MiR-217 Regulates SIRT1 Expression and Promotes Inflammatory and Apoptotic Responses in Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Articular Chondrocytes’ Cultures
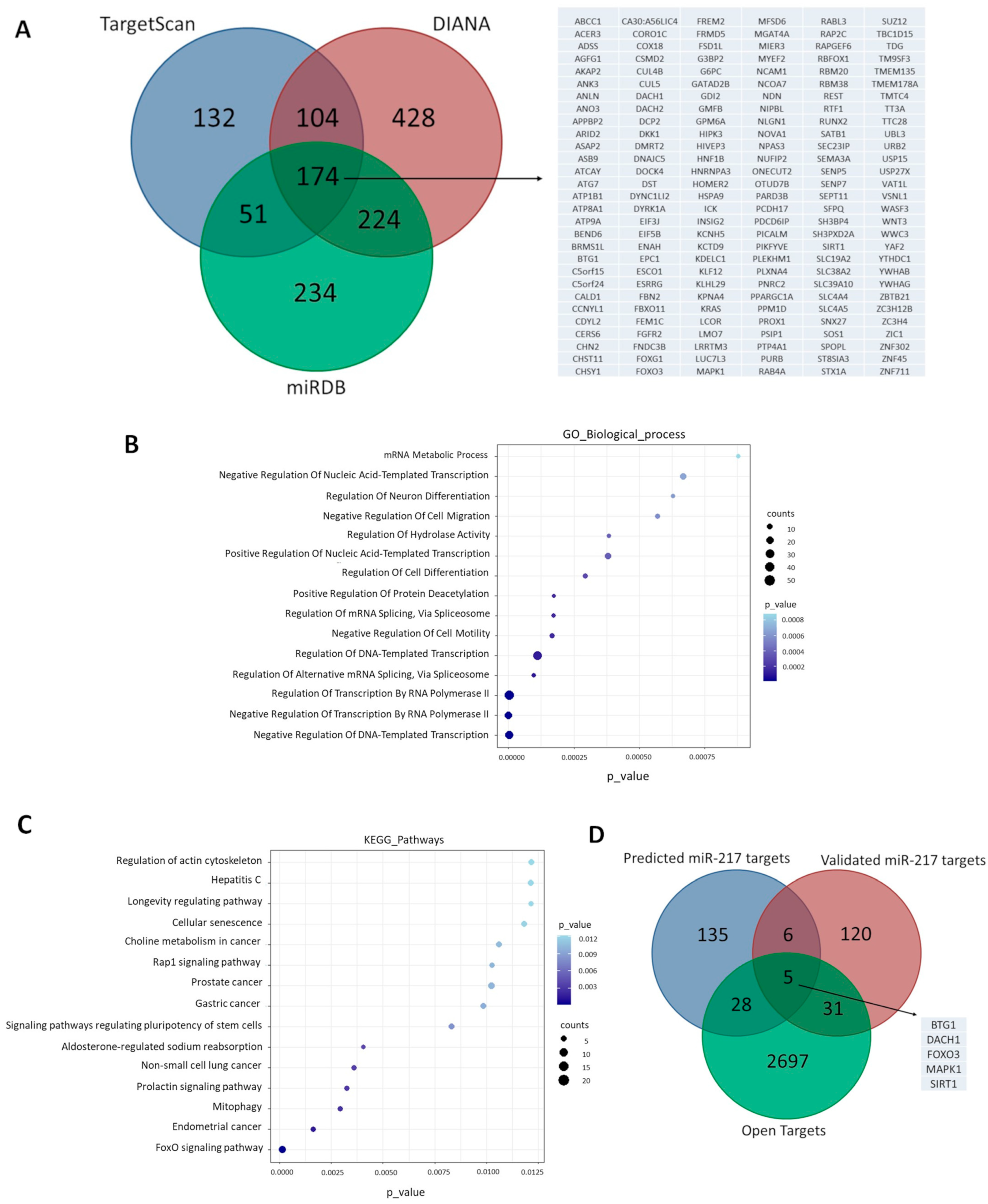
2.3. Prediction of miR-217-5p Target Genes and Functional Enrichment Analysis
2.4. Transfection of OA Chondrocytes with miR-217 Inhibitor and/or siRNA against SIRT1, or miR-217 Mimic
2.5. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot
2.7. Enzyme Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
3.1. Cellular miR-217-5p Target Genes Are Involved in Aging and OA-Related Pathways
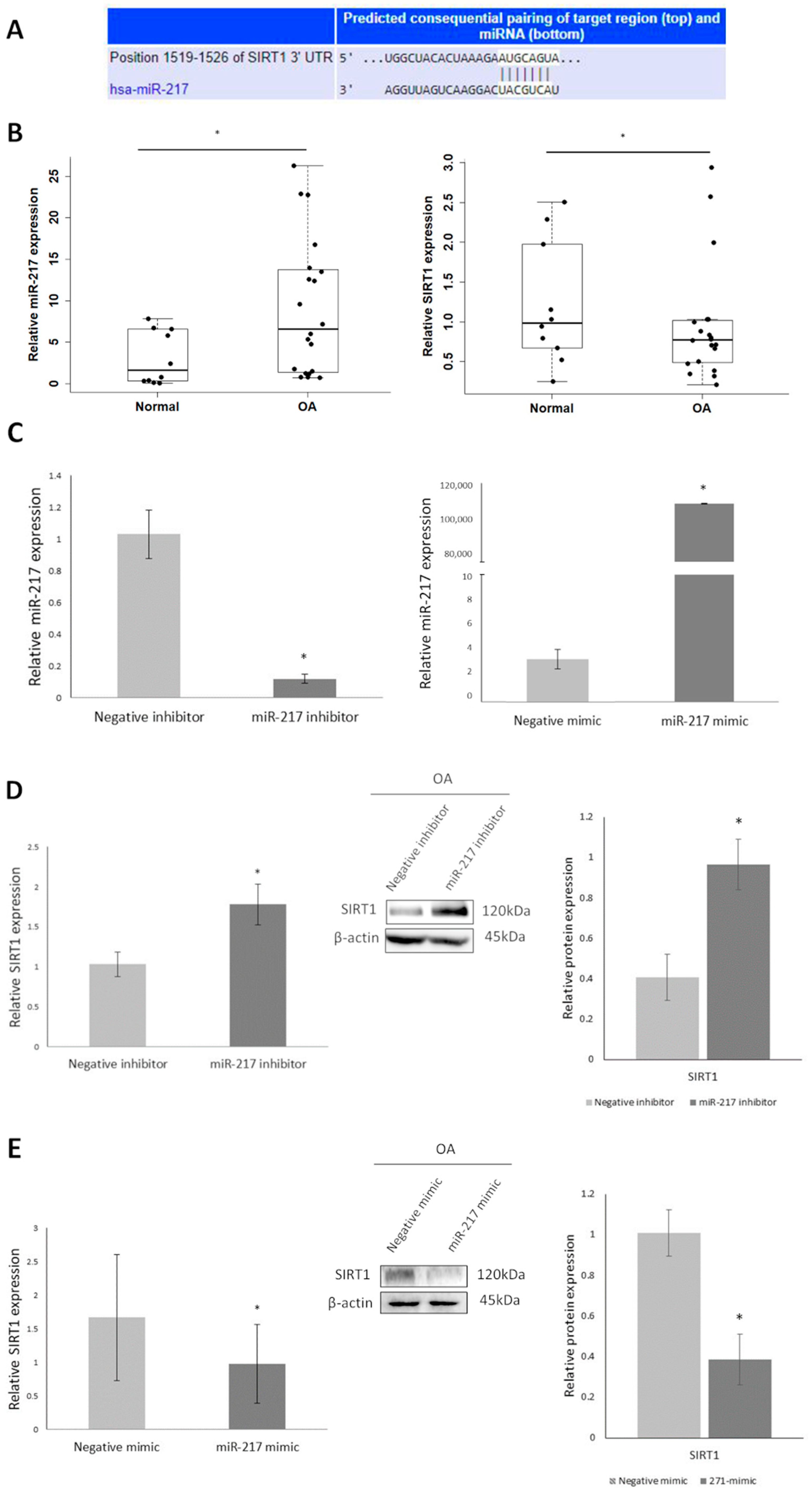
3.2. Differential Expression of miR-217 Affects SIRT1 Expression in OA Chondrocytes
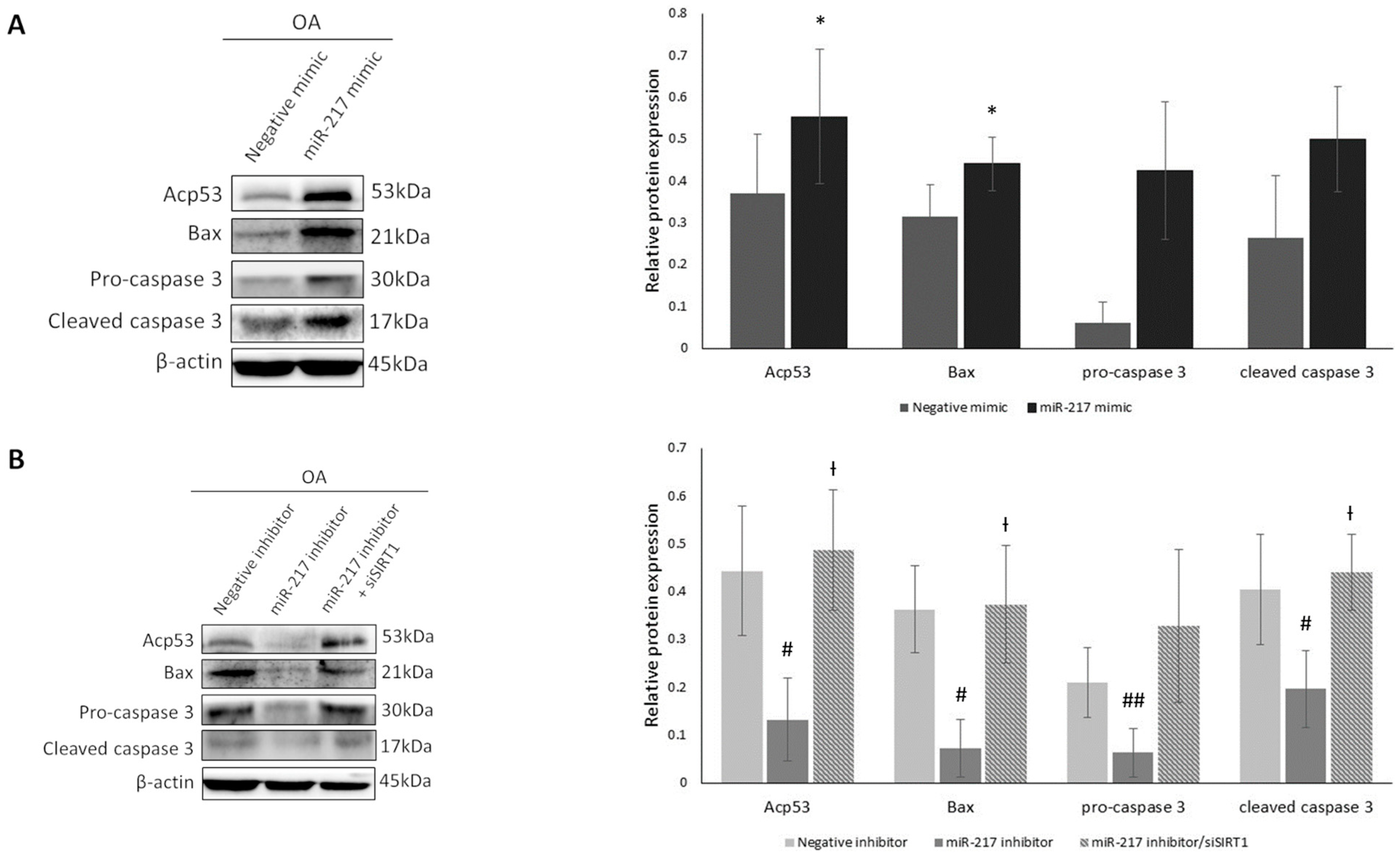
3.3. MiR-217 Overexpression Affects SIRT1 Deacetylase Activity Promoting the Expression of Inflammatory Markers in OA Chondrocytes
3.4. MiR-217 Overexpression Affects SIRT1 Deacetylase Activity Enhancing the Expression of Pro-Apoptotic Markers in OA Chondrocytes
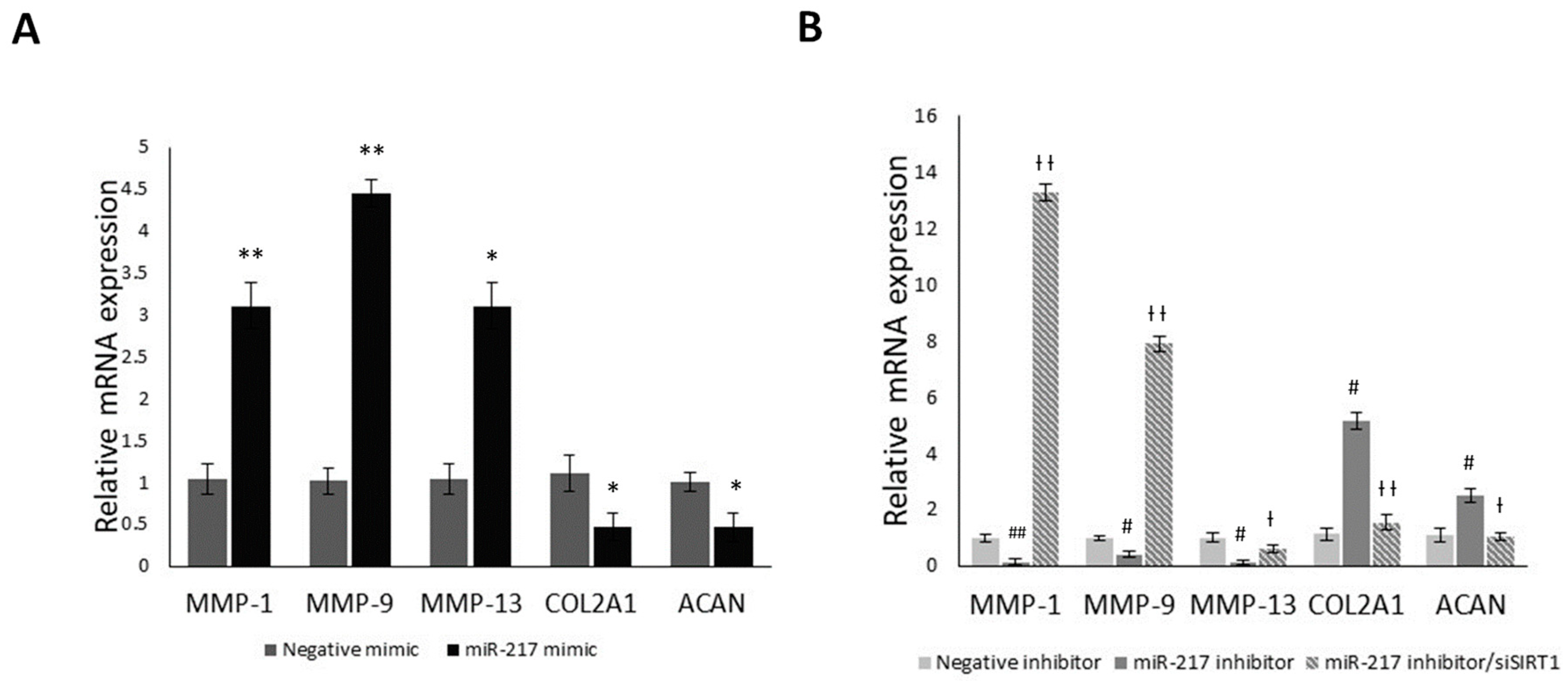
3.5. MiR-217 Overexpression Affects the Expression of Matrix Regulating Genes through SIRT1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr Cart. 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and Osteoarthritis. Subcell. Biochem. 2019, 91, 123–159. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Loeser, R.F. Why is Osteoarthritis an Age-Related Disease? Best. Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Lee, Y.J.; Seong, S.C.; Choe, K.W.; Song, Y.W. