Variants Identified in the HOXC13 and HOXD13 Genes Suggest Association with Cervical Cancer in a Cohort of Mexican Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Molecular Analysis
2.3. Statistical Analysis
2.4. Bioinformatics Analysis
3. Results
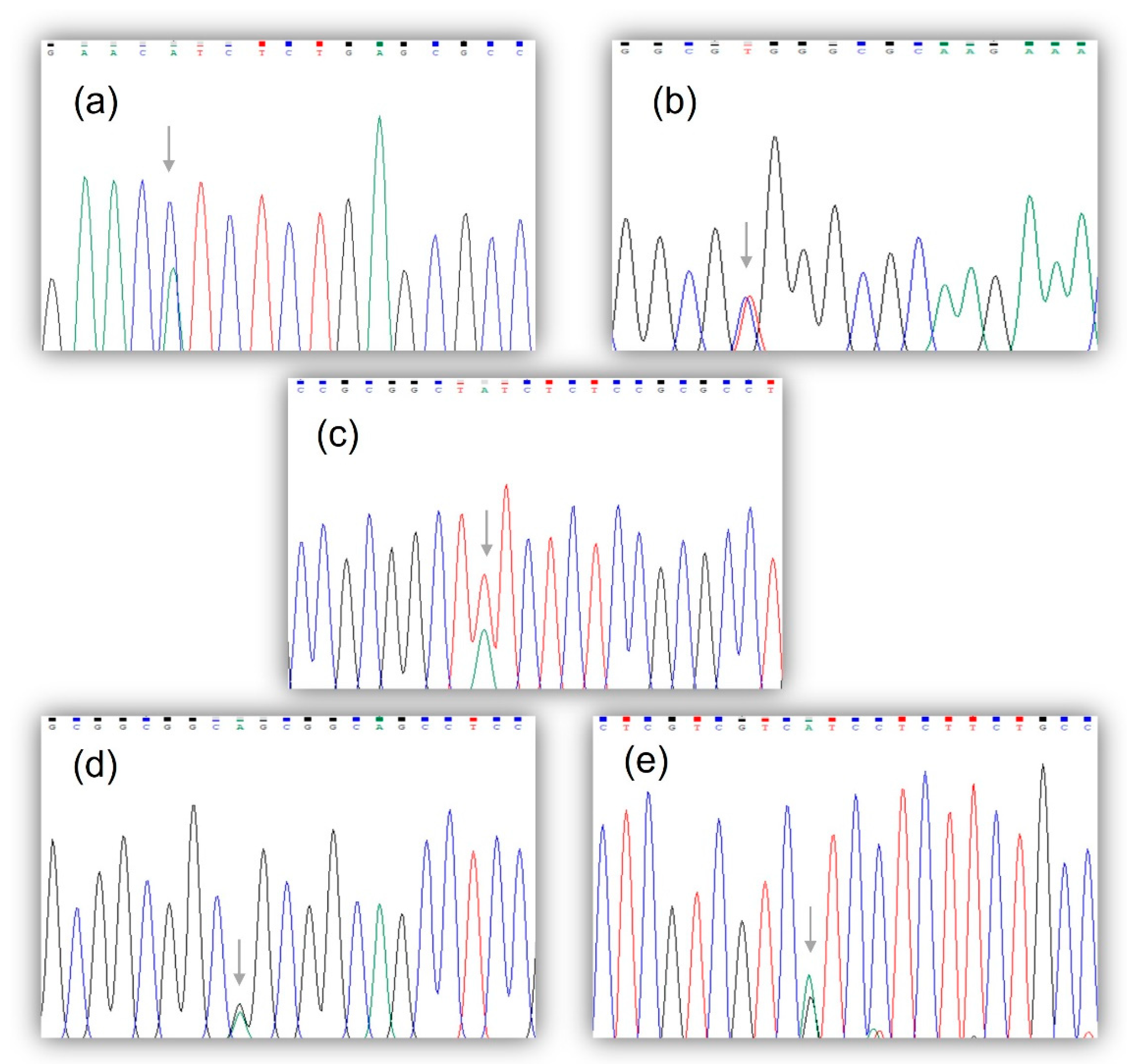
3.1. Molecular and Bioinformatics Analysis
3.2. Clinical Demographic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laengsri, V.; Kerdpin, U.; Plabplueng, C.; Treeratanapiboon, L.; Nuchnoi, P. Cervical cancer markers: Epigenetics and microRNAs. Lab. Med. 2018, 49, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Virarkar, M.; Javadi, S.; Elsherif, S.B.; de Castro Faria, S.; Bhosale, P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. AJR Am. J. Roentgenol. 2020, 214, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Mania-Pramanik, J. Molecular mechanisms in progression of HPV-associated cervical carcinogenesis. J. Biomed. Sci. 2019, 26, 8. [Google Scholar] [CrossRef]
- Buskwofie, A.; David-West, G.; Clare, C.A. A review of cervical cancer: Incidence and disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Isla-Ortiz, D.; Palomares-Castillo, E.; Mille-Loera, J.E.; Ramírez-Calderón, N.; Mohar-Betancourt, A.; Meneses-García, A.A.; Reynoso-Noverón, N. Cervical Cancer in Young Women: Do They Have a Worse Prognosis? A Retrospective Cohort Analysis in a Population of Mexico. Oncologist 2020, 25, e1363–e1371. [Google Scholar] [CrossRef]
- Burki, T.K. Novel mutations in cervical cancer. Lancet Oncol. 2017, 18, e137. [Google Scholar] [CrossRef]
- Marquina, G.; Manzano, A.; Casado, A. Targeted agents in cervical cancer: Beyond bevacizumab. Curr. Oncol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, D. The precision prevention and therapy of HPV-related cervical cancer: New concepts and clinical implications. Cancer Med. 2018, 7, 5217–5236. [Google Scholar] [CrossRef]
- Machalek, D.A.; Wark, J.D.; Tabrizi, S.N.; Hopper, J.L.; Bui, M.; Dite, G.S.; Cornall, A.M.; Pitts, M.; Gertig, D.; Erbas, B.; et al. Genetic and environmental factors in invasive cervical cancer: Design and methods of a classical twin study. Twin Res. Hum. Genet. 2017, 20, 10–18. [Google Scholar] [CrossRef]
- Mersakova, S.; Nachajova, M.; Szepe, P.; Kasajova, P.S.; Halasova, E. DNA methylation and detection of cervical cancer and precancerous lesions using molecular methods. Tumour Biol. 2016, 37, 23–27. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef]
- Tan, S.C.; Ankathil, R. Genetic susceptibility to cervical cancer: Role of common polymorphisms in apoptosis-related genes. Tumour Biol. 2015, 36, 6633–6644. [Google Scholar] [CrossRef]
