scDR: Predicting Drug Response at Single-Cell Resolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets Collection and Pre-Processing
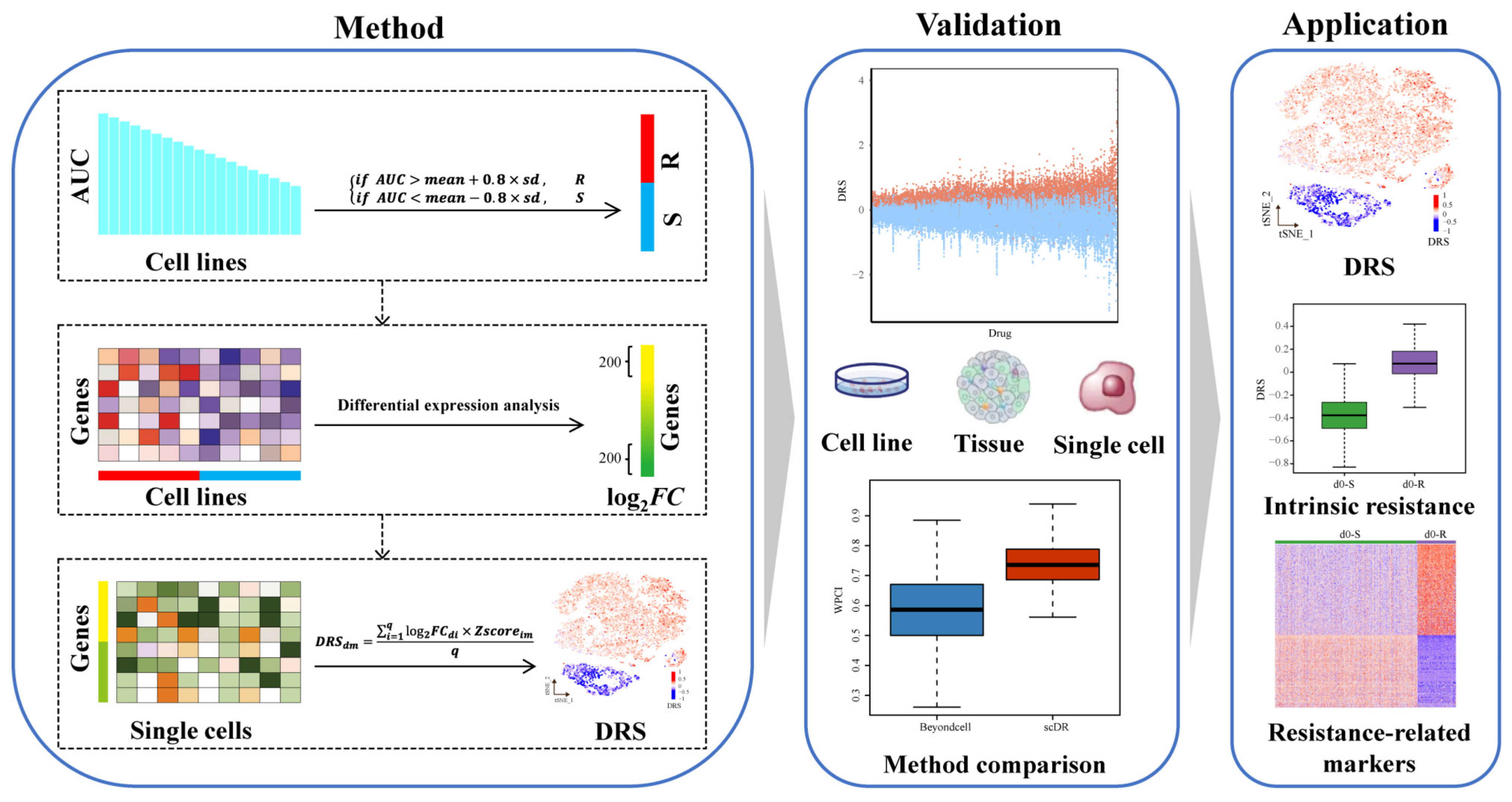
2.2. scDR Method
2.2.1. Step 1. Identifying Resistant and Sensitive Cell Lines
2.2.2. Step 2. Predicting Drug-Response Genes (DRGs)
2.2.3. Step 3. Calculating Drug-Response Scores (DRSs)
2.3. Validation of scDR Method
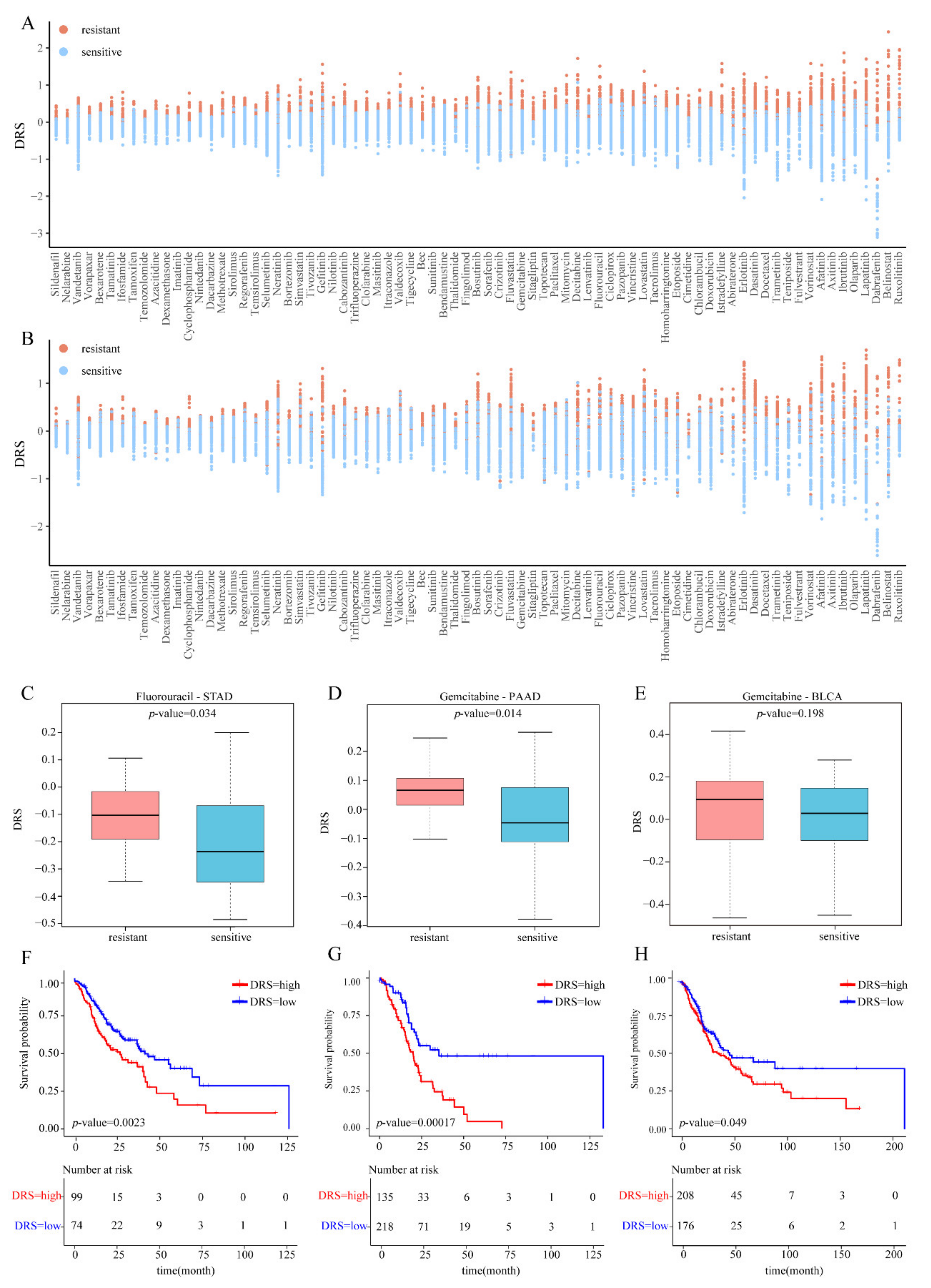
2.4. Survival Analysis
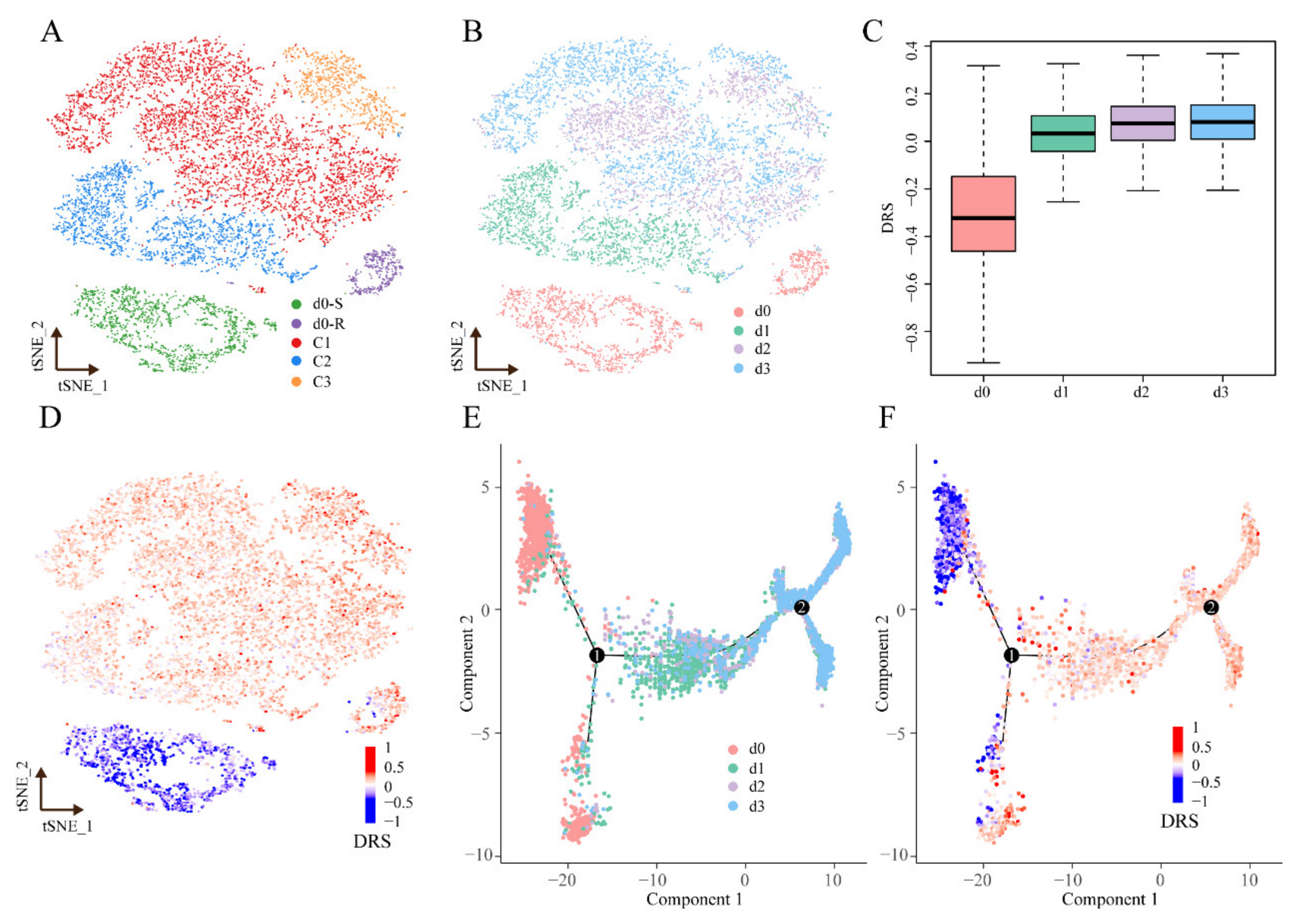
2.5. Pseudotime Analysis
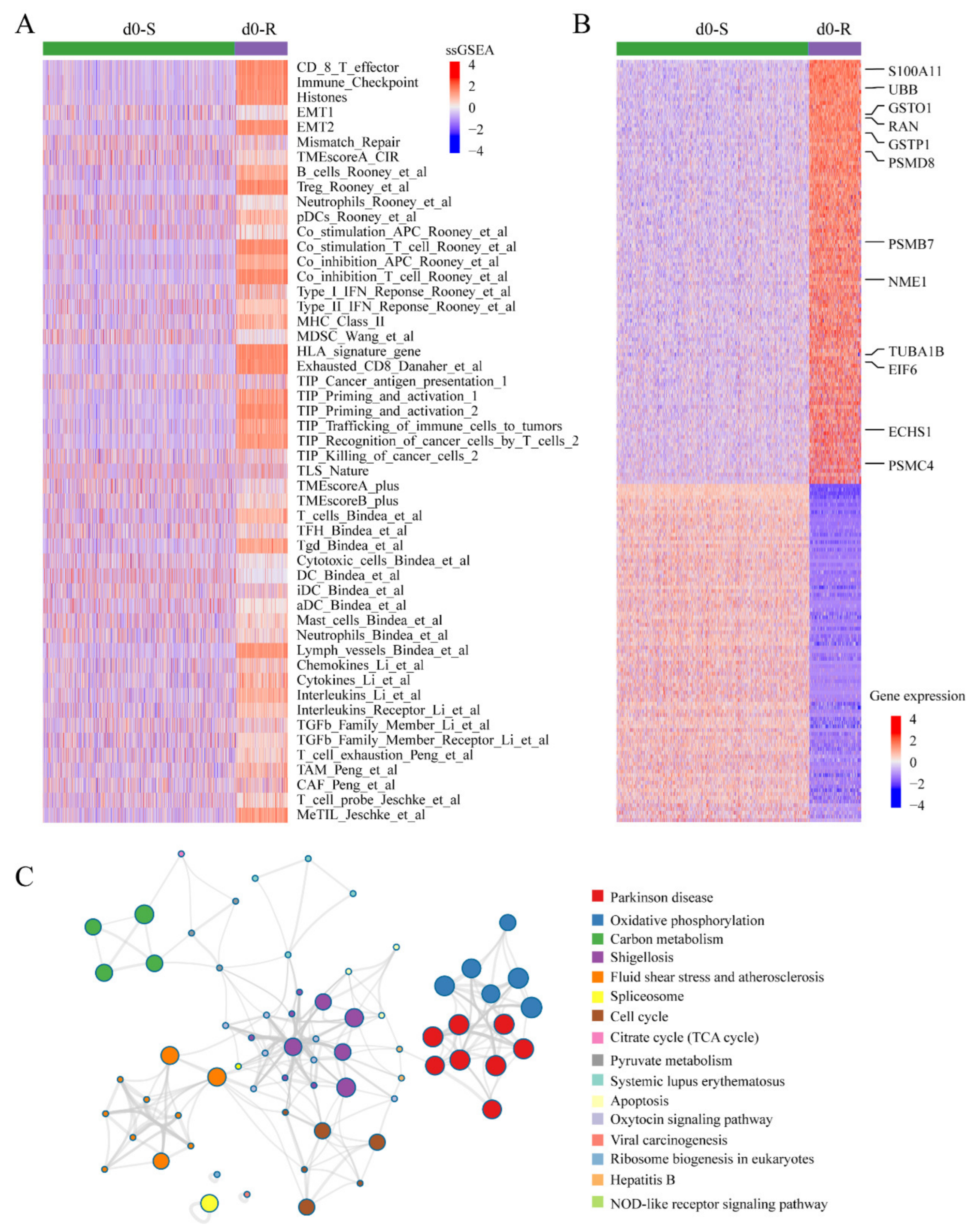
2.6. Gene Set Enrichment Analysis
2.7. Statistical Analysis
3. Results
3.1. Development of scDR
3.2. Internal Validation Based on Cell Lines
3.3. External Validation Using Bulk RNA-Seq Data of Cell Lines
3.4. External Validation Using Bulk RNA-Seq Data of TCGA Patients
3.5. Drug Response Prediction and Method Comparison in the scRNA-Seq Data
3.6. Applying scDR to Identify Intrinsic Resistant Cell Subgroups in Melanoma Based on scRNA-Seq Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baudino, T.A. Targeted cancer therapy: The next generation of cancer treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N. Drug discovery: Cell lines battle cancer. Nature 2012, 483, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehar, J.; Kryukov, G.V.; Sonkin, D.; et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Edelman, E.J.; Heidorn, S.J.; Greenman, C.D.; Dastur, A.; Lau, K.W.; Greninger, P.; Thompson, I.R.; Luo, X.; Soares, J.; et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575. [Google Scholar] [CrossRef]
