Abstract
Reproductive traits hold considerable economic importance in pig breeding and production. However, candidate genes underpinning the reproductive traits are still poorly identified. In the present study, we executed a genome-wide association study (GWAS) and runs of homozygosity (ROH) analysis using the PorcineSNP50 BeadChip array for 585 Yorkshire pigs. Results from the GWAS identified two genome-wide significant and eighteen suggestive significant single nucleotide polymorphisms (SNPs) associated with seven reproductive traits. Furthermore, we identified candidate genes, including ELMO1, AOAH, INSIG2, NUP205, LYPLAL1, RPL34, LIPH, RNF7, GRK7, ETV5, FYN, and SLC30A5, which were chosen due to adjoining significant SNPs and their functions in immunity, fertilization, embryonic development, and sperm quality. Several genes were found in ROH islands associated with spermatozoa, development of the fetus, mature eggs, and litter size, including INSL6, TAF4B, E2F7, RTL1, CDKN1C, and GDF9. This study will provide insight into the genetic basis for pig reproductive traits, facilitating reproduction improvement using the marker-based selection methods.
1. Introduction
Enhancing the fertility of pigs has been a perennially intriguing topic in the pig industry [1,2]. Molecular breeding, which involves the identification of SNPs and candidate genes associated with reproduction, has proven to be an efficacious approach to augment reproductive capacity [3]. However, the heritability of litter size is generally low [4]. Litter size directly reflects the productivity of pigs per sow per year [5]. Many researchers have dedicated their efforts to identifying SNPs and candidate genes that are associated with litter size [6,7,8], but there is still a long way to go to improve pig reproduction.
A genome-wide association study (GWAS) is a new method being used to accelerate the speed of progress in pig breeding [9]. The GWAS has been used to find new single nucleotide polymorphisms (SNPs) and to detect candidate genes for important economic traits in animals [10]. Improving reproductive traits including the number of teats [11], litter size [12], TNB, NBA [13], etc., is a great and helpful way to increase pig performance and economic benefits. Of course, decreasing the number of dead pigs [14], fetuses [15], and so on also cuts down the pecuniary losses. In this research, we collected more detailed pig litter records to perform a GWAS in a Yorkshire sow population and discover new SNPs and candidate genes from these traits.
Runs of homozygosity (ROH) are long, consecutive homozygous fragments in the genome [16] and are used to calculate the extent of identical haplotypes which are inherited from the parents [17], especially in inbred pigs. ROH are a crucial genome feature that provide a valuable reference for genome structure analysis. In animal genetics, the occurrence of homozygous genome segments is influenced by intensive selection, population history, and level of consanguinity [18]. Therefore, it is recognized that ROH hotspots exhibit a non-random distribution throughout the genome, and ROH islands are shown to be distributed and shared in individuals, which is likely a result of selection events [19,20]. In recent years, ROH analysis has been widely utilized to assess genomic inbreeding and detect selection signatures in various livestock populations [21], so ROH can be used as a way to detect signal selection in populations.
The present investigation endeavors to employ a GWAS to pinpoint noteworthy SNPs or genes linked to reproductive traits in Yorkshire pigs, a breed renowned for its superior reproductive performance, and to identify the genomic regions that have undergone selection for reproductivity via ROH analysis. Identifying reproduction-related genes in pigs can give perspective on the underlying molecular mechanisms of their reproductive capacity. The identification of genes associated with pig reproduction can provide insights into the underlying molecular mechanisms, and these results would help us to improve the understanding of reproductive capacity and performance in the breeding of pigs.
2. Materials and Methods
2.1. Sample Collection
A total of 585 female American Yorkshire pigs were obtained from the Beijing Swine Center in Beijing, China. These pigs were provided with a uniform diet and subjected to a standardized raising and management protocol, with ad libitum feeding. Throughout the entire duration of the experiment, none of the pigs experienced any physical or psychological distress, nor did they exhibit any signs of illness. The collection of samples was conducted following a specific procedure, which involved cleaning the area with an alcohol-soaked cotton ball (with a concentration of 75%) and subsequently cutting a small piece from the pig’s ear. Records of seven reproductive traits, including total number born (TNB), number born alive (NBA), number born strong (STRONG), number born weak (WEAK), number of born freak (FREAK), number of stillborn (DEAD), and number of mummified (MUMMY), were collected by professional breeders. The protocol for collecting ear tissue was approved by the Animal Welfare Committee of China Agricultural University (approval number XK257).
2.2. Genotyping and Quality Control
DNA was extracted from ear tissue using the phenol–chloroform method [22], and its quality and quantity were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Genotyping was carried out using the GeneSeek-Neogencine SNP60 BeadChip (Beijing Kangpusen Biological Technology Co., Ltd., Beijing, China). For other specific processing steps of genotyping we referred to previous articles [23]. In order to minimize false-positive associations resulting from genotyping errors, we used PLINK 1.9 (http://www.cog-genomics.org/plink/1.9 accessed on 14 May 2023) to perform quality control for 50K SNP chip data. We retained SNPs with a genotyping call rate greater than 99% and a minor allele frequency (MAF) of ≥1%. All autosomes were kept, and sex chromosomes were removed. After a serious filtration step, a total of 49,284 SNPs were retained to perform association analysis with reproductive traits.
2.3. Phenotypic Correlation Analysis
To calculate the correlation between the seven reproductive-related traits collected from Yorkshire pigs, we carried out correlation analysis. Correlation analysis of the seven reproductive traits of pigs was calculated using Spearman’s (rP) and Pearson’s (rS) correlation to ensure the reliability of the correlation results. The gcorrplot package version 0.1.4.1 (https://github.com/taiyun/corrplot accessed on 17 May 2023) was used for phenotypic correlation analysis, and ggplot2 version 3.4.4 (https://github.com/tidyverse/ggplot2 accessed on 18 May 2023) was used for visualization. The results of the association analysis retain two significant digits.
2.4. Principal Component Analysis (PCA) and Relationship Analysis
We then carried out population structure analysis, including principal component analysis and kinship analysis, to determine whether the population stratified. If the population stratified, the results of the GWAS analysis would have a great false-positive phenomenon. The results of the principal component analysis can be added to the GWAS analysis as a covariate to reduce false-positive results. Principal component analysis (PCA) was estimated using SNP data with GCTA version 1.94.0 (https://yanglab.westlake.edu.cn/software/gcta/ accessed on 8 May 2023). Then, pairwise kinship was estimated using genome-wide autosomal SNP information with the IBS [24] function in PLINK version 1.9. PCA and kinship visualization used scatterplot3d version 0.3.44 (https://cran.r-project.org/web/packages/scatterplot3d/ accessed on 10 May 2023) and pheatmap version 1.0.12 (https://CRAN.R-project.org/package=pheatmap accessed on 13 May 2023), respectively.
