Treatment Dilemma in Children with Late-Onset Pompe Disease
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.; Desai, A.K.; Kazi, Z.B.; Corey, K.; Austin, S.; Hobson-Webb, L.D.; Case, L.E.; Jones, H.N.; Kishnani, P.S. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol. Genet. Metab. 2017, 120, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Papadimas, G.K.; Michelakakis, H.; Kararizou, E.; Manta, P. Highlighting intrafamilial clinical heterogeneity in late-onset Pompe disease. Mol. Genet. Metab. Rep. 2014, 1, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Ausems, M.G.E.M.; Ten Berg, K.; Beemer, F.A.; Wokke, J.H.J. Phenotypic expression of late-onset glycogen storage disease type II: Identification of asymptomatic adults through family studies and review of reported families. Neuromuscul. Disord. 2000, 10, 467–471. [Google Scholar] [CrossRef]
- Martínez, J.R.B.; Martínez, A.C. Long-term enzyme-replacement therapy (ERT) with alglucosidase alfa: Evolution of two siblings with juvenile late-onset Pompe disease. J. Neurol. Sci. 2015, 358, 459–460. [Google Scholar] [CrossRef]
- van der Ploeg, A.T.; Clemens, P.R.; Corzo, D.; Escolar, D.M.; Florence, J.; Groeneveld, G.J.; Herson, S.; Kishnani, P.S.; Laforet, P.; Lake, S.L.; et al. A Randomized Study of Alglucosidase Alfa in Late-Onset Pompe’s Disease. N. Engl. J. Med. 2010, 362, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Corzo, D.; Nicolino, M.; Byrne, B.; Mandel, H.; Hwu, W.-L.; Leslie, N.; Levine, J.; Spencer, C.; McDonald, M.; et al. Recombinant human acid α-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology 2007, 68, 99–109. [Google Scholar] [CrossRef]
- Dornelles, A.D.; Junges, A.P.P.; Pereira, T.V.; Krug, B.C.; Gonçalves, C.B.T.; Llerena, J.C.; Kishnani, P.S.; de Oliveira, H.A.; Schwartz, I.V.D. A systematic review and meta-analysis of enzyme replacement therapy in late-onset pompe disease. J. Clin. Med. 2021, 10, 4828. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Goldenberg, P.C.; DeArmey, S.L.; Heller, J.; Benjamin, D.; Young, S.; Bali, D.; Smith, S.A.; Li, J.S.; Mandel, H.; et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010, 99, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Schuchman, E.H. Enzyme Replacement Therapy for Lysosomal Diseases: Lessons from 20 Years of Experience and Remaining Challenges. Hum. Genet. 2012, 13, 307–335. [Google Scholar] [CrossRef]