- Basu, A.; Bodycombe, N.E.; Cheah, J.H.; Price, E.V.; Liu, K.; Schaefer, G.I.; Ebright, R.Y.; Stewart, M.L.; Ito, D.; Wang, S.; et al. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell 2013, 154, 1151–1161. [Google Scholar] [CrossRef]
- East, P.; Kelly, G.P.; Biswas, D.; Marani, M.; Hancock, D.C.; Creasy, T.; Sachsenmeier, K.; Swanton, C.; Downward, J.; de Carne Trecesson, S. Ras oncogenic activity predicts response to chemotherapy and outcome in lung adenocarcinoma. Nat. Commun. 2022, 13, 5632. [Google Scholar] [CrossRef] [PubMed]
- Alhalabi, O.; Chen, J.; Zhang, Y.; Lu, Y.; Wang, Q.; Ramachandran, S.; Tidwell, R.S.; Han, G.; Yan, X.; Meng, J.; et al. Mtap deficiency creates an exploitable target for antifolate therapy in 9p21-loss cancers. Nat. Commun. 2022, 13, 1797. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, J.; Fan, Y.; Qi, Y.; Wang, S.; Zhao, S.; Guo, X.; Xue, H.; Deng, L.; Zhao, R.; et al. Pdia3p1 promotes temozolomide resistance in glioblastoma by inhibiting c/ebpbeta degradation to facilitate proneural-to-mesenchymal transition. J. Exp. Clin. Cancer Res. CR 2022, 41, 223. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Jiang, L.; Song, X.; Zheng, A.; Gao, H.; Wei, M.; Zhao, L. Lncrna cbr3-as1 regulates of breast cancer drug sensitivity as a competing endogenous RNA through the jnk1/mek4-mediated mapk signal pathway. J. Exp. Clin. Cancer Res. CR 2021, 40, 41. [Google Scholar] [CrossRef]