2.5. Genome-Wide Association Study
GWAS was performed using R statistical software, version 4.0.2, (https://www.r-project.org/ accessed on 15 September 2020) using the Fixed and random model Circulating Probability Unification (FarmCPU) method [25] in GAPIT, version 3.0 (https://www.maizegenetics.net/gapit accessed on 12 November 2020). The first three principal component analysis results were used as covariates in the GWAS. Bonferroni multiple tests were employed to identify the genome-wide significant (0.05/number of SNPs) and suggestive (1/number of SNPs) SNPs. Significant association between SNPs and reproductive traits was visualized in Manhattan plots and QQ-plots, which were generated using rMVP (https://github.com/xiaolei-lab/rMVP accessed on 23 November 2020) and CMplot (https://cran.r-project.org/web/packages/CMplot/ accessed on 22 October 2020). The functional genes were annotated using pig reference genome version 11.1 (http://asia.ensembl.org/Sus_scrofa/Info/Index accessed on 25 May 2023) in ENSEMBLE (http://asia.ensembl.org/ accessed on 25 May 2023).
2.6. Detection of ROH Segments and Common Runs of Homozygosity
The purpose of this part was to detect ROH segments and identify the genomic regions most commonly associated with ROHs, and the percentage occurrence of SNPs in ROH was computed by counting the number of SNPs detected in ROH for all individuals in the population. SNPs in the top 1% of frequency of occurrence were selected as potential ROH islands [26,27,28]. To determine these, we counted the number of SNPs detected in ROH for all individuals in the population and computed the percent occurrence of SNPs in ROH. Using a 50K SNP Chip and the R package detectRUNS (https://cran.r-project.org/web/packages/detectRUNS/index.html accessed on 11 November 2022), we calculated the frequency of occurrence for each SNP. The detectRUNS’s parameters of ROH island detection were as follows: We categorized the ROHs into five groups based on size: 0 to <6 Mb, 6 to <12 Mb, 12 to <24 Mb, 24 to <48 Mb, and ≥48 Mb. Then, we employed a sliding window analysis using a window size of 15 SNPs, with a minimum requirement of 20 homozygous SNPs. In addition, windows with a homozygous threshold of 0.05 were included, with a minimum of 1000 SNPs per kbps. Moreover, we set the maximum distance between two SNPs to 1,000,000 bps and the minimum length of a homozygous run to 250,000 bps. For the SNPs in the top 1% of frequency of occurrence, we also used ENSEMBLE, based on pig reference genome version 11.1, to annotate the functional genes in the ROH island.
3. Results and Discussion
3.1. Correlation Analysis between Phenotypes
In the Yorkshire pig population, we estimated phenotypic association analysis between the TNB, NBA, STRONG, WEAK, FREAK, DEAD, and MUMMY traits, and the results of the correlation analysis are shown in Figure 1. Additionally, the phenotype data of the seven reproductive traits are summarized in Supplementary Table S1.
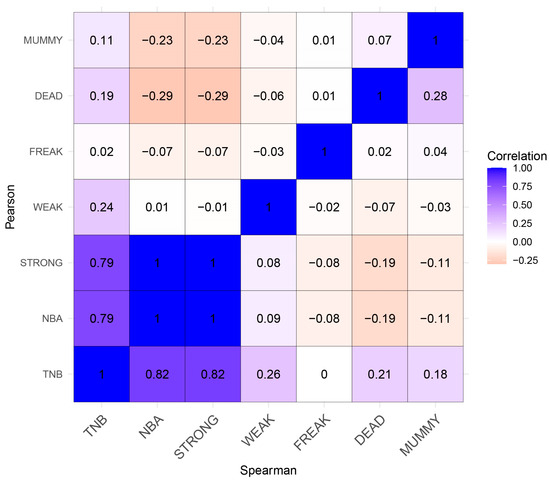
Figure 1.
Spearman (lower triangle) and Pearson (upper triangle) correlation coefficient among seven reproductive traits in Yorkshire pigs. In this diagram, positive correlations are depicted using the color blue, while negative correlations are represented by the color red.
The two correlations are basically consistent, and the details are as follows: three traits (TNB, NBA, and STRONG) were strongly correlated, ranging from 0.79 to1, among which the STRONG and NBA traits had the strongest positive correlation (1.00), while the TNB trait had a weak-positive correlation. To some extent, this illustrates the close relationship between the STRONG and NBA traits, and this confirms our understanding that as long as we can maximize the improvement of the STRONG trait, we can increase the NBA trait. In addition, genes affecting all three traits may have similar effects, so a gene affecting one trait may affect the other two. The DEAD and MUMMY traits were strongly negatively correlated with the NBA and STRONG traits, ranging from −0.11 to −0.29. The DEAD trait was the most negatively correlated with the NBA and STRONG traits, while the MUMMY trait was strongly correlated with the NBA trait (rS: −0.11, rP: −0.23); the STRONG trait (rS: −0.11, rP: −0.23) is second only to the DEAD trait. Therefore, during breeding, attention should be paid to the reduction of the DEAD and MUMMY traits, which are less correlated (rS: 0.28, rP: 0.07).
3.2. PCA and Kinship Analysis
Population stratification is a significant confounding factor in GWASs, as systematic ancestry differences can lead to false-positive associations [29]. Figure 2A depicts the population stratification of Yorkshire pigs based on PCA; the pig population was not divided into separate clusters of signaling, demonstrating no stratification in the reference population. The results of the principal component analysis (PCA) were consistent with the previous PLINK analysis [23] and did not reveal any significant clustering. The first three PCAs explain the 2.46%, 2.29%, and 1.98% of variation, respectively; about 4% of the variation is explained by the first three PCAs together. In addition, the first three principal components were selected as covariables to eliminate the influence of population stratification on the correlation analysis. The kinship heat map based on the IBS matrix (Figure 2B) also shows us a similar population structure to the PCA results, and there does not appear to be significant population stratification.