- Aalijahan, H.; Ghorbian, S. Long non-coding RNAs and cervical cancer. Exp. Mol. Pathol. 2019, 106, 7–16. [Google Scholar] [CrossRef]
- Javed, S.; Langley, S.E. Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int. 2014, 113, 535–540. [Google Scholar] [CrossRef]
- Shah, N.; Sukumar, S. The HOX genes and their roles in oncogenesis. Nat. Rev. Cancer 2010, 10, 361–371. [Google Scholar] [CrossRef]
- Ishii, Y.; Taguchi, A.; Kukimoto, I. The homeobox transcription factor HOXC13 upregulates human papillomavirus E1 gene expression and contributes to viral genome maintenance. FEBS Lett. 2020, 594, 751–762. [Google Scholar] [CrossRef]
- Platais, C.; Hakami, F.; Darda, L.; Lambert, D.W.; Morgan, R.; Hunter, K.D. The role of HOX genes in head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 239–247. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018, 2018, 3569493. [Google Scholar] [CrossRef]
- Shenoy, U.S.; Adiga, D.; Kabekkodu, S.P.; Hunter, K.D.; Radhakrishnan, R. Molecular implications of HOX genes targeting multiple signaling pathways in cancer. Cell Biol. Toxicol. 2022, 38, 1–30. [Google Scholar] [CrossRef]
- Decker, B.; Ostrander, E.A. Dysregulation of the homeobox transcription factor gene HOXB13: Role in prostate cancer. Pharmgenom. Pers. Med. 2014, 7, 193–201. [Google Scholar] [CrossRef]
- Morgan, R.; Simpson, G.; Gray, S.; Gillett, C.; Tabi, Z.; Spicer, J.; Harrington, K.J.; Pandha, H.S. HOX transcription factors are potential targets and markers in malignant mesothelioma. BMC Cancer 2016, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.; Franco, R.; Sabatino, R.; Mantia, E.L.; Scognamiglio, G.; Collina, F.; Longo, F.; Ionna, F.; Losito, N.S.; Liguori, G.; et al. Deregulation of paralogous 13 HOX genes in oral squamous cell carcinoma. Am. J. Cancer Res. 2015, 15, 3042–3055. [Google Scholar] [PubMed]
- Brison, N.; Tylzanowski, P.; Debeer, P. Limb skeletal malformations—What the HOX is going on? Eur. J. Med. Genet. 2012, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Song, J.; Wang, L.; Zhou, K.; Shu, L. HOXC13 promotes cervical cancer proliferation, invasion and Warburg effect through β-catenin/c-Myc signaling pathway. J. Bioenerg. Biomembr. 2021, 53, 597–608. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Huang, J.; Yao, Y.; Sun, Q.; Wang, J.; Shen, Y.; Xu, L.; Ren, B. HOXC13 promotes proliferation of esophageal squamous cell carcinoma via repressing transcription of CASP3. Cancer Sci. 2018, 109, 317–329. [Google Scholar] [CrossRef]