- Ballard, D.H.; Cho, J.; Zhao, H. Comparisons of multi-marker association methods to detect association between a candidate region and disease. Genet. Epidemiol. 2010, 34, 201–212. [Google Scholar] [CrossRef]
- D’Amico, T.A.; Massey, M.; Herndon, J.E., 2nd; Moore, M.B.; Harpole, D.H., Jr. A biologic risk model for stage i lung cancer: Immunohistochemical analysis of 408 patients with the use of ten molecular markers. J. Thorac. Cardiovasc. Surg. 1999, 117, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. Mrna-seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Kinker, G.S.; Greenwald, A.C.; Tal, R.; Orlova, Z.; Cuoco, M.S.; McFarland, J.M.; Warren, A.; Rodman, C.; Roth, J.A.; Bender, S.A.; et al. Pan-cancer single-cell RNA-seq identifies recurring programs of cellular heterogeneity. Nat. Genet. 2020, 52, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Kang, H.; Xu, T.; Hao, L.; Bao, Y.; Jia, P. Cedr atlas: A knowledgebase of cellular drug response. Nucleic Acids Res. 2022, 50, D1164–D1171. [Google Scholar] [CrossRef] [PubMed]
- Fustero-Torre, C.; Jimenez-Santos, M.J.; Garcia-Martin, S.; Carretero-Puche, C.; Garcia-Jimeno, L.; Ivanchuk, V.; Di Domenico, T.; Gomez-Lopez, G.; Al-Shahrour, F. Beyondcell: Targeting cancer therapeutic heterogeneity in single-cell RNA-seq data. Genome Med. 2021, 13, 187. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., 3rd; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive integration of single-cell data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Zhou, B.; Jin, W. Visualization of single cell RNA-seq data using t-sne in r. Methods Mol. Biol. 2020, 2117, 159–167. [Google Scholar]
- Costello, J.C.; Heiser, L.M.; Georgii, E.; Gonen, M.; Menden, M.P.; Wang, N.J.; Bansal, M.; Ammad-ud-din, M.; Hintsanen, P.; Khan, S.A.; et al. A community effort to assess and improve drug sensitivity prediction algorithms. Nat. Biotechnol. 2014, 32, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hill, A.; Packer, J.; Lin, D.; Ma, Y.A.; Trapnell, C. Single-cell mrna quantification and differential analysis with census. Nat. Methods 2017, 14, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Ye, Z.; Shen, R.; Yu, G.; Wu, J.; Xiong, Y.; Zhou, R.; Qiu, W.; Huang, N.; Sun, L.; et al. Iobr: Multi-omics immuno-oncology biological research to decode tumor microenvironment and signatures. Front. Immunol. 2021, 12, 687975. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, S.; Castelo, R.; Guinney, J. Gsva: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. Kegg: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. Gene ontology consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Suwala, A.K.; Stichel, D.; Schrimpf, D.; Kloor, M.; Wefers, A.K.; Reinhardt, A.; Maas, S.L.N.; Kratz, C.P.; Schweizer, L.; Hasselblatt, M. Primary mismatch repair deficient idh-mutant astrocytoma (pmmrdia) is a distinct type with a poor prognosis. Acta Neuropathol. 2021, 141, 85–100. [Google Scholar] [CrossRef]