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 2000, 27, 455–462. [Google Scholar]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Hata, A. Functions of MicroRNAs in Cardiovascular Biology and Disease. Annu. Rev. Physiol. 2013, 75, 69. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Palihaderu, P.; Mendis, B.; Premarathne, J.; Dias, W.; Yeap, S.K.; Ho, W.Y.; Dissanayake, A.S.; Rajapakse, I.H.; Karunanayake, P.; Senarath, U.; et al. Therapeutic Potential of miRNAs for Type 2 Diabetes Mellitus: An Overview. Epigenetics Insights 2022, 15, 25168657221130041. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Marchese, G.; Lanza, G.; Cosentino, F.; Salluzzo, M.G.; Schillaci, F.A.; Ventola, G.M.; Cordella, A.; Ravo, M.; Ferri, R. Role and Dysregulation of miRNA in Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 712. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The function of microRNAs in cartilage and osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 40–47. [Google Scholar] [PubMed]
- Nugent, M. MicroRNAs: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, D.; Zhang, N.; Yuan, T.; Tao, H. MiR-217 promotes endothelial cell senescence through the SIRT1/p53 signaling pathway. J. Mol. Histol. 2021, 52, 257–267. [Google Scholar] [CrossRef] [PubMed]
- De Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; Gonzalez-Amor, M.; Mendez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2408. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; He, Q.; Chao, Z.; Li, X.; Chen, M. MicroRNA-217 is involved in the progression of atherosclerosis through regulating inflammatory responses by targeting sirtuin 1. Mol. Med. Rep. 2019, 20, 3182. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Xue, H.; Zhou, Q.; Peng, L. MiR-217 Is a useful diagnostic biomarker and regulates human podocyte cells apoptosis via targeting tnfsf11 in membranous nephropathy. Biomed. Res. Int. 2017, 2017, 2168767. [Google Scholar] [CrossRef]
- Wang, L.P.; Wang, J.P.; Wang, X.P. HOTAIR contributes to the growth of liver cancer via targeting miR-217. Oncol. Lett. 2018, 15, 7963. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Chen, J.; Xiong, L.; Chen, G.; Li, P. Downregulated miR-217 expression predicts a poor outcome in acute myeloid leukemia. Cancer Biomark. 2018, 22, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, H.; Yao, Y.; Tang, X.; Wu, B. MicroRNA-217/138-5p downregulation inhibits inflammatory response, oxidative stress and the induction of neuronal apoptosis in MPP+-induced SH-SY5Y cells. Am. J. Transl. Res. 2019, 11, 6619. [Google Scholar] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Hung, C.H.; Shih, J.Y.; Chu, P.M.; Cheng, Y.H.; Lin, H.C.; Tsai, K.L. SIRT1 inhibition causes oxidative stress and inflammation in patients with coronary artery disease. Redox Biol. 2017, 13, 301–309. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Costa, C.; Hammes, T.O.; Rohden, F.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. SIRT1 Transcription Is Decreased in Visceral Adipose Tissue of Morbidly Obese Patients with Severe Hepatic Steatosis. Obes. Surg. 2010, 20, 633–639. [Google Scholar] [CrossRef]