- Cantile, M.; Scognamiglio, G.; Anniciello, A.; Farina, M.; Gentilcore, G.; Santonastaso, C.; Fulciniti, F.; Cillo, C.; Franco, R.; Ascierto, P.A.; et al. Increased HOXC13 expression in metastatic melanoma progression. J. Transl. Med. 2012, 10, 91. [Google Scholar] [CrossRef]
- Li, C.; Cui, J.; Zou, L.; Zhu, L.; Wei, W. Bioinformatics analysis of the expression of HOXC13 and its role in the prognosis of breast cancer. Oncol. Lett. 2020, 19, 899–907. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, J.; Sun, Q.; Xu, T.; Sun, S.; Chen, M.; Lin, X.; Qian, Q.; Zhang, Y.; Cao, L.; et al. HOXC13 promotes proliferation of lung adenocarcinoma via modulation of CCND1 and CCNE1. Am. J. Cancer Res. 2017, 7, 1820–1834. [Google Scholar] [PubMed]
- Cantile, M.; Franco, R.; Tschan, A.; Baumhoer, D.; Zlobec, I.; Schiavo, G.; Forte, I.; Bihl, M.; Liguori, G.; Botti, G.; et al. HOXD13 expression across 79 tumor tissue types. Int. J. Cancer 2009, 125, 1532–1541. [Google Scholar] [CrossRef]
- Xu, F.; Shangguan, X.; Pan, J.; Yue, Z.; Shen, K.; Ji, Y.; Zhang, W.; Zhu, Y.; Sha, J.; Wang, Y.; et al. HOXD13 suppresses prostate cancer metastasis and BMP4-induced epithelial-mesenchymal transition by inhibiting SMAD1. Int. J. Cancer 2021, 148, 3060–3070. [Google Scholar] [CrossRef]
- Miller, S.A.; Dikes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Attademo, L.; Tuninetti, V.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Tambaro, R.; Valabrega, G.; Musacchio, L.; Setola, S.V.; Piccirillo, P.; et al. Immunotherapy in cervix cancer. Cancer Treat. Rev. 2020, 90, 102088. [Google Scholar] [CrossRef]
- Liu, J.; Nie, S.; Gao, M.; Jiang, Y.; Wan, Y.; Ma, X.; Zhou, S.; Cheng, W. Identification of EPHX2 and RMI2 as two novel key genes in cervical squamous cell carcinoma by an integrated bioinformatic analysis. J. Cell. Physiol. 2019, 234, 21260–21273. [Google Scholar] [CrossRef]
- Yu, M.; Zhan, J.; Zhang, H. HOX family transcription factors: Related signaling pathways and post-translational modifications in cancer. Cell Signal. 2020, 66, 109469. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, T.; Wang, Y.; Xie, M.; Ji, X.; Luo, X.; Huang, W.; Xia, L. Homeobox Genes in Cancers: From Carcinogenesis to Recent Therapeutic Intervention. Front. Oncol. 2021, 14, 770428. [Google Scholar] [CrossRef]
- Jonkers, J.; Pai, P.; Sukumar, S. Multiple roles of HOX proteins in Metastasis: Let me count the ways. Cancer Metastasis Rev. 2020, 39, 661–679. [Google Scholar] [CrossRef]