- Llerena, J.C., Jr.; Nascimento, O.J.; Oliveira, A.S.B.; Dourado, M.E.T., Jr.; Marrone, C.D.; Siqueira, H.H.; Sobreira, C.F.R.; Dias-Tosta, E.; Werneck, L.C. Guidelines for the diagnosis, treatment and clinical monitoring of patients with juvenile and adult pompe disease. Arq. Neuropsiquiatr. 2016, 74, 166–176. [Google Scholar] [CrossRef]
- de Vries, J.M.; Kuperus, E.; Hoogeveen-Westerveld, M.; Kroos, M.A.; Wens, S.C.; Stok, M.; van der Beek, N.A.; Kruijshaar, M.E.; Rizopoulos, D.; van Doorn, P.A.; et al. Pompe disease in adulthood: Effects of antibody formation on enzyme replacement therapy. Genet. Med. 2017, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Manera, J.; Kishnani, P.S.; Kushlaf, H.; Ladha, S.; Mozaffar, T.; Straub, V.; Toscano, A.; van der Ploeg, A.T.; Berger, K.I.; Clemens, P.R.; et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): A phase 3, randomised, multicentre trial. Lancet Neurol. 2021, 20, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Sun, B.; Koeberl, D.D. Gene therapy for glycogen storage diseases. Hum. Mol. Genet. 2019, 28, R31–R41. [Google Scholar] [CrossRef] [PubMed]
- Schoser, B.; Roberts, M.; Byrne, B.J.; Sitaraman, S.; Jiang, H.; Laforêt, P.; Toscano, A.; Castelli, J.; Díaz-Manera, J.; Goldman, M.; et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): An international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021, 20, 1027–1037. [Google Scholar] [CrossRef]
- Niu, D.-M.; Lin, H.-Y.; Wu, T.J.-T.; Hsu, J.-H.; Yu, H.-C.; Lin, S.-P.; Chuang, C.-K.; Huang, C.-H. Novel human pathological mutations. Gene symbol: GAA. Disease: Glycogen storage disease 2. Hum. Genet. 2010, 127, 465. [Google Scholar]
- Niño, M.Y.; In’t Groen, S.L.; Bergsma, A.J.; van der Beek, N.A.; Kroos, M.; Hoogeveen-Westerveld, M.; Van Der Ploeg, A.T.; Pijnappel, W.P. Extension of the Pompe mutation database by linking disease-associated variants to clinical severity. Hum. Mutat. 2019, 40, 1954–1967. [Google Scholar] [CrossRef]
- Romero, M.B.; Cortes, E.B.; Lorite, J.B.; Rivas, E.G.; Sendra, I.I.; Jiménez, L.M.; Martos, M.L.; Arregui, A.L.D.M.; Fernández, J.P.; Pascual, S.I.P.; et al. Guía clínica de la enfermedad de Pompe de inicio tardío. Rev. Neurol. 2012, 54, 497. [Google Scholar] [CrossRef]
