosus as well as its potential application in agriculture as PGPR.
1. Introduction
As the world’s population is expected to exceed 9 billion over the next thirty years, an important question to address is how to meet the increasing demands for food [1,2,3]. The application of chemical fertilizers surely helped in increasing crop yields in the last fifty years, but their intensive and continuous use has brought about a lot of environmental problems, such as diverse pollutions and contamination of ecosystems but also soil quality and biodiversity reduction [1]. Plant growth-promoting rhizobacteria (PGPR) are not only able to increase the crop production, but also have the ability to help plants to resist to biotic or abiotic stresses. Thus, the use of PGPR to substitute for part the chemical fertilizers has been considered as an eco-friendly way [4,5].
Most strains from Paenibacillus genus isolated from soil promote plant growth by producing indole-3-acetic acid (IAA) and other auxin phytohormones. Such bacteria can solubilize inaccessible phophorous into forms that can be taken up by plant roots, and some strains can even fix atmospheric nitrogen [6].
P. mucilaginosus was first phylogenetically characterized as Bacillus mucilaginosus in 1967, and it was reclassified to the genus Paenibacillus in 2010 [7,8]. It is widely distributed in the soil or rhizosphere and produces high yield of exopolysaccharides [9,10,11,12,13]. The strains from this species can promote the growth of green gram, trifoliate orange, maize and apple seedling, and it has thus been described as an efficient PGPR [14,15,16,17,18,19].
As many genes are silenced under laboratory conditions, the whole-genome sequencing (WGS) and bioinformatics tools could help biologists investigate more functions and products of PGPR. With the development of genomic era, the next generation sequencing technology makes DNA sequencing faster and cheaper than the first-generation method [20]. Thus, more and more ecologically important metabolites for PGPR have been discovered by the extensive genomic studies [21]. Until now, the genomes of three strains (KNP414, 3016 and K02) from P. mucilaginosus have been sequenced [22,23,24]. However, it is necessary to provide detailed genome-level descriptions of essential features in P. mucilaginosus, including phosphate solubilization, plant hormone production, biofilm formation, and exopolysaccharide biosynthesis.
In this study, we sequenced the genome of a strain P. mucilaginosus G78, and we annotated the genes related to the ability of solubilizing the phosphate, releasing IAA, producing exopolysaccharides, and forming the biofilm. We also compared genomic regions implicated in association with plant hosts among 40 other strains from Paenibacillus genus. This study aimed to provide a foundation for the genetic studies and functions of P. mucilaginosus species and explore the potential ability of plant growth promotion of Paenibacillus genus at the genomic level.
2. Materials and Methods
2.1. Measurement of IAA Production and Phosphate Solubilization
For the measurement of IAA production, P. mucilaginosus G78 was grown in modified ACCC5 medium supplemented with 100 μg/mL Trp (IAA precursor). The modified ACCC5 medium contained sucrose 10 g/L, yeast extracts 0.5 g/L, K2HPO4·3H2O 0.5 g/L, NaCl 0.2 g/L, MgSO4·7H2O 0.2 g/L, CaCO3 1 g/L, at pH 7.2 [22]. The production of IAA was measured by using colorimetric assay, and the modified ACCC5 medium with Trp was used as negative control [25]. For the determination of phosphate solubilization, G78 strain was inoculated into Pikovskaya’s broth containing insoluble tri-calcium phosphate (0.5%) or soybean lecithin (0.02%) and cultured for 72 h, at 30 °C. Water-soluble phosphorus in the supernatant was determined by the chlorostannous-reduced molybdophosphoric acid blue method [26].
2.2. Biofilm Formation Assays
The formation of biofilm was measured applying the crystal violet (CV) method following the experimental procedure as described by Shang [27]. The strain was grown overnight in modified ACCC5 medium, and the N medium was used to develop the bacterial biofilm. The N medium contained maltose 2.5 g/L, MgSO4·7H2O 0.73 g/L, K2HPO4·3H2O 0.4 g/L, NaCl 0.06 g/L, FeCl3 0.6 mg/L, salicylic acid 10 mg/L and CaCO3 1 g/L, at pH 7.2.
2.3. Growth-Promoting Assay
The tomato seeds were surface sterilized by 1% (v/v) NaClO, germinated and transplanted in sterilized vermiculite moistened with Hoagland nutrient solution in Leonard jars, at 25 °C, and placed in a plant growth chamber [28]. The daylight illumination period was 12 h, and the light intensity was 1700 lx. The seedlings were inoculated with 10 mL of bacterial inoculum diluted with 10 mM sterilized MgSO4 solution (1 × 108 cfu/mL) on the 7th, 14th, and 21st days after transplanting. The control seedlings were incorporated with the same volume of 10 mM sterilized MgSO4. Shoot and root lengths, fresh weight were determined at 35 days post inoculation. The root length and scanning version were acquired and analyzed by Root Analysis WINRHIZO System (Regent, CAN). Data obtained were statically analyzed using SPSS software version 25.0 (IBM Corp., Armonk, NY, USA) and were presented in tables as the means ± standard error of mean (SEM). Significant differences between treatment were compared by Independent-samples t test.
2.4. Antagonistic Activity
The antagonistic effects of P. mucilaginosus G78 on the fungus were detected using the dual-culture plate approach by Deng et al. [29], with some modifications. P. mucilaginosus G78 was inoculated and incubated on modified ACCC5 agar medium for 24 h. Fungus inhibition tests were performed by placing the agar plug with Fusarium oxysporum f. sp. Momordicae or F. oxysporum f. sp. Cucumerinum in the center of PDA medium, and three agar plugs with P. mucilaginosus were placed 2.5 cm from the center. The agar plugs with no bacteria were selected as negative control. Plates were incubated, at 28 °C, for 5 days and checked for inhibition.