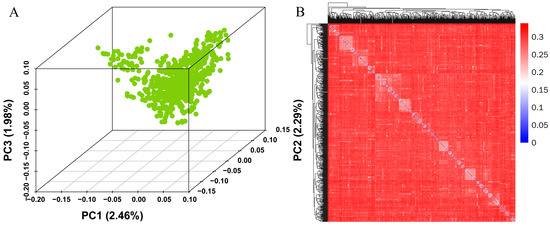
Figure 2.
The first three principal components (A) and kinship heat map (B) of the Yorkshire pig populations.
Kinship also verified the principal component analysis results, and the two confirmed each other. This suggests that there may be slight false positives in this population, which will be greatly reduced after adding PCA as a covariate to the GWAS calculation.
3.3. The Significant SNPs and Genes from GWAS
A total of 2 genome-wide significant and 18 suggestive significant SNPs were identified to be associated with five traits, including TNB, WEAK, FREAK, DEAD, and MUMMY (Figure 3 and Table 1). The QQ-plots for these GWAS results are also reported in Figure 3. No significant association was found between SNPs and the number of strong piglets (STRONG) or the number born alive (NBA).
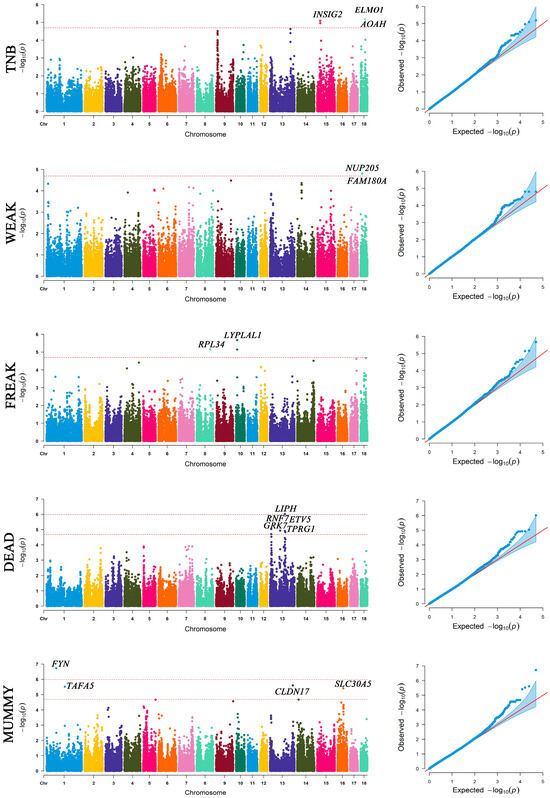
Figure 3.
Manhattan plots and quantile–quantile plots of GWAS results using FarmCPU, which confirm the TNB, WEAK, FREAK, DEAD, and MUMMY traits. The suggestive threshold is negative log10 2.03 × 10−5, and the genome-wide significant is negative log10 1.01 × 10−6. The two red dashed lines represent genome-level significance (up) and chromosomal significance (down).

Table 1.
The genome-wide significant and suggestive SNPs and candidate genes associated with reproductive traits. SNPs surpassing the genome-wide threshold are in the black and bold words, and the other unmarked SNPs reach the suggestive threshold.
The two genome-wide significant SNPs, ALGA0071870 relating to the DEAD trait (p = 9.56 × 10−7) and MARC0022221 relating to the MUMMY trait (p = 1.88 × 10−7), were located within the LIPH and FYN gene regions. Several studies have shown that LIPH is associated with immunity [30,31,32,33], and the presence of infections in pregnant mothers can lead to abnormal fetal development [34]. Therefore, it is speculated that LIPH may, to some extent, lead to stillbirths. FYN protein kinase has a crucial role in signal transduction after fertilization [35], and the FYN gene has been linked to abnormal sperm [36]. Although abnormal sperm and eggs can be fertilized normally, they can lead to abnormal embryo development and early miscarriage [37,38]. Thus, FYN may be closely related to the production of mummified fetuses in pigs.
TNB is an important indicator for predicting pig litter performance. We found that the ELMO1, AOAH, and INSIG2 genes were associated with the TNB trait. The engulfment and cell motility 1 (ELMO1) gene has been demonstrated to play significant roles in the clearance of apoptotic germ cells and spermatogenesis in mice [39], and the elimination of apoptotic germ cells and sperm is an important process to maintain reproductive health and function [40]. It is evident that ELMO1 likely has an impact on the total litter size of pigs. The acyloxyacyl hydrolase (AOAH) gene is expressed through AMPs (antimicrobial peptides and proteins) in placental cells, which is a result of a co-operation of leukocytes and cells from early embryonic development [41]. Therefore, AOAH may play an important role in early embryonic immunity, which might reduce the invasion of various viruses and germs in the embryo, ensure embryonic development, and thus ensure the survival of the embryo to a certain extent. Two suggestive significant SNPs are located within or near the INSIG2 gene (Table 1). The insulin-induced gene 2 (INSIG2) gene is involved in cholesterol anabolism and maintaining the homeostasis of cholesterol [42,43,44]. Homeostasis of cholesterol is possibly crucial for the maternal environment and for providing conditions for the normal development of the fetus, since cholesterol is an important component of the placenta and fetal development [45,46,47,48]. According to the above phenotypic correlation, the TNB trait has a strong correlation with the NBA and STRONG traits, and the above genes may also play an important role in the development of the NBA and STRONG traits (Table 2).

Table 2.
The genomic regions and functions of extended homozygosity (ROH islands) identified in Yorkshire pigs.
The nucleoporin 205 (NUP205) was related to the WEAK trait, and it was related to ensuring normal embryo development [65]. The quality of embryonic development directly affects the growth and development of the fetus [66], so NUP205 may play a certain role in fetal survival. The ribosomal protein L34 (RPL34) and lysophospholipase-like 1 (LYPLAL1) were also found to be associated with the FREAK trait. Some studies have found that RPL34 is associated with infertility triggered by sperm quality [67]. Female eggs can bind to abnormal sperm at the time of conception, which ultimately leads to fetal abnormalities, and RPL34 can serve as an entry point for studying these causes. LYPLAL1 is related to diet-induced obesity [68], which may make the sow too obese during pregnancy. Obesity in sows can lead to reduced uterine volume and affect embryo development [69,70], resulting in poor fetal malnutrition immunity and susceptibility to illness [71], which may also be the main cause of the increase in weak litters. Therefore, it is speculated that LYPLAL1 may increase the weight of the pregnant mother and affect fetal development.