- Schüller, A.; Kornblum, C.; Deschauer, M.; Vorgerd, M.; Schrank, B.; Mengel, E.; Lukacs, Z.; Gläser, D.; Young, P.; Plöckinger, U.; et al. Diagnose und Therapie des Late-onset-Morbus-Pompe. Nervenarzt 2013, 84, 1467–1472. [Google Scholar] [CrossRef]
- Hundsberger, T.; Rohrbach, M.; Kern, L.; Rösler, K.M. Swiss national guideline for reimbursement of enzyme replacement therapy in late-onset Pompe disease. J. Neurol. 2013, 260, 2279–2285. [Google Scholar] [CrossRef]
- Yang, C.-F.; Yang, C.C.; Liao, H.-C.; Huang, L.-Y.; Chiang, C.-C.; Ho, H.-C.; Lai, C.-J.; Chu, T.-H.; Yang, T.-F.; Hsu, T.; et al. Very Early Treatment for Infantile-Onset Pompe Disease Contributes to Better Outcomes. J. Pediatr. 2016, 169, 174–180.e1. [Google Scholar] [CrossRef]
- Laloui, K.; Wary, C.; Carlier, R.-Y.; Hogrel, J.-Y.; Caillaud, C.; Laforet, P. Making diagnosis of Pompe disease at a presymptomatic stage: To treat or not to treat? Neurology 2011, 77, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Echaniz-Laguna, A.; Carlier, R.-Y.; Laloui, K.; Carlier, P.; Salort-Campana, E.; Pouget, J.; Laforet, P. Should patients with asymptomatic pompe disease be treated? A nationwide study in France. Muscle Nerve 2015, 51, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Ashe, K.M.; Taylor, K.M.; Chu, Q.; Meyers, E.; Ellis, A.; Jingozyan, V.; Klinger, K.; Finn, P.F.; Cooper, C.G.; Chuang, W.-L.; et al. Inhibition of glycogen biosynthesis via mTORC1 suppression as an adjunct therapy for Pompe disease. Mol. Genet. Metab. 2010, 100, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Li, L.; Shirihai, O.S.; Trudeau, K.M.; Puertollano, R.; Raben, N. Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. EMBO Mol. Med. 2017, 9, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-W.; Xu, X.-B.; Ji, K.-Q.; Zhang, N.; Sun, Y.; Zhao, D.-D.; Zhao, Y.-Y.; Yan, C.-Z. Activated mTOR signaling pathway in myofibers with inherited metabolic defect might be an evidence for mTOR inhibition therapies. Chin. Med. J. 2019, 132, 805–810. [Google Scholar] [CrossRef]
- Kronn, D.F.; Day-Salvatore, D.; Hwu, W.-L.; Jones, S.A.; Nakamura, K.; Okuyama, T.; Swoboda, K.J.; Kishnani, P.S. Management of confirmed newborn-screened patients with pompe disease across the disease spectrum. Pediatrics 2017, 140, S24–S45. [Google Scholar] [CrossRef]
- van der Ploeg, A.T.; Kruijshaar, M.E.; Toscano, A.; Laforêt, P.; Angelini, C.; Lachmann, R.H.; Pascual, S.I.P.; Roberts, M.; Rösler, K.; Stulnig, T.; et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: A 10-year experience. Eur. J. Neurol. 2017, 24, 768-e31. [Google Scholar] [CrossRef]