2.5. Antibiotic Susceptibility Tests
Bacteria was cultured for 24 h, centrifuged, resuspended and diluted 102 times with the modified ACCC5 medium, and then spread onto modified ACCC5 agar medium containing different antibiotics. Bacteria spread onto the medium without any antibiotics was used as a control [30]. The antibiotics used in this study included ampicillin, bacitracin, polymyxin, chloramphenicol, vancomycin, tetracycline, streptomycin and getamicin, with 1 mg/L, 5 mg/L, 10 mg/L, 50 mg/L, 100 mg/L and 150 mg/L, respectively. Plates were incubated, at 28 °C, and checked for inhibition.
2.6. Genome Sequencing and Analysis
The genomic DNA was extracted using a Qiagen Genomic-tip kit and following a modified manufacturer’s protocol as previously described [31]. Sequencing libraries were generated using NEBNext® UltraTM DNA Library Prep Kit for Illumina (Lincoln, NE, USA) following manufacturer’s recommendations, and index codes were added to attribute sequences to the sample. The whole genome of P. mucilaginosus G78 was sequenced using Illumina NovaSeq PE150 at the Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). The predicted CDSs were annotated from NR (NCBI non-redundant protein sequences; Version 202210, Swiss-Prot (A manually annotated and reviewed protein sequence database; Version 202210), Pfam (Protein family; Version Pfam v35.0), GO (Gene Ontology; Version 20220915), COG (Clusters of Orthologous Groups of proteins; Version 202006), and KEGG (Kyoto Encyclopedia of Genes and Genomes; Version 202210) database using sequence alignment tools such as RPS-BLAST, Diamond and HMMER. Briefly, each set of query proteins were aligned with the databases, and annotations of best-matched subjects (e-value < 10−5) were obtained for gene annotation. Secondary metabolites synthesis clusters were identified using antiSMASH (Version 5.1.2). Antibiotic resistance genes were predicted using CARD (Comprehensive Antibiotic Resistance Database, version 1.1.3). The genomic analyses were also performed using the online platform of Majorbio Cloud Platform (http://cloud.majorbio.com accessed on 1 October 2022) from Shanghai Majorbio Bio-pharm. The GenBank accession number of the sequence for P. mucilaginosus G78 is JAKQYK000000000.
2.7. Comparative Genomic and Phylogenetic Analysis
The core-orthologs from 41 strains were detected by PGAP pipeline-based protein similarity method [32]. The core-orthologs were clustered with at least 50% similarity for protein sequence to each other and 50% overlap with the longest sequence. The total genes within 41 genomes weres defined as the pan genome, and the shared genes among 41 strains was defined as their core genome [26]. Multiple alignment of amino acid sequences was carried out by using ClustalW (version 2.1) [33]. Conserved blocks from multiple alignments of test proteins were selected by using Gblocks [34]. Phylogenetic trees were inferred with 309 sing-copy core genes shared by 41 taxa. Maximum Likelihood (ML) method were inferred with PhyML (version 3.0) using the LG model with 1000 bootstrap replicates to construct the phylogenetic trees [35].
3. Results
3.1. Assessment of Plant Growth-Promoting Traits
Our results indicated significant effect of P. mucilaginosus G78 having the ability to form biofilm, solubilizing the inorganic and organic phosphate, and produce IAA (Table 1). Furthermore, the plant height and fresh weight determined after 35 days of inoculation of P. mucilaginosus G78 showed significant differences (p < 0.05), as presented in Table 2. The inoculation treatment improved the plant height and fresh weight of the tomato plant. The plant height and fresh weight of G78-treated tomato plants increased by 44.1% and 90.0% compared to the control plant, respectively (Table 2, Figure 1a,c), indicating the growth-promoting effect of P. mucilaginosus G78 inoculation.

Table 1.
Plant growth-promoting traits of P. mucilaginosus G78 strain.

Table 2.
Effects of P. mucilaginosus G78 strain on the growth of tomato seedlings.
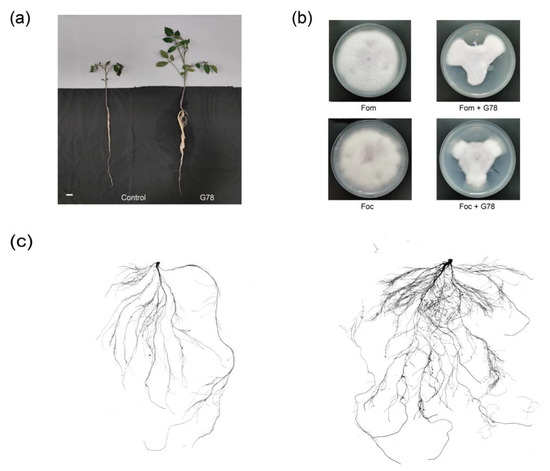
Figure 1.
The plant growth promotion ability and antagonistic activity against pathogenic fungi through the dual-culture test of P. mucilaginosus G78 strain. (a) The effects of G78 strain on the growth of tomato plant. Bar, 2 cm. (b) The growth of F. oxysporum f. sp. momordicae and F. oxysporum f. sp. cubense with or without G78. Fom, F. oxysporum f. sp. momordicae; Foc, F. oxysporum f. sp. cubense; Fom+G78 or Foc+G78, F. oxysporum f. sp. momordicae or F. oxysporum f. sp. cubense with G78. (c) The root scanning image of tomato plants. G78, the treatment which was inoculated by G78; control, the uninoculated treatment.