The genes found to be associated with the DEAD trait include the ring finger protein 7 (RNF7), G protein-coupled receptor kinase (GRK7), and ets variant gene 5 (ETV5). The RNF7 gene, identified as the gene most similar to the expression of PCOS in transgender people, may lead to ubiquitination of the androgen receptor and eventually lead to antral follicular growth stagnation [72]. This may affect the mother’s conception and pregnancy, which is not good for the fetus. It has been found that GRK7 can perform highly effective photo-oxidation of photoactivated visual pigments in cone cells [73]. This may lead to an increase in the body’s biochemical reaction phosphate bond generation and reconstruction of high-energy bonds, increase the body’s energy reserves, and may also provide conditions for the body to produce more fat. This will lead to weight gain in the mother, which is not conducive to maternal pregnancy. Pathways of ETV5 are deeply related to the epithelial-to-mesenchymal transition (EMT) process in endometrial cancer (EC) [74]. ETV5 plays an important role in many aspects of development including embryonic and perinatal survival, postnatal growth, limb patterning, kidney development, and fertility [75]. Moreover, the loss of ETV5 reduces the proliferation and RET levels of testicular germ cells in newborn mice and leads to abnormalities in the first wave of spermatogenesis [76]. Maternal pregnancy is affected by obesity and abnormal sperm, and these factors can be the leading cause of fetal death.
Regarding the solute carrier family 30 member 5 (SLC30A5) related to the MUMMY trait, SLC30A5 has a relation to zinc homeostasis [77] and zinc transporters [78]. Zinc is a key element in embryonic development, and if the mother is deficient in zinc during pregnancy, it may cause a variety of complications for the fetus [79]. In addition, relevant studies have shown that enteral zinc supplementation prevents preterm birth and death of fetuses [80]. Therefore, SLC30A5 may affect the development of the fetus in the mother by affecting the synthesis and transport of zinc. Based on the results of the phenotypic correlation analysis, we observed a significant correlation between the traits FREAK, DEAD, and MUMMY. This suggests that the genes associated with these traits may also contribute to the expression of the other two traits and thus, they should be further investigated. Unexpectedly, four genes, including FAM180A, ENSSSCG00000047980, TPRG1, and CLDN17, adjacent to suggestive significant SNPs, have no published research on their biological function.
3.4. ROH Detection and Candidate Genes within Runs of Homozygosity Islands
The results of ROH detection show that the number of five kinds of ROH segments were: 141,334 (0–6 Mb), 8573 (6–12 Mb), 3853 (12–24 MB), 1249 (24–48 MB), and 292 (>48 Mb), respectively. The number and proportion of ROH on each chromosome are shown in Supplementary Figure S1. These results show that Yorkshire pigs had more long ROH fragments than in previous research of Chinese native pigs [81], indicating that Yorkshire pigs have a higher inbred coefficient, which may also be one of the reasons for their good performance. Long runs of homozygosity (ROH) can serve as an indicator of recent generation kinship due to the lower probability of interruption recombination events in shorter generations, so the longer ROH fragments suggest a higher likelihood of inbreeding within the population [82]. The percentage of SNPs in ROHs are plotted against the chromosome position in Figure 4. The top 1% of SNPs in the ROH with an over 68.38% occurrence were selected for further analysis. Based on these SNPs, we identified the genomic regions and genes that are most associated with ROH across all individuals. The 12 candidate genes associated with production traits including spermatogenesis, embryonic development, and litter size were identified in the ROH islands.
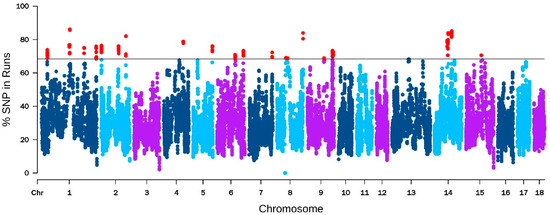
Figure 4.
Manhattan plot of the frequency (%) of each SNP in the runs of homozygosity among the total pig population. The solid line represents the 68.38% threshold and the red dots represent the top 1%.
Six genes related to spermatozoa include INSL6, PSMA8, SPATA6, TAF4B, TPD52L3, and HERC4. INSL6 ensures sperm motility and is required for the progression of spermatogenesis [49,50]. PSMA8 is a component of the sperm proteasome, and its deletion reduces proteasome abundance in the testis [51,52]. SPATA6 is required to connect the sperm head and tail during spermatogenesis [53]. TAF4B plays a role in spermatogenesis and oogenesis [54], and it maintains the development of mouse sperm stem cells [83,84]. Some studies indicated a potential role for TPD52L3 in testis development and spermatogenesis [55]. Moreover, HERC4 is required for sperm maturation [56]. Improving the quality of sperm can improve the fecundity of sows, and targeting these genes may be important in improving the fecundity of sows.
Next on the list are genes involved in embryonic and fetal development, including AGBL4, AHI1, E2F7, RTL1, and CDKN1C. AGBL4 is involved in KLF4 deglutamylation which promotes KLF4 proteasome-mediated degradation, thereby negatively regulating embryogenesis [57]. As a positive modulator of classical Wnt signaling, AHI1 may play a crucial role in ciliary signaling during cerebellum embryonic development [58]. The function of E2F7 in the extra-embryonic trophoblast is critical in developing the fetus [59,60]. RTL1 can maintain the fetal and maternal interface and placental development [61]. CDKN1C may cause fetal dysplasia and miscarriage [62]. These genes may play an important role in the fecundity of pigs.
Furthermore, GDF9 is a factor secreted by oocytes, which can promote the development of primordial follicles and regulate the quality and developmental ability of eggs [63,64]. GDF9 mutation can affect ewe fertility by affecting follicles and oocytes [85]. Previous studies showed that GDF9 had some connection with the litter size in sheep, and GDF9 may affect the ovulation rate and litter size in sheep by promoting ovulation [86,87,88]. These results indicate that GDF9 may be an important gene affecting porcine calving traits.