- Al Jasmi, F.; Al Jumah, M.; Alqarni, F.; Al-Sanna’A, N.; Al-Sharif, F.; Bohlega, S.; Cupler, E.J.; Fathalla, W.; Hamdan, M.A. Diagnosis and treatment of late-onset Pompe disease in the Middle East and North Africa region: Consensus recommendations from an expert group. BMC Neurol. 2015, 15, 205. [Google Scholar] [CrossRef]
- Pichiecchio, A.; Uggetti, C.; Ravaglia, S.; Egitto, M.G.; Rossi, M.; Sandrini, G.; Danesino, C. Muscle MRI in adult-onset acid maltase deficiency. Neuromuscul. Disord. 2004, 14, 51–55. [Google Scholar] [CrossRef]
- Khan, A.; Boggs, T.; Rt, M.B.; Austin, S.; Bs, M.S.; Case, L.; Kishnani, P.S. Whole-body magnetic resonance imaging in late-onset Pompe disease: Clinical utility and correlation with functional measures. J. Inherit. Metab. Dis. 2020, 43, 549–557. [Google Scholar] [CrossRef]
- Díaz-Manera, J.; Walter, G.; Straub, V. Skeletal muscle magnetic resonance imaging in Pompe disease. Muscle Nerve 2021, 63, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Bonaparte, S.; Segovia, S.; Llauger, J.; Belmonte, I.; Pedrosa, I.; Alejaldre, A.; Mayos, M.; Suárez-Cuartín, G.; Gallardo, E.; Illa, I.; et al. Muscle MRI findings in childhood/adult onset pompe disease correlate with muscle function. PLoS ONE 2016, 11, e0163493. [Google Scholar] [CrossRef] [PubMed]
- Pichiecchio, A.; Poloni, G.; Ravaglia, S.; Ponzio, M.; Germani, G.; Maranzana, D.; Costa, A.; Repetto, A.; Tavazzi, E.; Danesino, C.; et al. Enzyme replacement therapy in adult-onset glycogenosis II: Is quantitative muscle MRI helpful? Muscle Nerve 2009, 40, 122–125. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 7 Months Old | Patient 2 8 Years Old | Patient 3 10 Years Old | Mother | |
|---|---|---|---|---|
| Symptoms | None | Slight fatigability in the previous four months | None | Muscle fatigability and cramps from a young age |
| Signs of disease are present at evaluation | Slight hypotonia of the upper back | Difficulty in whistling and blowing properly | None | None |
| Serum Creatine Phosphokinase (CPK, U/L) Normal value < 190 | 372 | 153 | 183 | 134 |
| Lactate Dehydrogenase (LDH, U/L) Normal value 120–300 | 342 | 357 | 242 | Not available |
| Aspartate Transaminase (AST, U/L) Normal value < 40 | 40 | 25 | 20 | Not available |