- Brotto, D.B.; Siena, Á.D.D.; de Barros, I.I.; Carvalho, S.D.C.E.S.; Muys, B.R.; Goedert, L.; Cardoso, C.; Plaça, J.R.; Ramão, A.; Squire, J.A.; et al. Contributions of HOX genes to cancer hallmarks: Enrichment pathway analysis and review. Tumour Biol. 2020, 42, 1010428320918050. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The role of HOX genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (synonymous) SNPs: Should we care about them? Methods Mol. Biol. 2009, 578, 23–39. [Google Scholar] [CrossRef]
- Hecht, M.; Bromberg, Y.; Rost, B. Better prediction of functional effects for sequence variants. BMC Genom. 2015, 16, S1. [Google Scholar] [CrossRef]
- Tang, H.; Thomas, P.D. PANTHER-PSEP: Predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinformatics 2016, 32, 2230–2232. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, A.M.U.B.; Khan, M.A.; Deb, P.; Ansary, S.J.; Jahan, R. Identification of deleterious single nucleotide polymor-phism (SNP)s in the human TBX5 gene & prediction of their structural & functional consequences: An in silico approach. Biochem. Biophys. Rep. 2021, 28, 101179. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, D.; Newell, A.C.; Komarova, N.L. Passenger mutations can accelerate tumour suppressor gene inactivation in cancer evolution. J. R. Soc. Interface 2018, 15, 20170967. [Google Scholar] [CrossRef] [PubMed]
- McFarland, C.D.; Yaglom, J.A.; Wojtkowiak, J.W.; Scott, J.G.; Morse, D.L.; Sherman, M.Y.; Mirny, L.A. The Damaging Effect of Passenger Mutations on Cancer Progression. Cancer Res. 2017, 15, 4763–4772. [Google Scholar] [CrossRef]
- Tamborero, D.; Rubio-Perez, C.; Deu-Pons, J.; Schroeder, M.P.; Vivancos, A.; Rovira, A.; Tusquets, I.; Albanell, J.; Rodon, J.; Tabernero, J.; et al. Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. 2018, 28, 25. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Wu, X. The role of galectins in cervical cancer biology and progression. Biomed. Res. Int. 2018, 2018, 2175927. [Google Scholar] [CrossRef]
- Maia, S.; Cardoso, M.; Pinto, P.; Pinheiro, M.; Santos, C.; Peixoto, A.; Bento, M.J.; Oliveira, J.; Henrique, R.; Jerónimo, C.; et al. Identification of two novel HOXB13 germline mutations in Portuguese prostate cancer patients. PLoS ONE 2015, 10, e0132728. [Google Scholar] [CrossRef]
- Musselwhite, L.W.; Oliveira, C.M.; Kwaramba, T.; de Paula Pantano, N.; Smith, J.S.; Fregnani, J.H.; Reis, R.M.; Mauad, E.; Vazquez, F.L.; Longatto-Filho, A. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta Cytol. 2016, 60, 518–526. [Google Scholar] [CrossRef]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human papilloma virus-associated cervical cancer and health disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef]