4. Conclusions
In this study, we performed a GWAS and ROH analysis to identify reproduction-related genes with a 50K BeadChip of Yorkshire pigs. We identified a total of 20 candidate SNPs for seven reproductive traits in Yorkshire pigs using the GWAS. Furthermore, the genes, including ELMO1, AOAH, INSIG2, NUP205, LYPLAL1, RPL34, LIPH, RNF7, GRK7, ETV5, FYN, and SLC30A5, identified by these SNPs have a range of functions associated with immunity, sperm, embryonic development, and pregnancy. Moreover, we identified 12 ROH islands containing genes (INSL6, TAF4B, E2F7, RTL1, CDKN1C, and GDF9) with molecular functions involving the spermatozoa, development of the fetus, mature eggs, and litter size. These findings can contribute to the understanding of reproductive performance and enhance future breeding strategies by providing insight for animal husbandry production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14122133/s1, Table S1: Statistical information on seven reproductive traits of Yorkshire pigs; Figure S1: The number and proportion of ROHs on each chromosome.
Author Contributions
K.W. and M.F. contributed to the conception of the study. K.W. and L.Z. wrote and revised the manuscript. L.Z., S.Z., F.Z., M.Y. and M.S. performed data analysis. K.W., M.F., X.L. (Xinjian Li), X.H., R.Q., X.L. (Xiuling Li), P.S. and F.Y. contributed to data collection. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program (2021YFD1301205), National Science Foundation of China (32002142),the Starting Foundation for Outstanding Young Scientists of Henan Agricultural University (30501280), the Grand Science and Technology Special Project in Tibet (XZ202101ZD0005N), and Extraction of Fining gene in Zaozhuang Black Cover Pig (2022TSGC1196).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Association of Henan Agricultural University (approval number HNND2023031439).
Informed Consent Statement
Not applicable.
Data Availability Statement
All animals involved in this study were managed according to the instructions of the care and use of experimental animals established. The genotype data for Yorkshire pigs are owned by Henan Agricultural University. Data from the current study are available via https://osf.io/jqtw8/ (accessed on 14 July 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Onteru, S.K.; Fan, B.; Du, Z.Q.; Garrick, D.J.; Stalder, K.J.; Rothschild, M.F. A whole-genome association study for pig reproductive traits. Anim. Genet. 2012, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, K.; Zhou, J.; Chen, D.; Yang, Q.; Yang, X.; Liu, Y.; Feng, B.; Jiang, A.; Shen, L.; et al. GWAS on Imputed Whole-Genome Resequencing From Genotyping-by-Sequencing Data for Farrowing Interval of Different Parities in Pigs. Front. Genet. 2019, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Fortes, M.; Bresolin, T.; Mota, L.; Albuquerque, L.; Carvalheiro, R. Multitrait meta-analysis identified genomic regions associated with sexual precocity in tropical beef cattle. J. Anim. Sci. 2018, 96, 4087–4099. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Rempel, L.; Rohrer, G. Genome-wide association study of swine farrowing traits. Part I: Genetic and genomic parameter estimates. J. Anim. Sci. 2012, 90, 3353–3359. [Google Scholar] [CrossRef] [PubMed]
- Leman, A. Optimizing farrowing rate and litter size and minimizing nonproductive sow days. Vet. Clin. N. Am. Food Anim. Pract. 1992, 8, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Vashi, Y.; Magotra, A.; Kalita, D.; Banik, S.; Sahoo, N.; Gupta, S.; Naskar, S. Evaluation of candidate genes related to litter traits in Indian pig breeds. Reprod. Domest. Anim. Zuchthyg. 2021, 56, 577–585. [Google Scholar] [CrossRef]
- Ding, R.; Qiu, Y.; Zhuang, Z.; Ruan, D.; Wu, J.; Zhou, S.; Ye, J.; Cao, L.; Hong, L.; Xu, Z.; et al. Genome-wide association studies reveals polygenic genetic architecture of litter traits in Duroc pigs. Theriogenology 2021, 173, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, K.; Yang, Q.; Zhou, J.; Chen, D.; Ma, J.; Tang, Q.; Jin, L.; Xiao, W.; Jiang, A.; et al. Identifying SNPs and candidate genes for three litter traits using single-step GWAS across six parities in Landrace and Large White pigs. Physiol. Genom. 2018, 50, 1026–1035. [Google Scholar] [CrossRef]
- Schiavo, G.; Bovo, S.; Tinarelli, S.; Bertolini, F.; Dall’Olio, S.; Gallo, M.; Fontanesi, L. Genome-wide association analyses for several exterior traits in the autochthonous Casertana pig breed. Livest. Sci. 2019, 230, 103842. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bosse, Y.; Pare, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ding, R.; Peng, L.; Wu, J.; Ye, Y.; Zhou, S.; Wang, X.; Quan, J.; Zheng, E.; Cai, G.; et al. Genome-wide association analyses identify known and novel loci for teat number in Duroc pigs using single-locus and multi-locus models. BMC Genom. 2020, 21, 344. [Google Scholar] [CrossRef]
- Guo, X.; Su, G.; Christensen, O.F.; Janss, L.; Lund, M.S. Genome-wide association analyses using a Bayesian approach for litter size and piglet mortality in Danish Landrace and Yorkshire pigs. BMC Genom. 2016, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, X.; Tan, Z.; Xing, K.; Yang, T.; Wang, Y.; Sun, D.; Wang, C. Genome-wide association study for reproductive traits in a Large White pig population. Anim. Genet. 2018, 49, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, S.; Teng, J.; Diao, S.; Yuan, X.; Chen, Z.; Zhang, H.; Li, J.; Zhang, Z. Genome-wide association studies for the number of animals born alive and dead in duroc pigs. Theriogenology 2019, 139, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wilkinson, J.; Wang, Z.; Ladinig, A.; Harding, J.; Plastow, G. A genome-wide association study of fetal response to type 2 porcine reproductive and respiratory syndrome virus challenge. Sci. Rep. 2016, 6, 20305. [Google Scholar] [CrossRef]