| Alanine Transaminase (ALT, U/L) Normal value < 41 | 24 | 17 | 25 | Not available |
| Creatine Kinase Muscle Band (CK-MB, ng/mL) Normal value < 6.22 | 11.8 | 3.41 | 3.3 | Not available |
| Pro-B-type Natriuretic Peptide (pro-BNP, pg/mL) Normal value < 320 | 31 | 79 | 52 | Not available |
| Acid maltase activity on leukocytes Normal value > 0.35 | 0.33 | Not performed | Not performed | Not performed |
| Enzyme activity on a dry blood spot (µmol/L/h) Normal value > 2 | 1.9 | 1.2 | 1.2 | |
| Urinary Tetrasaccharid (Glc4, mmol/mol/cr) Normal value 0.08–1.37 | 3.21 | 0.78 | 0.69 | Not performed |
| GAA sequencing | c.2284G>A p.(Glu762Lys); c.1994G>A p.(Gly665Glu) | c.2284G>A p.(Glu762Lys); c.1994G>A p.(Gly665Glu) | c.2284G>A p.(Glu762Lys); c.1994G>A p.(Gly665Glu) | c.1994G>A p.(Gly665Glu) |
| Electromyography | Normal | Normal | Normal | Normal |
| Heart ultrasound | Normal | Normal | Normal | Not performed |
| Spirometry | Not performed | Normal | Normal | Not performed |
| Six Minute Walking Test | Not performed | Normal | Normal | Not performed |
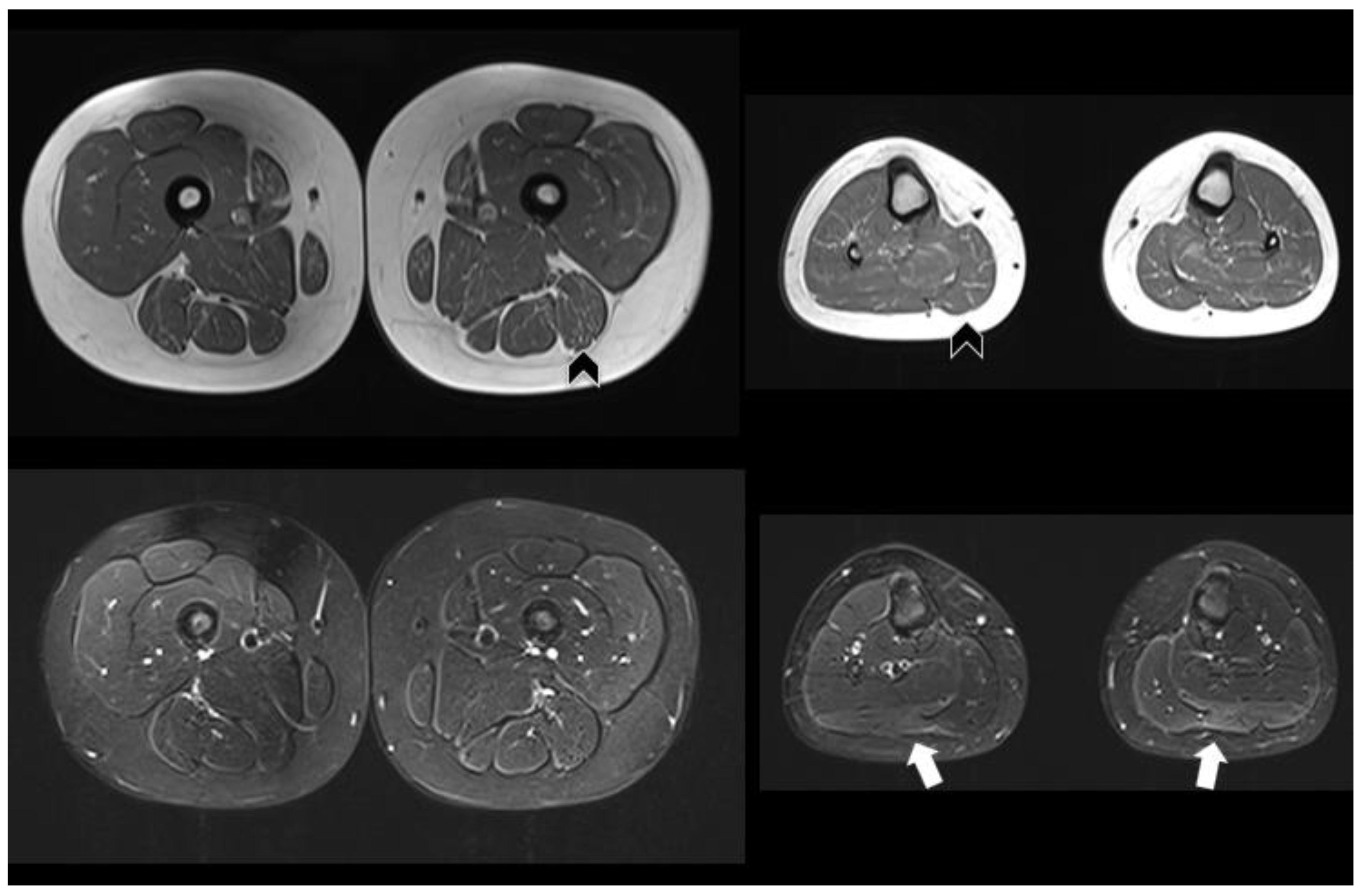
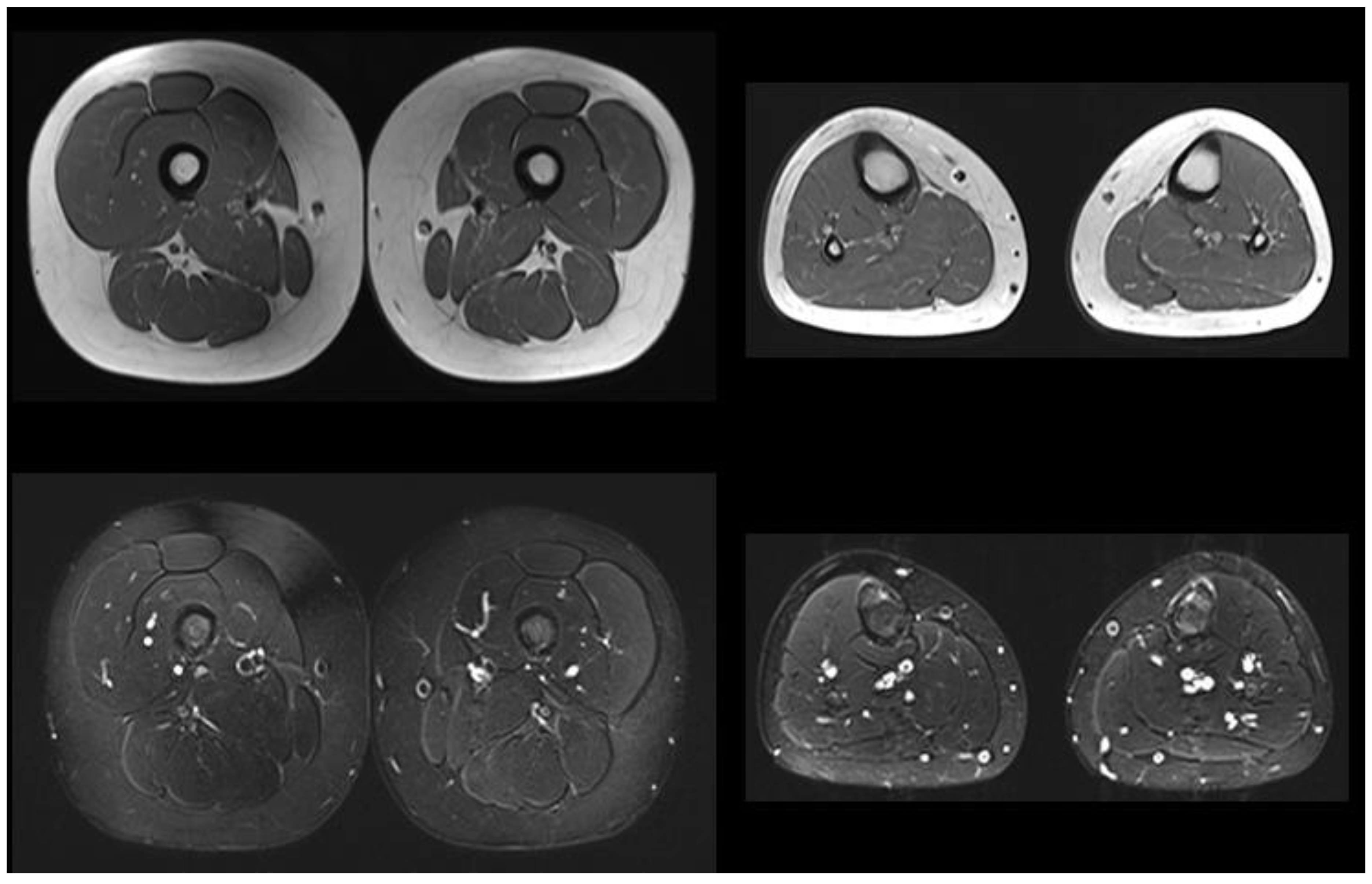
| Muscle MRI | Not performed | Pathologic (Figure 1) | Normal | Not performed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraguna, M.C.; Crescitelli, V.; Fornari, A.; Barzaghi, S.; Savasta, S.; Foiadelli, T.; Veraldi, D.; Paoletti, M.; Pichiecchio, A.; Gasperini, S. Treatment Dilemma in Children with Late-Onset Pompe Disease. Genes 2023, 14, 362. https://doi.org/10.3390/genes14020362
Faraguna MC, Crescitelli V, Fornari A, Barzaghi S, Savasta S, Foiadelli T, Veraldi D, Paoletti M, Pichiecchio A, Gasperini S. Treatment Dilemma in Children with Late-Onset Pompe Disease. Genes. 2023; 14(2):362. https://doi.org/10.3390/genes14020362
Chicago/Turabian StyleFaraguna, Martha Caterina, Viola Crescitelli, Anna Fornari, Silvia Barzaghi, Salvatore Savasta, Thomas Foiadelli, Daniele Veraldi, Matteo Paoletti, Anna Pichiecchio, and Serena Gasperini. 2023. "Treatment Dilemma in Children with Late-Onset Pompe Disease" Genes 14, no. 2: 362. https://doi.org/10.3390/genes14020362
APA StyleFaraguna, M. C., Crescitelli, V., Fornari, A., Barzaghi, S., Savasta, S., Foiadelli, T., Veraldi, D., Paoletti, M., Pichiecchio, A., & Gasperini, S. (2023). Treatment Dilemma in Children with Late-Onset Pompe Disease. Genes, 14(2), 362. https://doi.org/10.3390/genes14020362