- Ratna, A.; Mandrekar, P. Alcohol and cancer: Mechanisms and therapies. Biomolecules 2017, 7, 61. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse | Exon | Fragment Size | Conditions |
|---|---|---|---|---|---|
| HOXC13 | 5′-GTGTCTCCGC ATGCGTAGAG-3′ | 5′GGAAGGGAGA CTTCCAGAGG-3′ | 1 | 907 pb | 95 °C for 5 min, followed by 35 cycles at 95 °C for 1 min; 59, 53, and 54 °C, respectively, for 1 min; 72 °C for 52 s; and 72 °C for 7 min. |
| 5′-ACTTCTTCCC GCTTGCCTTA-3′ | 5′-GAAATCTTGC CTAAGGAGTG-3′ | 2A | 789 pb | ||
| 5′-AATTCTTGCC TCATCCTATG-3′ | 5′-AGTACATTGT CATTCAGACA-3′ | 2B | 848 pb | ||
| HOXD13 | 5′-AGAGAGGGCT AGAGGAAGAG-3′ | 5′-GGCTGGTCCT TGGTGCAGTA-3′ | 1 | 874 pb | 95 °C for 5 min, followed by 35 cycles at 95 °C for 1 min; 59, 53, and 53 °C, respectively, for 1 min; 72 °C for 52 s; and 72 °C for 7 min. |
| 5′-GCTCCGAATA TCCCAGCCTA-3′ | 5′-GAAGATAATC AGTGCTGGGA-3′ | 2A | 701 pb | ||
| 5′GAAGTGCCAT TCTGATTTAA-3′ | 5′-AAGAGTTCTG TTTATTGGCA-3′ | 2B | 872 pb |
| Gene | Variant | Amino Acid Change | Exon | Genotype | CC Patient Frequency (%) | Healthy Women Frequency (%) | p Value | Allele | Allelic Frequency (CC Patients %) | Allelic Frequency (Healthy Women %) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HOXC13 | c.895C>A | p.Leu299Ile | 2A | CC | 49 (98) | 50 (100) | 0.315 | C A | 99 (99) | 100 (0) | 0.316 |
| CA | 1 (2) | 0 (0) | 1 (1) | 0 (0) | |||||||
| AA | 0 (0) | 0 (0) | |||||||||
| Total | 50 (100) | 50 (100) | 100 (100) | 100(100) | |||||||
| c.777C>T | p.Arg259Arg | 2A | CC | 49 (98) | 50 (100) | 0.315 | C T | 99 (99) | 100 (0) | 0.316 | |
| CT | 1 (2) | 0 (0) | 1 (1) | 0 (0) | |||||||
| TT | 0 (0) | 0 (0) | |||||||||
| Total | 50 (100) | 50 (100) | 100 (100) | 100(100) | |||||||
| HOXD13 | c.128T>A | p.Phe43Tyr | 1 | TT | 39 (78) | 50 (100) | 0.000 * | T A | 89 (89) | 100 (0) | 0.001 * |
| TA | 11 (22) | 0 (0) | 11 (11) | 0 (0) | |||||||
| AA | 0 (0) | 0 (0) | |||||||||
| Total | 50 (100) | 50 (100) | 100 (100) | 100(100) | |||||||
| c.204G>A | p.Ala68Ala | 1 | GG | 48 (96) | 46 (92) | 0.360 | G A | 98 (98) | 94 (94) | 0.149 | |
| GA | 2 (4) | 2 (4) | 2 (2) | 6 (6) | |||||||
| AA | 0 (0) | 2 (4) | |||||||||
| Total | 50 (100) | 50 (100) | 100 (100) | 100(100) | |||||||
| c.267G>A | p.Ser89Ser | 1 | GG | 49 (98) | 47 (94) | 0.360 | G A | 99 (99) | 95 (95) | 0.097 | |
| GA | 1 (2) | 1 (2) | 1 (1) | 5 (5) | |||||||
| AA | 0 (0) | 2 (4) | |||||||||
| Total | 50 (100) | 50 (100) | 100 (100) | 100(100) |
| Gene | Exon | Variant | Bioinformatics Server | Score | Effect | Interpretation |
|---|---|---|---|---|---|---|