3.2. Genomic Features
After assembly, the draft genome size of the P. mucilaginosus G78 was 8,576,872 bp with a GC content of 58.5% and 77 scaffolds with the N50 of 250,045 bp. The mean scaffold size was 111,388 bp and the longest scaffold was 778,093 bp. Additionally, a total of 7337 genes with 143 tRNAs, 41 rRNAs, and 5 ncRNAs were identified. The predicted genes included 2274 genes involved in metabolism, 753 genes involved in environmental information processing, and 253 genes in cellular processes. COG function classification showed that 904 genes are involved in carbohydrate transport and metabolism, 701 genes involved in the transcription, 651 genes involved in general function, and 497 genes involved in signal transduction. A total of 423 carbohydrate-active enzyme-encoding genes were identified in G78, including glycosyl hydrolysis (GHs, 58.4%), glycosyl transferases (GTs, 11.8%), carbohydrate esterases (CEs, 31.3%), carbohydrate-binding modules (CBMs, 1.2%), polysaccharide lyases (PLs, 5.7%), and auxiliary activities (AAs, 5%). The circular genome visualization for the P. mucilaginosus G78 was produced by the circular viewer, as shown in Figure 2a.
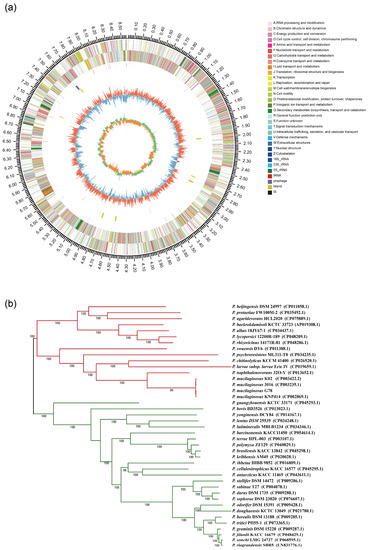
Figure 2.
Genomic features of P. mucilaginosus G78 and phylogenetic relationship of 41 Paenibacillus strains. (a) Genome map of P. mucilaginosus G78. Circles (from outside to inside) as follows: (1) scale marks (unit, Mb), (2) protein-coding sequences on the forward strand colored by COG category, (3) protein-coding sequences on the reverse strand (same color scheme as the second circle), (4) rRNA genes, (5) tRNA genes, (6) GC content (deviation from average), and (7) positive (green) and negative (orange) GC skew. (b) ML phylogenetic tree was constructed using based on 309 single-copy core proteins shared by 41 genomes.
3.3. Phylogenetic Tree and Comparative Genomic Analysis
A phylogenetic tree based on single-copy core genes was reconstructed using the whole genome sequence (Figure 2b). The information about Paenibacillus strains was shown in Table S1. It was inferred among the 41 Paenibacillus strains that the G78 strain was very closely related to the P. mucilaginosus strain KNP414 and to two other P. mucilaginosus strains: K02 and 3016. The ANI value between P. mucilaginosus G78 and KNP414 equals 99.9%, 98.9% for strain 3016 and 98.49% for K02. It also indicated that the P. mucilaginosus strains grouped closely to P. naphthalenovorans strain 32O-Y.
To visualize the similarity of encoded proteins, the whole-genome alignments of protein coding sequences were conducted for 41 Paenibacillus species strains. Average amino acid identities were calculated using the pair-wise orthologous sets of CDSs. Only 0.13% of the total 234,857 putative protein-coding genes were identified as core genes, which suggests that genetic differentiation and horizontal gene acquisition from other taxa are high. G78 contained a total of 229 strain specific genes, while P. mucilaginosus strain KNP414 has 728 strain specific CDS.
3.4. Secondary Metabolites Production and Antimicrobial Resistance Genes
As shown by the dual-cultural plate, P. mucilaginosus G78 exhibits prohibition of the growth of the plant pathogen, F. oxysporum f. sp. momordicae and F. oxysporum f. sp. cubense (Figure 1b) following incubation for 5 d. Such findings demonstrated the capability of G78 strain to inhibit the growth of F. oxysporum f. sp. momordicae and F. oxysporum f. sp. cubense, with the inhibition rate of 51.2% and 47.3%, respectively. In P. mucilaginosus G78, the genome analysis identified several gene clusters that encode secondary metabolites. The putative natural products include terpene, siderophore, ladderane, flaviolins, polyketides, and NRPS. The NRPS contains some proposed peptide antibiotics, such as icosalide, paenibacterin, tridecaptin, locillomycin. The representative gene clusters encoding putative secondary metabolites were summarized in Table 3. G78 can grow under ampicillin, bacitracin, and polymyxin at a low concentration level, suggesting it contains antimicrobial resistance-related genes (Table 4). These were predicted based on the CARD database (Table 5). G78 was found to contain 429 genes related to the resistance to different antibiotics (Table S2).

Table 3.
The putative gene cluster encoding secondary metabolites in P. mucilaginosus G78.

Table 4.
Antimicrobial susceptibility test of P. mucilaginosus G78.

Table 5.
The putative antibiotic resistance-related genes.
3.5. EPS Synthesis Genes
Exopolysaccharides (EPS) play key structural and functional roles in P. mucilaginosus, and were reported to protect the bacteria against the host defense during the plant–microbe interaction. We found an EPS gene cluster in G78 strain, mainly comprising 35 putative genes on a ~39.1 kb DNA fragment, which includes glycosyl transferases, polymerases, enzymes involved in the synthesis of nucleotide precursors and enzymes responsible for sugar modification or the addition of sugar substituents (Table 6, Figure 3). We further blast the putative EPS biosynthetic gene cluster among the sequenced strains from this species, and found P. mucilaginosus KNP414 and K02 have very similar gene structures with strain G78, while strain 3016 showed some of the truncated and non-homologous sequences (Figure S1).

Table 6.
Putative exopolysaccharides production-related gene cluster in G78.
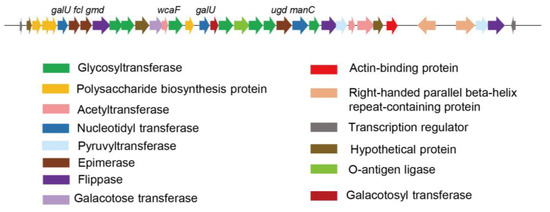
Figure 3.
Predicted exopolysaccharide gene cluster for P. mucilaginosus G78. The predicted functions of each color-coded ORF are indicated at the lower bottom panel.