- Ceballos, F.; Joshi, P.; Clark, D.; Ramsay, M.; Wilson, J. Runs of homozygosity: Windows into population history and trait architecture. Nat. Rev. Genet. 2018, 19, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Purfield, D.; Berry, D.; McParland, S.; Bradley, D. Runs of homozygosity and population history in cattle. BMC Genet. 2012, 13, 70. [Google Scholar] [CrossRef]
- Peripolli, E.; Munari, D.; Silva, M.; Lima, A.; Irgang, R.; Baldi, F. Runs of homozygosity: Current knowledge and applications in livestock. Anim. Genet. 2017, 48, 255–271. [Google Scholar] [CrossRef]
- Xu, Z.; Mei, S.; Zhou, J.; Zhang, Y.; Qiao, M.; Sun, H.; Li, Z.; Li, L.; Dong, B.; Oyelami, F.; et al. Genome-Wide Assessment of Runs of Homozygosity and Estimates of Genomic Inbreeding in a Chinese Composite Pig Breed. Front. Genet. 2021, 12, 720081. [Google Scholar] [CrossRef]
- Peripolli, E.; Stafuzza, N.; Munari, D.; Lima, A.; Irgang, R.; Machado, M.; Panetto, J.; Ventura, R.; Baldi, F.; da Silva, M. Assessment of runs of homozygosity islands and estimates of genomic inbreeding in Gyr (Bos indicus) dairy cattle. BMC Genom. 2018, 19, 34. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Xiao, Q.; Sun, H.; Gao, H.; Yang, Y.; Chen, J.; Li, Z.; Xue, M.; Ma, P.; et al. Distribution of runs of homozygosity in Chinese and Western pig breeds evaluated by reduced-representation sequencing data. Anim. Genet. 2018, 49, 579–591. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.B.; Xue, Y.; Peng, Y.L.; Chen, G.; Fang, M.Y. Differential expression of CYB5A in Chinese and European pig breeds due to genetic variations in the promoter region. Anim. Genet. 2015, 46, 16–22. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, K.; Liu, X.; Zhou, H.; Xu, L.; Wang, Z.; Fang, M. Identification of growth trait related genes in a Yorkshire purebred pig population by genome-wide association studies. Asian-Australas. J. Anim. Sci. 2017, 30, 462–469. [Google Scholar] [CrossRef]
- Price, A.; Patterson, N.; Plenge, R.; Weinblatt, M.; Shadick, N.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Listgarten, J.; Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLOS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Fang, Y.; Hao, X.; Xu, Z.; Sun, H.; Zhao, Q.; Cao, R.; Zhang, Z.; Ma, P.; Sun, Y.; Qi, Z.; et al. Genome-Wide Detection of Runs of Homozygosity in Laiwu Pigs Revealed by Sequencing Data. Front. Genet. 2021, 12, 629966. [Google Scholar] [CrossRef]
- Pemberton, T.; Absher, D.; Feldman, M.; Myers, R.; Rosenberg, N.; Li, J. Genomic patterns of homozygosity in worldwide human populations. Am. J. Hum. Genet. 2012, 91, 275–292. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, S.; Ganguly, I.; Bhatia, A.; Sharma, A.; Kumar, N.; Dang, A.; Jayakumar, S. Bos indicusGenome-Wide Runs of Homozygosity Revealed Selection Signatures in. Front. Genet. 2020, 11, 92. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, J.; Chen, C.; Zhang, J.; Wen, W.; Tian, J.; Zhang, Z.; Gu, Y. GWAS-Based Identification of New Loci for Milk Yield, Fat, and Protein in Holstein Cattle. Animals 2020, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Chen, X.; Wang, Y.; Huang, S.; Chen, B.; Zhang, C.; Hou, B. Identification of LIPH as an unfavorable biomarkers correlated with immune suppression or evasion in pancreatic cancer based on RNA-seq. Cancer Immunol. Immunother. CII 2022, 71, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, C.; Chen, H.; Li, R.; Chong, Q.; Xiao, H.; Chen, S. Genome-wide scan of selection signatures in Dehong humped cattle for heat tolerance and disease resistance. Anim. Genet. 2020, 51, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qu, K.; Ma, X.; Hanif, Q.; Zhang, J.; Liu, J.; Chen, N.; Suolang, Q.; Lei, C.; Huang, B. Whole-Genome Analyses Reveal Genomic Characteristics and Selection Signatures of Lincang Humped Cattle at the China-Myanmar Border. Front. Genet. 2022, 13, 833503. [Google Scholar] [CrossRef]
- Orozco-terWengel, P.; Barbato, M.; Nicolazzi, E.; Biscarini, F.; Milanesi, M.; Davies, W.; Williams, D.; Stella, A.; Ajmone-Marsan, P.; Bruford, M. Revisiting demographic processes in cattle with genome-wide population genetic analysis. Front. Genet. 2015, 6, 191. [Google Scholar] [CrossRef]
- Littauer, E.; Skountzou, I. Hormonal Regulation of Physiology, Innate Immunity and Antibody Response to H1N1 Influenza Virus Infection During Pregnancy. Front. Immunol. 2018, 9, 2455. [Google Scholar] [CrossRef]
- McGinnis, L.; Albertini, D.; Kinsey, W. Localized activation of Src-family protein kinases in the mouse egg. Dev. Biol. 2007, 306, 241–254. [Google Scholar] [CrossRef]
- Luo, J.; Gupta, V.; Kern, B.; Tash, J.; Sanchez, G.; Blanco, G.; Kinsey, W. Role of FYN kinase in spermatogenesis: Defects characteristic of Fyn-null sperm in mice. Biol. Reprod. 2012, 86, 1–8. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Li, C.; Xu, C. Progress in Research on Sperm DNA Fragmentation. Med. Sci. Monit. 2020, 26, e918746. [Google Scholar] [CrossRef]
- Zini, A.; Libman, J. Sperm DNA damage: Clinical significance in the era of assisted reproduction. CMAJ 2006, 175, 495–500. [Google Scholar] [CrossRef][Green Version]
- Elliott, M.; Zheng, S.; Park, D.; Woodson, R.; Reardon, M.; Juncadella, I.; Kinchen, J.; Zhang, J.; Lysiak, J.; Ravichandran, K. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337. [Google Scholar] [CrossRef]