| HOXC13 | 2A | c.895 C>A (p.Leu299Ile) | SIFT | 0 | Damaging | Amino acid substitution is deleterious. Affects protein function. |
| PolyPhen-2 | 1 | Probably Damaging | Amino acid substitution is damaging. Affects protein structure and function. | |||
| HOXD13 | 1 | c.128T>A (p.Phe43Tyr) | SIFT | 0.002 | Damaging | Amino acid substitution is deleterious. Affects protein function. |
| PolyPhen-2 | 0.898 | Possibly Damaging | Amino acid substitution is damaging. Possibly affects protein structure and function. |
| Characteristic | N° of Cases | % |
|---|---|---|
| Age at diagnosis | ||
| ≤30 years | 8 | 16 |
| 31–50 years | 17 | 34 |
| 51–65 years | 15 | 30 |
| >65 years | 10 | 20 |
| Family history | ||
| Yes | 18 | 36 |
| No | 32 | 64 |
| Schooling | ||
| None | 16 | 32 |
| Elementary | 27 | 54 |
| High school | 4 | 8 |
| Bachelor degree | 3 | 6 |
| Socioeconomic status | ||
| Low | 13 | 26 |
| Lower-middle | 30 | 60 |
| High | 7 | 14 |
| HPV | ||
| Yes | 2 | 4 |
| No | 48 | 96 |
| Alcohol | ||
| Yes | 10 | 20 |
| No | 40 | 80 |
| Tobacco | ||
| Yes | 8 | 16 |
| No | 42 | 84 |
| FIGO system | ||
| I | 10 | 20 |
| II | 23 | 46 |
| III | 6 | 12 |
| IV | 11 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez-Rendón, K.J.; Castro-García, M.A.; Prada-Ortega, D.G.; Rivera, G.; Ruíz-Godoy, L.M.; Enríquez-Cárcamo, V.I.; Reyes-Lopez, M.A. Variants Identified in the HOXC13 and HOXD13 Genes Suggest Association with Cervical Cancer in a Cohort of Mexican Women. Genes 2023, 14, 358. https://doi.org/10.3390/genes14020358
Juárez-Rendón KJ, Castro-García MA, Prada-Ortega DG, Rivera G, Ruíz-Godoy LM, Enríquez-Cárcamo VI, Reyes-Lopez MA. Variants Identified in the HOXC13 and HOXD13 Genes Suggest Association with Cervical Cancer in a Cohort of Mexican Women. Genes. 2023; 14(2):358. https://doi.org/10.3390/genes14020358
Chicago/Turabian StyleJuárez-Rendón, Karina Janett, Manuel Alejandro Castro-García, Diddier Giovanni Prada-Ortega, Gildardo Rivera, Luz María Ruíz-Godoy, Virginia Isabel Enríquez-Cárcamo, and Miguel Angel Reyes-Lopez. 2023. "Variants Identified in the HOXC13 and HOXD13 Genes Suggest Association with Cervical Cancer in a Cohort of Mexican Women" Genes 14, no. 2: 358. https://doi.org/10.3390/genes14020358
APA StyleJuárez-Rendón, K. J., Castro-García, M. A., Prada-Ortega, D. G., Rivera, G., Ruíz-Godoy, L. M., Enríquez-Cárcamo, V. I., & Reyes-Lopez, M. A. (2023). Variants Identified in the HOXC13 and HOXD13 Genes Suggest Association with Cervical Cancer in a Cohort of Mexican Women. Genes, 14(2), 358. https://doi.org/10.3390/genes14020358