3.6. Biofilm Formation Genes
The key genes involved in the formation of biofilm were investigated using the KAAS database, and 28 genes were explored, including metabolic pathway regulators, diguanylate or adenylate cyclase, matrix protein-coding genes, and putative matrix polysaccharide synthesis genes (Table 7). It was shown that pel-like operon encoded the biofilm polysaccharide in Bacillus cereus [36]. We explored the pel-like genes among 41 Paenibacillus strains, and found that P. mucilaginosus strains had more Pel polysaccharide biosynthetic genes, which indicated that Pel polysaccharide is not a common biofilm matrix component among the genus of Paenibacillus (Figure 4).

Table 7.
Putative biofilm formation-related genes in P. mucilaginosus G78.
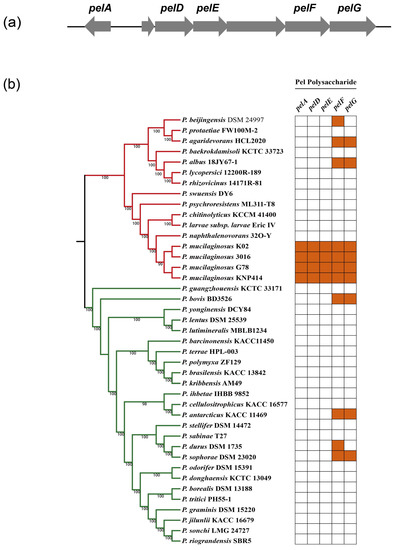
Figure 4.
pel-like operon of P. mucilaginosus G78 and genes involved in Pel-polysaccharide of 41 Paenibacillus strains. (a) pel-like operon architectures of P. mucilaginosus G78. Arrows are used to denote open reading frames, with the direction of each arrow indicating the direction of transcription. (b) pelAEDFG genes of 41 Paenibacillus strains. Colored box represents the presence of a gene within a genome and white box indicates absence of a gene.
3.7. Plant Growth-Promoting Ability Genes
Indole-3-acetic acid (IAA) has been reported as an important phytohormone with the capacity to control plant development, which can be produced by many rhizosphere bacteria [37]. In this study, the indolepyruvate decarboxylase (encoded by ipdC gene) and auxin carrier protein were identified in strain G78 (Figure 5). However, the genes encoding tryptophan monooxygenas or indole-3-acetamide hydrolase were not detected in the G78 strain. Furthermore, the gene ipdC exists among all tested strains, suggesting these bacteria are all capable of IAA production following the indole-3-pyruvic acid pathway, even if auxin carrier proteins are deficient in some strains.
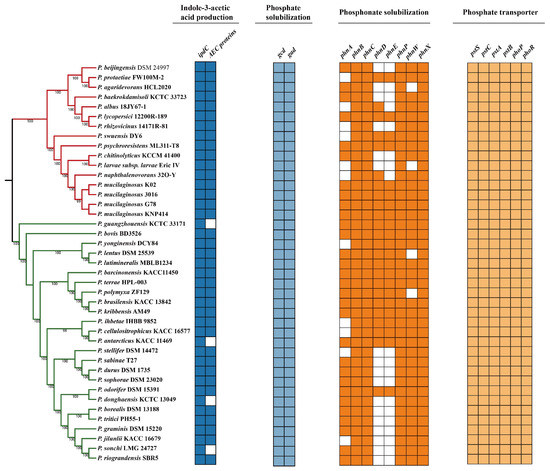
Figure 5.
Genes involved in IAA production, organic and inorganic phosphate solubilization of 41 Paenibacillus strains. Colored box represents the presence of a gene within a genome and white box indicates absence of a gene.
Considering the phosphate-providing ability of strain G78, we found eight phn genes (phnABCDEWXM)and two genes encoding glucose-1-dehydragenase (gcd) and gluconic acid dehydrogenase (gad). We also identified the putative pst operon (pstS, ptsC, pstA, pstB) and PhoP-PhoR system in the genome of G78 (Figure 5). In addition, we screened 20 pathways in total related with the organic acid metabolic pathway, and all above can explain the phosphate solubilization and secretion ability of this strain (Table S2). Additionally, the mineral phosphate solubilizations genes (gcd, gad) and phosphate transport system are present in all 41 sequenced Paenibacillus strains, indicating that all these strains have the ability to promote plant growth on phosphate-limited soil.
4. Discussion
P. mucilaginosus has been widely reported as a plant growth-promoting rhizobacteria (PGPR) [14,15,16,17,18,19]. Until now, only three strains from P. mucilaginosus were sequenced, and the PGPR traits at the genome level has not been described in detail. In this study, we sequenced a PGPR strain, P. mucilaginosus G78, and explored the genes related to microbe-–plant interaction, such as secondary metabolites synthesis, exopolysaccharides biosynthesis, biofilm formation, IAA production, and phosphate-dissolving ability. The genome size of P. mucilaginosus G78 was 8,576,872 bp with a GC content of 58.5%, which is very similar in size with other P. mucilaginosus strains. As shown in Table S1, P. mucilaginosus showed high GC content. Moreover, it presents the second largest genome size than other 37 Paenibacillus strains. Focusing on the influence of several properties including biochemical, genetic flows, selection biases, and the biochemical-energetic properties shaping genome composition, it indicated a trend toward high GC content and larger genomes in free-living organisms, as a result of more complex and varied environments [38,39]. The genes related to the glycoside hydrolase family are much abundant in this strain in comparison to other Paenibacillus, which is consistent with their reported importance for Paenibaicllus survival [40].