- Elliott, M.; Ravichandran, K. ELMO1 signaling in apoptotic germ cell clearance and spermatogenesis. Ann. N. Y. Acad. Sci. 2010, 1209, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Klaffenbach, D.; Friedrich, D.; Strick, R.; Strissel, P.L.; Beckmann, M.W.; Rascher, W.; Gessner, A.; Dotsch, J.; Meissner, U.; Schnare, M. Contribution of different placental cells to the expression and stimulation of antimicrobial proteins (AMPs). Placenta 2011, 32, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Vanwong, N.; Sukasem, C.; Unaharassamee, W.; Jiratjintana, N.; Na Nakorn, C.; Hongkaew, Y.; Puangpetch, A. SREBF2Associations of the Gene and Polymorphisms with Obesity and Dyslipidemia in Thai Psychotic Disorder Patients Treated with Risperidone. J. Pers. Med. 2021, 11, 943. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Qiu, L.; Miao, Y. Negative effect of insulin-induced gene 2 on milk fat synthesis in buffalo mammary epithelial cells. J. Dairy Res. 2021, 88, 401–406. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Z.; Ren, X.; Wang, Y.; Yang, R.; Luo, J.; Strappe, P. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis. 2015, 14, 49. [Google Scholar] [CrossRef]
- Tint, G.; Yu, H.; Shang, Q.; Xu, G.; Patel, S. The use of the Dhcr7 knockout mouse to accurately determine the origin of fetal sterols. J. Lipid Res. 2006, 47, 1535–1541. [Google Scholar] [CrossRef]
- Shehu, A.; Mao, J.; Gibori, G.; Halperin, J.; Le, J.; Devi, Y.; Merrill, B.; Kiyokawa, H.; Gibori, G. Prolactin receptor-associated protein/17beta-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol. Endocrinol. 2008, 22, 2268–2277. [Google Scholar] [CrossRef]
- Herrera, E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 2000, 54 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef]
- Woollett, L. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32, S218–S221. [Google Scholar] [CrossRef]
- Anand-Ivell, R.; Dai, Y.; Ivell, R. Neohormones as biomarkers of reproductive health. Fertil. Steril. 2013, 99, 1153–1160. [Google Scholar] [CrossRef]
- Burnicka-Turek, O.; Shirneshan, K.; Paprotta, I.; Grzmil, P.; Meinhardt, A.; Engel, W.; Adham, I. Inactivation of insulin-like factor 6 disrupts the progression of spermatogenesis at late meiotic prophase. Endocrinology 2009, 150, 4348–4357. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, S.; Busayavalasa, K.; Shao, J.; Yu, C. Meiosis I progression in spermatogenesis requires a type of testis-specific 20S core proteasome. Nat. Commun. 2019, 10, 3387. [Google Scholar] [CrossRef]
- Gómez-H, L.; Felipe-Medina, N.; Condezo, Y.; Garcia-Valiente, R.; Ramos, I.; Suja, J.; Barbero, J.; Roig, I.; Sánchez-Martín, M.; de Rooij, D.; et al. The PSMA8 subunit of the spermatoproteasome is essential for proper meiotic exit and mouse fertility. PLoS Genet. 2019, 15, e1008316. [Google Scholar] [CrossRef]
- Yuan, S.; Stratton, C.; Bao, J.; Zheng, H.; Bhetwal, B.; Yanagimachi, R.; Yan, W. Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction. Proc. Natl. Acad. Sci. USA 2015, 112, E430–E439. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, E.; Seymour, K.; Sigrist, K.; Rooij, D.; Freiman, R. ZFP628 Is a TAF4b-Interacting Transcription Factor Required for Mouse Spermiogenesis. Mol. Cell. Biol. 2020, 40, e00228-19. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, J.; Zhu, L.; Liu, Y.; Zhou, Z.; Sha, J.; Wang, S.; Li, J. A testis-specific and testis developmentally regulated tumor protein D52 (TPD52)-like protein TPD52L3/hD55 interacts with TPD52 family proteins. Biochem. Biophys. Res. Commun. 2006, 344, 798–806. [Google Scholar] [CrossRef]
- Li, S.; Lan, K.; Hung, H.; Huang, W.; Lai, Y.; Cheng, H.; Tsai, C.; Huang, K.; You, H.; Hsu, T. HSPA4 Is a Biomarker of Placenta Accreta and Enhances the Angiogenesis Ability of Vessel Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 5682. [Google Scholar] [CrossRef]
- Ye, B.; Liu, B.; Hao, L.; Zhu, X.; Yang, L.; Wang, S.; Xia, P.; Du, Y.; Meng, S.; Huang, G.; et al. Klf4 glutamylation is required for cell reprogramming and early embryonic development in mice. Nat. Commun. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Lancaster, M.; Gopal, D.; Kim, J.; Saleem, S.; Silhavy, J.; Louie, C.; Thacker, B.; Williams, Y.; Zaki, M.; Gleeson, J. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat. Med. 2011, 17, 726–731. [Google Scholar] [CrossRef]
- Ouseph, M.; Li, J.; Chen, H.; Pécot, T.; Wenzel, P.; Thompson, J.; Comstock, G.; Chokshi, V.; Byrne, M.; Forde, B.; et al. Atypical E2F repressors and activators coordinate placental development. Dev. Cell 2012, 22, 849–862. [Google Scholar] [CrossRef]
- Li, J.; Ran, C.; Li, E.; Gordon, F.; Comstock, G.; Siddiqui, H.; Cleghorn, W.; Chen, H.; Kornacker, K.; Liu, C.; et al. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev. Cell 2008, 14, 62–75. [Google Scholar] [CrossRef]
- Fujioka, K.; Nishida, K.; Ashina, M.; Abe, S.; Fukushima, S.; Ikuta, T.; Ohyama, S.; Morioka, I.; Iijima, K. DNA methylation of the Rtl1 promoter in the placentas with fetal growth restriction. Pediatr. Neonatol. 2019, 60, 512–516. [Google Scholar] [CrossRef]
- Suntharalingham, J.; Ishida, M.; Buonocore, F.; Del Valle, I.; Solanky, N.; Demetriou, C.; Regan, L.; Moore, G.; Achermann, J. CDKN1CAnalysis of in fetal growth restriction and pregnancy loss. F1000Research 2019, 8, 90. [Google Scholar] [CrossRef]
- Belli, M.; Shimasaki, S. Molecular Aspects and Clinical Relevance of GDF9 and BMP15 in Ovarian Function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar] [CrossRef]
- Hobeika, E.; Armouti, M.; Fierro, M.; Winston, N.; Scoccia, H.; Zamah, A.; Stocco, C. Regulation of Insulin-Like Growth Factor 2 by Oocyte-Secreted Factors in Primary Human Granulosa Cells. J. Clin. Endocrinol. Metab. 2020, 105, 327–335. [Google Scholar] [CrossRef]