- Elibol, B.; Kilic, U. High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public. Heal. 2017, 5, 335. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, C.; Pang, Q.; Liu, H.C. MicroRNA-217: A regulator of human cancer. Biomed. Pharmacother. 2021, 133, 110943. [Google Scholar] [CrossRef]
- Kramer, M.F. Stem-Loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 2011, 95, 15.10.1–15.10.15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wątroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Matsushita, T.; Takayama, K.; Matsumoto, T.; Nishida, K.; Kuroda, R.; Kurosaka, M. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann. Rheum. Dis. 2014, 73, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.A.; Litsaki, M.; Mourmoura, E.; Papathanasiou, I.; Tsezou, A. DNA methylation regulates Sirtuin 1 expression in osteoarthritic chondrocytes. Adv. Med. Sci. 2023, 68, 101–110. [Google Scholar] [CrossRef]
- Moon, M.H.; Jeong, J.K.; Lee, Y.J.; Seol, J.W.; Jackson, C.J.; Park, S.Y. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Johansen, T.; Berger, S.L.; Dou, Z. SIRT1—A new mammalian substrate of nuclear autophagy. Autophagy 2021, 17, 593–595. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef]
- Elayyan, J.; Lee, E.J.; Gabay, O.; Smith, C.A.; Qiq, O.; Reich, E.; Mobasheri, A.; Henrotin, Y.; Kimber, S.J.; Dvir-Ginzberg, M. LEF1-mediated MMP13 gene expression is repressed by SIRT1 in human chondrocytes. FASEB J. 2017, 31, 3116–3125. [Google Scholar] [CrossRef]
- Fujita, N.; Matsushita, T.; Ishida, K.; Kubo, S.; Matsumoto, T.; Takayama, K.; Kurosaka, M.; Kuroda, R. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J. Orthop. Res. 2011, 29, 511–515. [Google Scholar] [CrossRef]
- Papageorgiou, A.A.; Goutas, A.; Trachana, V.; Tsezou, A. Dual Role of SIRT1 in Autophagy and Lipid Metabolism Regulation in Osteoarthritic Chondrocytes. Medicina 2021, 57, 1203. [Google Scholar] [CrossRef]
- Liu, X.; Gao, F.; Wang, W.; Yan, J. Expression of miR-204 in patients with osteoarthritis and its damage to chondrocytes. J. Musculoskelet. Neuronal Interact. 2020, 20, 265. [Google Scholar] [PubMed]
- Hamilton, D.W.; Riehle, M.O.; Monaghan, W.; Curtis, A.S.G. Articular chondrocyte passage number: Influence on adhesion, migration, cytoskeletal organisation and phenotype in response to nano- and micro-metric topography. Cell Biol. Int. 2005, 29, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R.; et al. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Pulai, J.I.; Chen, H.; Im, H.J.; Kumar, S.; Hanning, C.; Hegde, P.S.; Loeser, R.F. NF-κB Mediates the Stimulation of Cytokine and Chemokine Expression by Human Articular Chondrocytes in Response to Fibronectin Fragments. J. Immunol. 2005, 174, 5781. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, W.; Cui, Z.M.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-κB signaling pathway. J. Orthop. Surg. Res. 2020, 15, 424. [Google Scholar] [CrossRef]