Secondary metabolites (SM) produced by plant-associated biocontrol bacteria can directly reduce the pathogen’s ability to cause disease, induce plant defense mechanisms, or promote plant development [41]. P. mucilaginosus G78 showed antifungal activity against phytopathogens such as F. oxysporum, and has the genomic potential to produce a lot of SMs. Recent extensive bacterial genome sequencing and bioinformatic analysis showed that terpene synthases are widely distributed in bacteria [42,43]. The ability to produce or capture siderophores makes the bacteria competitive advantages to colonize plant tissues [44]. Antismash analysis showed that strain G78 has asb operon, which is responsible for petrobactin biosynthesis in Bacillus anthracis [45,46]. Kedarcidin (KED) is an aromatic enediyne that may be produced by strain G78. It was reported to be chromoprotein antitumor antibiotic and was isolated from Streptoalloteichus sp. ATCC 53560 but rarely reported in Paenibacillus or Bacillus genus [47,48]. Bacteriocins are ribosomally synthesized peptides (RSPs) that contain 12~50 amino acid residues which exhibited a broad spectrum of antimicrobial activity. Many of the polyketides (PKs) produced by Bacillus and Paenibacillus species have been described as bioactive natural products that had medical value and can be potentially applied in agriculture for controlling plant pathogens [49,50]. In total, there are 18 NRPS or NRPS-like metabolites proposed gene clusters in strain G78, including 10 unknown ones. The NRPS contains some proposed peptide antibiotics, such as icosalide, paenibacterin, tridecaptin, locillomycin and other new products. However, whether the metabolites mentioned above were produced still needs further determination. In this study, we demonstrated that P. mucilaginosus G78 could grow on the medium supplemented with ampicillin, bacitracin, polymyxin and chloramphenicol. The putative genes which have a role in the resistance to these antibiotics are listed in Table 5. The G78 strain remains susceptible to vancomycin, tetracycline and streptomycin (Table 4), although partial genes participating in these antibiotic resistances were identified by CARD analysis, indicating that the completed operon is necessary for the antibiotic resistance.
Exopolysaccharides secreted by P. mucilaginosus strains was reported to have strong antioxidant abilities [12] and was hypothesized to play an important role during the process of wastewater treatment [13,51]. Studying the genes responsible to EPS synthesis will be helpful to explore its potential functions and structures. Exopolysaccharide biosynthesis for bacteria usually includes the following steps: uptake of substance, nucleotide sugar precursors synthesis, assembling and polymerization, modification, and release [52]. In this study, we explored the potential EPS biosynthesis gene cluster from strain G78. It was shown that the potential monosaccharides include glucose, mannose, galactose, fucose, that can probably be acetylated and pyruvated according to its genetic features. The chemical structure of EPS from this species is strain specific, as different strains from P. mucilaginosus produced various EPS, consistently with the reported biosynthetic genes variations (Figure S1). The reported partial structure of the EPS from P. mucilaginosus SM-01 was mainly composed of β-1, 4-linked-Glc and β-1, 4-linked-Man as the backbone and branched at C-2 position of β-1, - 4-linked-Glc residue by the acetyl esters [13]. The possible structure of polysaccharide from P. mucilaginosus WL412 was identified as [→4)α-Glc(1 → 2)α-Man(1 → 3)β-Glc(1 → 3)α-Man(6-Ac)(1 → 3)β-Gal(1→] [53].
Biofilms are surface-associated microbial communities in which the cells are embedded within an extracellular matrix, and they can help the microorganisms to defend against biotic or abiotic stress, to colonize the plant host and to acquire nutrients or genetic traits [54,55,56]. The major genes for the formation of biofilm include those encoding for important biofilm transcriptional regulators, the matrix structural synthesis (matrix protein, putative matrix polysaccharide), extracellular DNA synthesis and cyclic-di-GMP metabolisms [57,58]. The genome of G78 strain contained several genes that participate in biofilm formation, including transcriptional regulators, matrix structural synthesis genes, eDNA synthesis genes and diguanylate or adenylate cyclase-encoding genes. Pel polysaccharide was reported to play an important role in the biofilm formation of Pseudomonas aeruginosa and Bacillus cereus, and their biosynthesis requires an inner membrane complex comprising of PelD, PelE, PelF, and PelG [34,59,60]. Conservation of the pelADEFG among 41 studied strains from the Paenibacillus genus suggests that Pel may not be a common biofilm matrix component in this genus except for the species of P. mucilaginosus. However, further investigation via a gene deletion approach is required to characterize the function of Pel polysaccharide in P. mucilaginosus G78.
Paenibacillus strains are well known for their beneficial effects of plant growth, including production of IAA and mineral solubilization [6,61,62]. The biosynthesis of indole-3-acetic acid (IAA) is often related to beneficial effects of PGPR on plant development including cell division, elongation, tropism, apical dominance, senescence, flowering, and response to stress [35,63,64,65]. In this study, P. mucilaginosus G78 was demonstrated to produce indole-3-acetic acid (IAA), and to promote the growth of tomato seedlings by increasing the root length, fresh weight, and height of plants. The genes encoding putative indole pyruvate decarboxylase (IpdC) and auxin efflux carrier (AEC) protein are present in the genome of P. mucilaginosus G78. Knocking out the ipdC gene in Bacillus thuringiensis RZ2MS9 resulted in the decreasing production of IAA and significantly reduced its ability to promote maize growth, indicating that IAA biosynthesis by this PGPR is a major mechanism to promote plant growth [66]. Xie et al. found that ipdC homologies are present in all analyzed P. polymyxa genomes, with over 96% amino acid identity between strains across 98% of the sequence [26]. We explored the ipdC and auxin efflux carrier protein-encoding genes among the genomes of 41 Paenibacillus strains, and found that ipdC is present in all analyzed genomes as well. In contrast, not all strains have the auxin efflux carrier protein. This could indicate that their capability of exporting IAA is not common in this genus.