- Labade, A.S.; Karmodiya, K.; Sengupta, K. HOXA repression is mediated by nucleoporin Nup93 assisted by its interactors Nup188 and Nup205. Epigenetics Chromatin 2016, 9, 54. [Google Scholar] [CrossRef]
- Broere-Brown, Z.; Schalekamp-Timmermans, S.; Jaddoe, V.; Steegers, E. Deceleration of fetal growth rate as alternative predictor for childhood outcomes: A birth cohort study. BMC Pregnancy Childbirth 2019, 19, 216. [Google Scholar] [CrossRef]
- Bansal, S.; Gupta, N.; Sankhwar, S.; Rajender, S. Differential Genes Expression between Fertile and Infertile Spermatozoa Revealed by Transcriptome Analysis. PLoS ONE 2015, 10, e0127007. [Google Scholar] [CrossRef]
- Lei, X.; Callaway, M.; Zhou, H.; Yang, Y.; Chen, W. Obesity associated Lyplal1 gene is regulated in diet induced obesity but not required for adipocyte differentiation. Mol. Cell. Endocrinol. 2015, 411, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Pellicer, A.; García-Velasco, J.; Ballesteros, A.; Remohí, J.; Meseguer, M. Obesity reduces uterine receptivity: Clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil. Steril. 2013, 100, 1050–1058. [Google Scholar] [CrossRef]
- Campos, P.; Silva, B.; Donzele, J.; Oliveira, R.; Knol, E. Effects of sow nutrition during gestation on within-litter birth weight variation: A review. Animal 2012, 6, 797–806. [Google Scholar] [CrossRef]
- Tenenbaum-Gavish, K.; Hod, M. Impact of maternal obesity on fetal health. Fetal Diagn. Ther. 2013, 34, 1–7. [Google Scholar] [CrossRef]
- Dong, R.; Gao, S.; Shan, M. Identification of the similarly expressed genes in patients with polycystic ovary syndrome and transsexuals. Medicine 2021, 100, e26990. [Google Scholar] [CrossRef]
- Tachibanaki, S.; Arinobu, D.; Shimauchi-Matsukawa, Y.; Tsushima, S.; Kawamura, S. Highly effective phosphorylation by G protein-coupled receptor kinase 7 of light-activated visual pigment in cones. Proc. Natl. Acad. Sci. USA 2005, 102, 9329–9334. [Google Scholar] [CrossRef]
- Colas, E.; Pedrola, N.; Devis, L.; Ertekin, T.; Campoy, I.; Martinez, E.; Llaurado, M.; Rigau, M.; Olivan, M.; Garcia, M.; et al. The EMT signaling pathways in endometrial carcinoma. Clin. Transl. Oncol. 2012, 14, 715–720. [Google Scholar] [CrossRef]
- Jamsai, D.; Clark, B.J.; Smith, S.J.; Whittle, B.; Goodnow, C.C.; Ormandy, C.J.; O’Bryan, M.K. A missense mutation in the transcription factor ETV5 leads to sterility, increased embryonic and perinatal death, postnatal growth restriction, renal asymmetry and polydactyly in the mouse. PLoS ONE 2013, 8, e77311. [Google Scholar] [CrossRef]
- Tyagi, G.; Carnes, K.; Morrow, C.; Kostereva, N.; Ekman, G.; Meling, D.; Hostetler, C.; Griswold, M.; Murphy, K.; Hess, R.; et al. Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol. Reprod. 2009, 81, 258–266. [Google Scholar] [CrossRef]
- Malavolta, M.; Costarelli, L.; Giacconi, R.; Basso, A.; Piacenza, F.; Pierpaoli, E.; Provinciali, M.; Ogo, O.A.; Ford, D. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp. Gerontol. 2017, 99, 35–45. [Google Scholar] [CrossRef]
- Kumar, L.; Michalczyk, A.; McKay, J.; Ford, D.; Kambe, T.; Hudek, L.; Varigios, G.; Taylor, P.E.; Ackland, M.L. Altered expression of two zinc transporters, SLC30A5 and SLC30A6, underlies a mammary gland disorder of reduced zinc secretion into milk. Genes Nutr. 2015, 10, 487. [Google Scholar] [CrossRef]
- Brion, L.; Heyne, R.; Lair, C. Role of zinc in neonatal growth and brain growth: Review and scoping review. Pediatr. Res. 2021, 89, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Staub, E.; Evers, K.; Askie, L. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane Database Syst. Rev. 2021, 3, CD012797. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Zhan, F.; Song, M.; Shang, P.; Zhu, F.; Li, J.; Yang, F.; Li, X.; Qiao, R.; et al. Population Genetic Analysis of Six Chinese Indigenous Pig Meta-Populations Based on Geographically Isolated Regions. Animals 2023, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Shi, L.; Liu, J.; Deng, T.; Wang, L.; Liu, Y.; Zhao, F. Genome-Wide Scan for Runs of Homozygosity Identifies Candidate Genes in Three Pig Breeds. Animals 2019, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Lovasco, L.; Gustafson, E.; Seymour, K.; de Rooij, D.; Freiman, R. TAF4b is required for mouse spermatogonial stem cell development. Stem Cells 2015, 33, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Falender, A.; Freiman, R.; Geles, K.; Lo, K.; Hwang, K.; Lamb, D.; Morris, P.; Tjian, R.; Richards, J. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005, 19, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutar, H.; Younis, L. Effect of Point Mutation in the Growth Differentiation Factor 9 Gene of Oocytes on the Sterility and Fertility of Awassi Sheep. Arch. Razi Inst. 2020, 75, 101–108. [Google Scholar] [CrossRef]
- Wang, F.; Chu, M.; Pan, L.; Wang, X.; He, X.; Zhang, R.; Tao, L.; La, Y.; Ma, L.; Di, R. Polymorphism Detection of GDF9 Gene and Its Association with Litter Size in Luzhong Mutton Sheep (Ovis aries). Animals 2021, 11, 571. [Google Scholar] [CrossRef]
- Das, A.; Shaha, M.; Gupta, M.; Dutta, A.; Miazi, O. Polymorphism of fecundity genes (BMP15 and GDF9) and their association with litter size in Bangladeshi prolific Black Bengal goat. Trop. Anim. Health Prod. 2021, 53, 230. [Google Scholar] [CrossRef]
- Li, Y.; Jin, W.; Wang, Y.; Zhang, J.; Meng, C.; Wang, H.; Qian, Y.; Li, Q.; Cao, S. GDF9 Three Complete Linkage SNPs of Gene Affect the Litter Size Probably Mediated by OCT1 in Hu Sheep. DNA Cell Biol. 2020, 39, 563–571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).