- Takayama, K.; Ishida, K.; Matsushita, T.; Fujita, N.; Hayashi, S.; Sasaki, K.; Tei, K.; Kubo, S.; Matsumoto, T.; Fujioka, H.; et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum 2009, 60, 2731–2740. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, Z.; Kang, N.; Yang, J.C.; Wei, X.; Hai, Y. Circ-VANGL1 promotes the progression of osteoporosis by absorbing miRNA-217 to regulate RUNX2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 923–931. [Google Scholar] [CrossRef]
- Liu, J.; Xue, N.; Guo, Y.; Niu, K.; Gao, L.; Zhang, S.; Gu, H.; Wang, X.; Zhao, D.; Fan, R. CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. Aging 2019, 11, 12412–12427. [Google Scholar] [CrossRef] [PubMed]

| (a) Stem-loop primer sequences | ||
| U6 stem-loop | 5′-CACGGAAGCCCTCACACCGTGTCGTTC-3′ | |
| miR-217-5p stem-loop | 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCCAAT-3′ | |
| (b) qRT-PCR primer sequences | ||
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
| U6 | GCTTCGGCAGCACATATACTAAAAT | CTCACACCGTGTCGTTCCA |
| miR-217-5p | CGCTCTACTGCATCAGGAACTGA | GTGCAGGGTCCGAGGT |
| GAPDH | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
| SIRT1 | CTGCCTGGATCCCCTTAGTT | TATAATCAGGGGCCTGTTGC |
| IL-1β | ATGGACAAGCTGAGGAAGATG | CCTCGTTATCCCATGTGTCG |
| IL-6 | CAACCTGAACCTTCCAAAGATG | ACCTCAAACTCCAAAAGACCAG |
| TNFα | CCTGAAAACAACCCTCAGA | AAGAGGCTGAGGAACAAGCA |
| MMP-1 | ACGTTCCCAAAATCCTGTCC | GGGTAGAAGGGATTTGTGCG |