- Lv, P.; Man, S.; Xie, L.; Ma, L.; Gao, W. Pathogenesis and therapeutic strategy in platinum resistance lung cancer. Biochim. Biophys. Acta. Rev. Cancer 2021, 1876, 188577. [Google Scholar] [CrossRef]
- Barrueto, L.; Caminero, F.; Cash, L.; Makris, C.; Lamichhane, P.; Deshmukh, R.R. Resistance to checkpoint inhibition in cancer immunotherapy. Transl. Oncol. 2020, 13, 100738. [Google Scholar] [CrossRef]
- Carazo-Salas, R.E.; Guarguaglini, G.; Gruss, O.J.; Segref, A.; Karsenti, E.; Mattaj, I.W. Generation of gtp-bound ran by rcc1 is required for chromatin-induced mitotic spindle formation. Nature 1999, 400, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liu, H.; Liu, X.; Ruan, K.; Zhang, Y.; Zhang, Z.; Hu, Q.; Liu, Y.; Akram, S.; Zhang, J.; et al. Mitosis-specific acetylation tunes ran effector binding for chromosome segregation. J. Mol. Cell Biol. 2018, 10, 18–32. [Google Scholar] [CrossRef] [PubMed]
- LaFrance, B.J.; Roostalu, J.; Henkin, G.; Greber, B.J.; Zhang, R.; Normanno, D.; McCollum, C.O.; Surrey, T.; Nogales, E. Structural transitions in the gtp cap visualized by cryo-electron microscopy of catalytically inactive microtubules. Proc. Natl. Acad. Sci. USA 2022, 119, e2114994119. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zeng, L.; Zhao, Z.; Chen, T. Functionalized multiwalled carbon nanotubes as carriers of ruthenium complexes to antagonize cancer multidrug resistance and radioresistance. ACS Appl. Mater. Interfaces 2015, 7, 14933–14945. [Google Scholar] [CrossRef]
- Yu, Y.; Song, Y.; Cheng, L.; Chen, L.; Liu, B.; Lu, D.; Li, X.; Li, Y.; Lv, F.; Xing, Y. Circcemip promotes anoikis-resistance by enhancing protective autophagy in prostate cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Ma, A.; Wang, Q.E.; Liu, B.; Li, L.; Xu, D.; Ma, Q. Deep transfer learning of cancer drug responses by integrating bulk and single-cell RNA-seq data. Nat. Commun. 2022, 13, 6494. [Google Scholar] [CrossRef]
- Yu, F.; Cato, L.D.; Weng, C.; Liggett, L.A.; Jeon, S.; Xu, K.; Chiang, C.W.K.; Wiemels, J.L.; Weissman, J.S.; de Smith, A.J.; et al. Variant to function mapping at single-cell resolution through network propagation. Nat. Biotechnol. 2022, 40, 1644–1653. [Google Scholar] [CrossRef]
- Dohmen, J.; Baranovskii, A.; Ronen, J.; Uyar, B.; Franke, V.; Akalin, A. Identifying tumor cells at the single-cell level using machine learning. Genome Biol. 2022, 23, 123. [Google Scholar] [CrossRef]
- Sun, D.; Guan, X.; Moran, A.E.; Wu, L.Y.; Qian, D.Z.; Schedin, P.; Dai, M.S.; Danilov, A.V.; Alumkal, J.J.; Adey, A.C.; et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat. Biotechnol. 2022, 40, 527–538. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]

| Data Set | Data Source | Cancer | Data Type | Samples |