A large proportion of organic and inorganic phosphate is present in the soil, but they cannot be absorbed directly by plants because of the insoluble forms. Phosphate solubilizing bacteria has the ability to convert insoluble phosphates and to make it accessible to the plants [67,68]. It was proved that mineral phosphates solubilization is achieved through gluconic acid production and that the phn genes are responsible for solubilizing organic phosphate [69,70,71]. The glucose-1-dehydrogenase (gcd) and gluconic acid dehydrogenase (gad) are implicated in the production of gluconic acid [68,72,73]. The phosphate transportation is mostly related to the Pst (phosphate-specific transport) system and to the PhoP-PhoR system [24,74,75,76]. The gcd, gad, phnABCDEPWX, pst SCAB and phoPR were all present in the genome of G78, which is consistent with its ability to dissolve both organic or inorganic phosphorus compounds. All the analyzed Paenibacillus strains exhibit the genes for mineral phosphorus solubilization and phosphorus transport, indicating the potential application of Paenibacillus strains as phosphorus activator in the plant rhizosphere. In addition, although G78 strain can grow on the free-nitrogen medium, we did not find the nif genes present in this species (Figure S2), indicating that it could employ an unknown metabolic pathway to survive under nitrogen deficient condition, which needs to be clarified by further investigation.
5. Conclusions
The genome size of the P. mucilaginosus G78 was 8,576,872 bp with a GC content of 58.5%. Additionally, a total of 7337 genes with 143 tRNAs, 41 rRNAs, 5 ncRNAs were identified. It contained 26 gene clusters encoding secondary metabolites and 20 proteins related to the resistance to ampicillin, bacitracin, polymyxin and chloramphenicol, which is in accordance with its antagonist activity and antibiotic resistance ability. According to the genetic features, the potential monosaccharides of its exopolysaccharides for P. mucilaginosus G78 may include glucose, mannose, galactose, fucose, that can probably be acetylated and pyruvated. Conservation of the pelADEFG compared with other 40 Paenibacillus species suggests that Pel may be a specific biofilm matrix component in P. mucilaginosus. The containing genes encoding IAA production and phosphate solubilization associated with the phenotypic analysis highlighted the capability of P. mucilaginosus G78 strain to promote the plant growth. P. mucilaginosus species showed high GC content, and it presents the second largest genome size than other 37 studied Paenibacillus strains. Several genes associated with plant growth-promoting traits, i.e., IAA production and phosphate solubilization, are well conserved among 41 Paenibacillus strains, suggesting their potential uses in agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14020392/s1, Table S1: The genome information of Paenibacillus strains in this study; Table S2. Putative genes related to phosphate solubilization ability in G78; Predicted exopolysaccharide gene clusters for four P. mucilaginosus strains (3016, K02, KNP414 and G78); Figure S1. Predicted exopolysaccharide gene clusters for four Paenibacillus mucilaginosus strains (3016, K02, KNP414 and G78); Figure S2. Genes involved in nitrogen fixation of 41 Paenibacillus strains.
Author Contributions
D.W., W.G. and V.P. conceived and designed the experiment. D.W. and V.P. wrote the paper. D.W. and W.L. (Wangxi Li) performed the experiment. W.G. supervised the study. Y.L. (Yusheng Lu), C.L., Y.L. (Yaying Li), K.X., L.S., C.S., H.P., W.L. (Wanling Li) and C.Z. contributed in reagents/materials/analysis tools. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (31800102), Guangdong Basic and Applied Basic Research Foundation (2021A1515011331, 2021A1515011211), The Science and Technology Program of Guangdong Province (2021B1212050022), The open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG08, 2022SDZG09), Modern Agricultural Industrial Technology System of Guangdong Province (The task of Innovation team-building of key generic technologies in agricultural resources and environment) (2022KJ118, 2022KJ111), High-level Foreign Expert Project of Guangdong Province (2019, 2021), Science and Technology Program of Guangzhou, China (201904010262, 202002020075), Dean project funding of the Guangdong Academy of Agricultural Sciences, China (201934, 201935), Agricultural competitive industry discipline team building project of Guangdong Academy of Agricultural Sciences (202121TD), Low carbon agriculture and carbon neutralization Research Center, GDAAS (XT202220), and Special fund for scientific innovation strategy-construction of high-level Academy of Agriculture Science (R2019PY-QF010, R2020PY-JG012, R2021YJ-YB1003, R2021YJ-QG007, R2022YJ-YB3009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Muhammad Kamran (College of Pastoral Agriculture Science and Technology, Lanzhou University) for his suggestion about the data analyses and the comments on our manuscript. We acknowledge Aiting Lin (Shanghai Biozeron Biotech Co., Ltd.) for her assistance in bioinformatic analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Van Kernebeek, H.R.J.; Oosting, S.J.; Van Ittersum, M.K.; Bikker, P.; De Boer, I.J.M. Saving land to feed a growing population: Consequences for consumption of crop and livestock products. Int. J. Life Cycle Assess. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Factories 2016, 15, 203. [Google Scholar] [CrossRef]
- Avakyan, Z.; Pivovarova, T.; Karavaiko, G. Properties of a new species, Bacillus mucilaginosus. Microbiology 1986, 55, 369–374. [Google Scholar]