| MMP-9 | TTGACAGCGACAAGAAGTGG | GCCATTCACGTCGTCCTTAT |
| MMP-13 | TGGCATTGCTGACATCATGA | GCCAGAGGGCCCATCAA |
| COL2A1 | ATGACAATCTGGCTCCCAACACTGC | GACCGGCCCTATGTCCACACCGAAT |
| ACAN | TGAGGAGGGCTGGAACAAGTACC | GGAGGTGGTAATTGCAGGGAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, A.-A.; Roussos, A.; Papathanasiou, I.; Balis, C.; Karachalios, T.; Varitimidis, S.E.; Malizos, K.N.; Tsezou, A. MiR-217 Regulates SIRT1 Expression and Promotes Inflammatory and Apoptotic Responses in Osteoarthritis. Genes 2023, 14, 2155. https://doi.org/10.3390/genes14122155
Papageorgiou A-A, Roussos A, Papathanasiou I, Balis C, Karachalios T, Varitimidis SE, Malizos KN, Tsezou A. MiR-217 Regulates SIRT1 Expression and Promotes Inflammatory and Apoptotic Responses in Osteoarthritis. Genes. 2023; 14(12):2155. https://doi.org/10.3390/genes14122155
Chicago/Turabian StylePapageorgiou, Aliki-Alexandra, Athanasios Roussos, Ioanna Papathanasiou, Charalampos Balis, Theophilos Karachalios, Sokratis E. Varitimidis, Konstantinos N. Malizos, and Aspasia Tsezou. 2023. "MiR-217 Regulates SIRT1 Expression and Promotes Inflammatory and Apoptotic Responses in Osteoarthritis" Genes 14, no. 12: 2155. https://doi.org/10.3390/genes14122155
APA StylePapageorgiou, A.-A., Roussos, A., Papathanasiou, I., Balis, C., Karachalios, T., Varitimidis, S. E., Malizos, K. N., & Tsezou, A. (2023). MiR-217 Regulates SIRT1 Expression and Promotes Inflammatory and Apoptotic Responses in Osteoarthritis. Genes, 14(12), 2155. https://doi.org/10.3390/genes14122155