|---|---|---|---|---|
| Discovery Cohort | CTRP | Pan-Cancer | Drug response | 664 |
| CCLE | Pan-Cancer | RNA-seq | 1019 | |
| Validation Cohort I | GDSC | Pan-Cancer | RNA-seq | 424 |
| Validation Cohort II | TCGA | STAD | RNA-seq | 353 |
| PAAD | RNA-seq | 173 | ||
| BLCA | RNA-seq | 385 | ||
| Validation Cohort III | Kinker et al. | Pan-Cancer | scRNA-seq | 53,502 |
| Application Cohort | GSE162045 | Melanoma | scRNA-seq | 10,050 |
| Cancer | Drug | PD | CR |
|---|---|---|---|
| STAD | Fluorouracil | 16 | 17 |
| PAAD | Gemcitabine | 28 | 19 |
| BLCA | Gemcitabine | 19 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Yuan, M.; Long, M.; Zhang, T.; Huang, Y.-e.; Liu, H.; Jiang, W. scDR: Predicting Drug Response at Single-Cell Resolution. Genes 2023, 14, 268. https://doi.org/10.3390/genes14020268
Lei W, Yuan M, Long M, Zhang T, Huang Y-e, Liu H, Jiang W. scDR: Predicting Drug Response at Single-Cell Resolution. Genes. 2023; 14(2):268. https://doi.org/10.3390/genes14020268
Chicago/Turabian StyleLei, Wanyue, Mengqin Yuan, Min Long, Tao Zhang, Yu-e Huang, Haizhou Liu, and Wei Jiang. 2023. "scDR: Predicting Drug Response at Single-Cell Resolution" Genes 14, no. 2: 268. https://doi.org/10.3390/genes14020268
APA StyleLei, W., Yuan, M., Long, M., Zhang, T., Huang, Y.-e., Liu, H., & Jiang, W. (2023). scDR: Predicting Drug Response at Single-Cell Resolution. Genes, 14(2), 268. https://doi.org/10.3390/genes14020268