- Hu, X.-F.; Li, S.-X.; Wu, J.-G.; Wang, J.-F.; Fang, Q.-L.; Chen, J.-S. Transfer of Bacillus mucilaginosus and Bacillus edaphicus to the genus Paenibacillus as Paenibacillus mucilaginosus comb. nov. and Paenibacillus edaphicus comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 8–14. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Guo, J. Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Wu, J.G.; Wang, J.F.; Zhang, X.H.; Zhang, S.S.; Hu, X.F.; Chen, J.S. A gyrB-targeted PCR for rapid identification of Paenibacillus mucilaginosus. Appl. Microbiol. Biotechnol. 2010, 87, 739–747. [Google Scholar] [CrossRef]
- Tang, J.; Qi, S.; Li, Z.; An, Q.; Xie, M.; Yang, B.; Wang, Y. Production, purification and application of polysaccharide-based bioflocculant by Paenibacillus mucilaginosus. Carbohydr. Polym. 2014, 113, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.-W.; Tseng, S.-C.; Wang, S.-L. Production and characterization of antioxidant properties of exopolysaccharide(s) from Peanibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, S.; Deng, C.; Li, H.; Yang, Q.; Xu, Z.; Chen, J. Preparation and partial structural characterization of the exopolysaccharide from Bacillus mucilaginosus SM-01. Carbohydr. Polym. 2016, 146, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Parmar, S.; Vaghela, H.; Dhandhukia, P.; Thakker, J.N.; Moral, M.T. Describing Paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR). Cogent Food Agric. 2015, 1, 1000714. [Google Scholar] [CrossRef]
- Wang, P.; Wu, S.-H.; Wen, M.-X.; Wang, Y.; Wu, Q.-S. Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Sci. Hortic. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Attar, A. Effects of Azorhizophilus paspali and Paenibacillus mucilaginosus as biofertilizer and determination of nutritional efficiency by sensors. Arab. J. Sci. Eng. 2018, 43, 3477–3484. [Google Scholar] [CrossRef]
- Mercl, F.; Tejnecký, V.; Ságová-Marečková, M.; Dietel, K.; Kopecký, J.; Břendová, K.; Kulhánek, M.; Košnář, Z.; Száková, J.; Tlustoš, P. Co-application of wood ash and Paenibacillus mucilaginosus to soil: The effect on maize nutritional status, root exudation and composition of soil solution. Plant Soil 2018, 428, 105–122. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yang, X.-Z.; Li, Z.; An, X.-H.; Ma, R.-P.; Li, Y.-Q.; Cheng, C.-G. Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J. Integr. Agric. 2020, 19, 2458–2469. [Google Scholar] [CrossRef]
- Koryagin, Y.; Kulikova, E.; Efremova, S.; Sukhova, N. The influence of microbiological fertilisers on the productivity and quality of winter wheat. Plant Soil Environ. 2020, 66, 564–568. [Google Scholar] [CrossRef]
- Kchouk, M.; Gibrat, J.F.; Elloumi, M. Generations of sequencing technologies: From first to next generation. Biol. Med. 2017, 9, 395. [Google Scholar] [CrossRef]
- Paterson, J.; Jahanshah, G.; Li, Y.; Wang, Q.; Mehnaz, S.; Gross, H. The contribution of genome mining strategies to the understanding of active principles of PGPR strains. FEMS Microbiol. Ecol. 2017, 93, 3. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, Z.; Li, L.; Jiang, X.; Guan, D.; Cao, F.; Chen, H.; Wang, X.; Shen, D.; Du, B.; et al. Complete genome sequence of Paenibacillus mucilaginosus 3016, a bacterium functional as microbial fertilizer. J. Bacteriol. 2012, 194, 2777–2778. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Wang, J.F.; Hu, X.F. Genome sequence of growth-improving Paenibacillus mucilaginosus strain KNP414. Genome Announc. 2013, 1, e00881-13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, Y.F.; Lian, B.; Chen, T.M. Complete genome sequence and comparative genome analysis of the Paenibacillus mucilaginosus K02. Microb. Pathog. 2016, 93, 194–203. [Google Scholar] [CrossRef]
- Xie, B.; Xu, K.; Zhao, H.X.; Chen, S.F. Isolation of transposon mutants from Azospirillum brasilense Yu62 and characterization of genes involved in indole-3-acetic acid biosynthesis. FEMS Microbiol. Lett. 2005, 248, 57–63. [Google Scholar] [CrossRef]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef]
- Shang, L.; Yan, Y.; Zhan, Y.; Ke, X.; Shao, Y.; Liu, Y.; Yang, H.; Wang, S.; Dai, S.; Lu, J.; et al. A regulatory network involving Rpo, Gac and Rsm for nitrogen-fixing biofilm formation by Pseudomonas stutzeri. NPJ Biofilms Microbiomes 2021, 7, 54. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Ext. Publ. 1938, 347, 35–37. [Google Scholar] [CrossRef]
- Deng, J.; Kong, S.; Wang, F.; Liu, Y.; Jiao, J.; Lu, Y.; Zhang, F.; Wu, J.; Wang, L.; Li, X. Identification of a new Bacillus sonorensis strain KLBC GS-3 as a biocontrol agent for postharvest green mould in grapefruit. Biol. Control. 2020, 151, 104393. [Google Scholar] [CrossRef]
- Syal, K.; Mo, M.; Yu, H.; Iriya, R.; Jing, W.; Guodong, S.; Wang, S.; Grys, T.E.; Haydel, S.E.; Tao, N. Current and emerging techniques for antibiotic susceptibility tests. Theranostics 2017, 7, 1795–1805. [Google Scholar] [CrossRef]
- Yan, X.; Fratamico, P.M.; Bono, J.L.; Baranzoni, G.M.; Chen, C.Y. Genome sequencing and comparative genomics provides insights on the evolutionary dynamics and pathogenic potential of different H-serotypes of Shiga toxin-producing Escherichia coli O104. BMC Microbiol. 2015, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Yang, J.; Sun, S.; Xiao, J.; Yu, J. PGAP: Pan-genomes analysis pipeline. Bioinformatics 2012, 28, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2.3.1–2.3.2. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2020, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Whitfield, G.B.; Marmont, L.S.; Bundalovic-Torma, C.; Razvi, E.; Roach, E.J.; Khursigara, C.M.; Parkinson, J.; Howell, P.L. Discovery and characterization of a Gram-positive Pel polysaccharide biosynthetic gene cluster. PLoS Pathog. 2020, 16, e1008281. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Mann, S.; Chen, Y.P. Bacterial genomic G + C composition-eliciting environmental adaptation. Genomics 2010, 95, 7–15. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Huang, W.C.; Hu, Y.; Zhang, G.; Li, M. Comparative genomic analysis reveals metabolic diversity of different Paenibacillus groups. Appl. Microbiol. Biotechnol. 2020, 104, 10133–10143. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; Garcia-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-Ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Loaces, I.; Ferrando, L.; Scavino, A.F. Dynamics, diversity and function of endophytic siderophore-producing bacteria in rice. Microb. Ecol. 2011, 61, 606–618. [Google Scholar] [CrossRef]
- Koppisch, A.T.; Browder, C.C.; Moe, A.L.; Shelley, J.T.; Kinkel, B.A.; Hersman, L.E.; Iyer, S.; Ruggiero, C.E. Petrobactin is the primary siderophore synthesized by Bacillus anthracis str. Sterne under conditions of iron starvation. Biometals 2005, 18, 577–585. [Google Scholar] [CrossRef]
- Lee, J.Y.; Janes, B.K.; Passalacqua, K.D.; Pfleger, B.F.; Bergman, N.H.; Liu, H.; Hakansson, K.; Somu, R.V.; Aldrich, C.C.; Cendrowski, S.; et al. Biosynthetic analysis of the petrobactin siderophore pathway from Bacillus anthracis. J. Bacteriol. 2007, 189, 1698–1710. [Google Scholar] [CrossRef]
- Hofstead, S.J.; Matson, J.A. Kedarcidin, a new chromoprotein antitumor antibiotic II. isolation, purification and physico-chemical properties. J. Antibiot. 1992, 45, 1250–1254. [Google Scholar] [CrossRef]
- Lohman, J.R.; Huang, S.; Horsman, G.P.; Dilfer, P.E.; Huang, T.; Chen, Y.; Wendt-Pienkowski, E.; Shen, B. Cloning and sequencing of the kedarcidin biosynthetic gene cluster from Streptoalloteichus sp. ATCC 53650 revealing new insights into biosynthesis of the enediyne family of antitumor antibiotics. Mol. Biosyst. 2013, 9, 478–491. [Google Scholar] [CrossRef]
- Olishevska, S.; Nickzad, A.; Deziel, E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215. [Google Scholar] [CrossRef]
- Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 2010, 27, 996–1047. [Google Scholar] [CrossRef]
- Nyanikova, G.G.; Kuprina, E.E.; Pestova, O.V.; Vodolazhskaya, S.V. Immobilization of Bacillus mucilaginosus a producer of exopolysaccharides, on chitin. Appl. Biochem. Microbiol. 2002, 38, 259–262. [Google Scholar] [CrossRef]
- Mishra, A.; Jha, B. Microbial Exopolysaccharides. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 179–192. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Wang, L.; Fu, R.; Cheng, R.; Wang, S.; Zhang, J. Purification and characterization of a highly viscous polysaccharide produced by Paenibacillus strain. Eur. Polym. J. 2018, 101, 314–323. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 2008, 251, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Heikal, A.; Finke, S.; Hofgaard, A.; Rehman, Y.; Sabri, A.N.; Økstad, O.A. Bacillus cereus biofilm formation on central venous catheters of hospitalised cardiac patients. Biofouling 2019, 35, 204–216. [Google Scholar] [CrossRef]
- Sornchuer, P.; Saninjuk, K.; Prathaphan, P.; Tiengtip, R.; Wattanaphansak, S. Antimicrobial susceptibility Profile and Whole-Genome Analysis of a Strong Biofilm-Forming Bacillus Sp. B87 Strain Isolated from Food. Microorganisms 2022, 10, 252. [Google Scholar] [CrossRef]
- Whitfield, G.B.; Marmont, L.S.; Ostaszewski, A.; Rich, J.D.; Whitney, J.C.; Parsek, M.R.; Harrison, J.J.; Howell, P.L. Pel Polysaccharide Biosynthesis Requires an Inner Membrane Complex Comprised of PelD, PelE, PelF, and PelG. J. Bacteriol. 2020, 202, e00684-19. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.; Parsek, M.R. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef]
- Xiao, L.; Hao, J.; Wang, W.; Lian, B.; Shang, G.; Yang, Y.; Liu, C.; Wang, S. The up-regulation of carbonic anhydrase genes of Bacillus mucilaginosus under soluble Ca2+ deficiency and the heterologously expressed enzyme promotes calcite dissolution. Geomicrobiol. J. 2014, 31, 632–641. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Li, W.; Yan, R.; Li, L.; Li, J.; Li, Y.; Li, M. Growth promoting effect of a transgenic Bacillus mucilaginosus on tobacco planting. Appl. Microbiol. Biotechnol. 2007, 74, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, M.; Okon, Y.; Broek, A.V.; Vanderleyden, J. Indole-3-acetic acid a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol. 2000, 8, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Facella, P.; Daddiego, L.; Giuliano, G.; Perrotta, G. Gibberellin and auxin influence the diurnal transcription pattern of photoreceptor genes via CRY1a in tomato. PLoS ONE 2012, 7, e30121. [Google Scholar] [CrossRef]
- Figueredo, E.F.; Cruz, T.A.D.; Almeida, J.R.; Batista, B.D.; Marcon, J.; Andrade, P.A.M.; Hayashibara, C.A.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the CRISPR-Cas9 system. Microbiol. Res. 2022, 266, 127218. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Metcalf, W.W.; Wanner, B.L. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene 1993, 129, 27–32. [Google Scholar] [CrossRef]
- Metcalf, W.W.; Wanner, B.L. Mutational analysis of an Eschenichia coli fourteen-gene operon for phosphonate degradation, using TnphoA’ elements. J. Bacteriol. 1993, 175, 3430–3442. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef]
- de Werra, P.; Pechy-Tarr, M.; Keel, C.; Maurhofer, M. Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 2009, 75, 4162–4174. [Google Scholar] [CrossRef] [PubMed]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Kobayashi, Y.; Hulett, F.M. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the pho regulon. J. Bacteriol. 1997, 179, 2534–2539. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.N.; Torriani, A. Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol. 1990, 4, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Groisman, E.A. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 2001, 183, 1835–1842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).