β-Aminobutyric Acid and Powdery Mildew Infection Enhanced the Activation of Defense-Related Genes and Salicylic Acid in Cucumber (Cucumis sativus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Host Plant and Infection
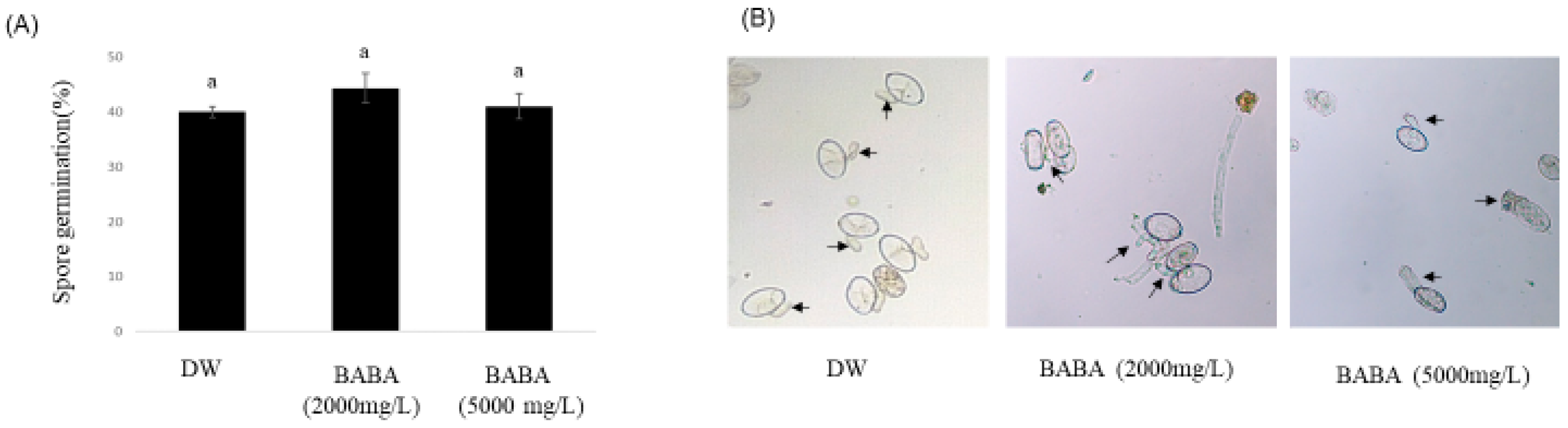
2.2. Spore Germination
2.3. qRT-PCR
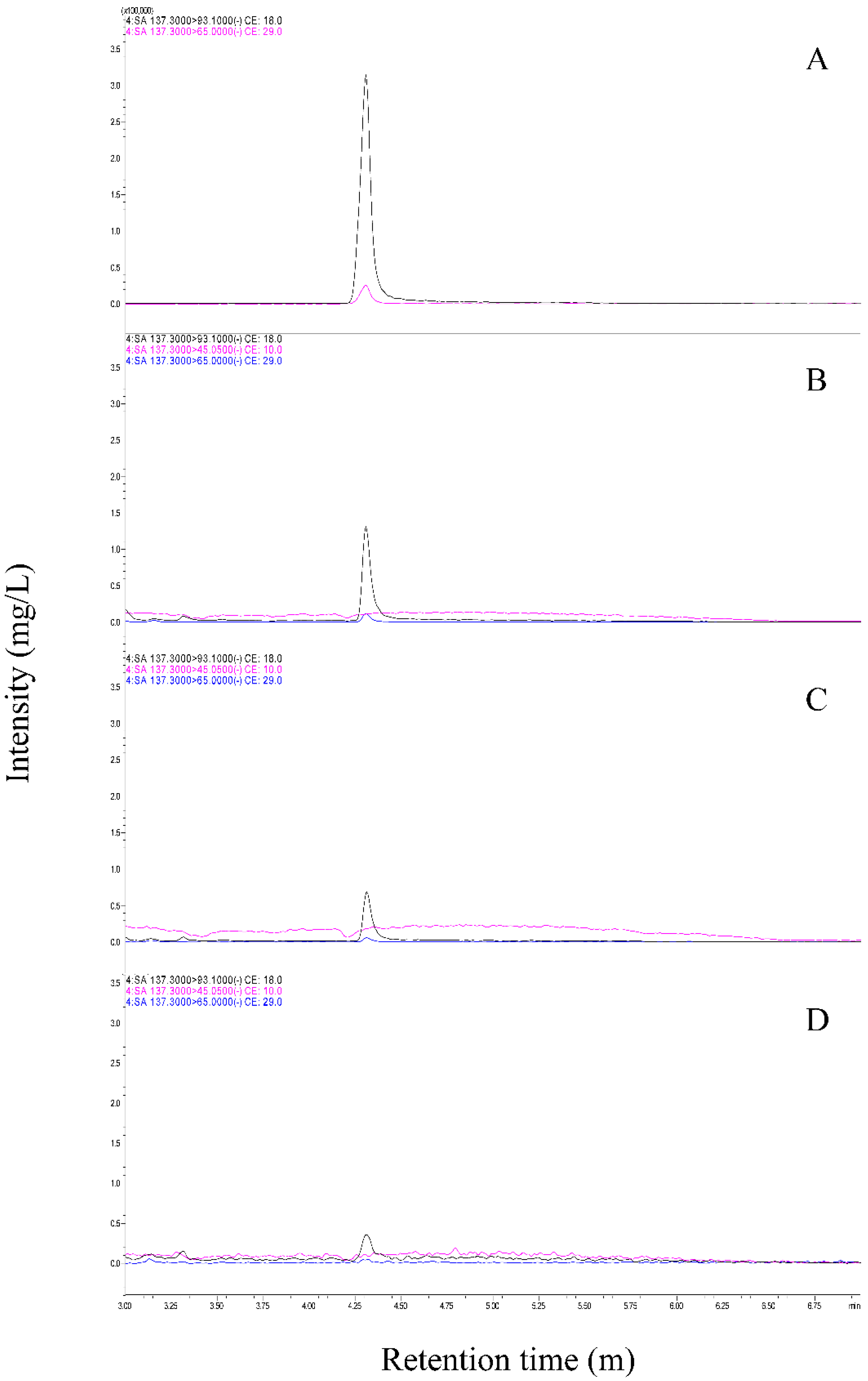
2.4. Extraction and Measurement of SA from Cucumber Leaves
2.5. Statistical Analysis
3. Results
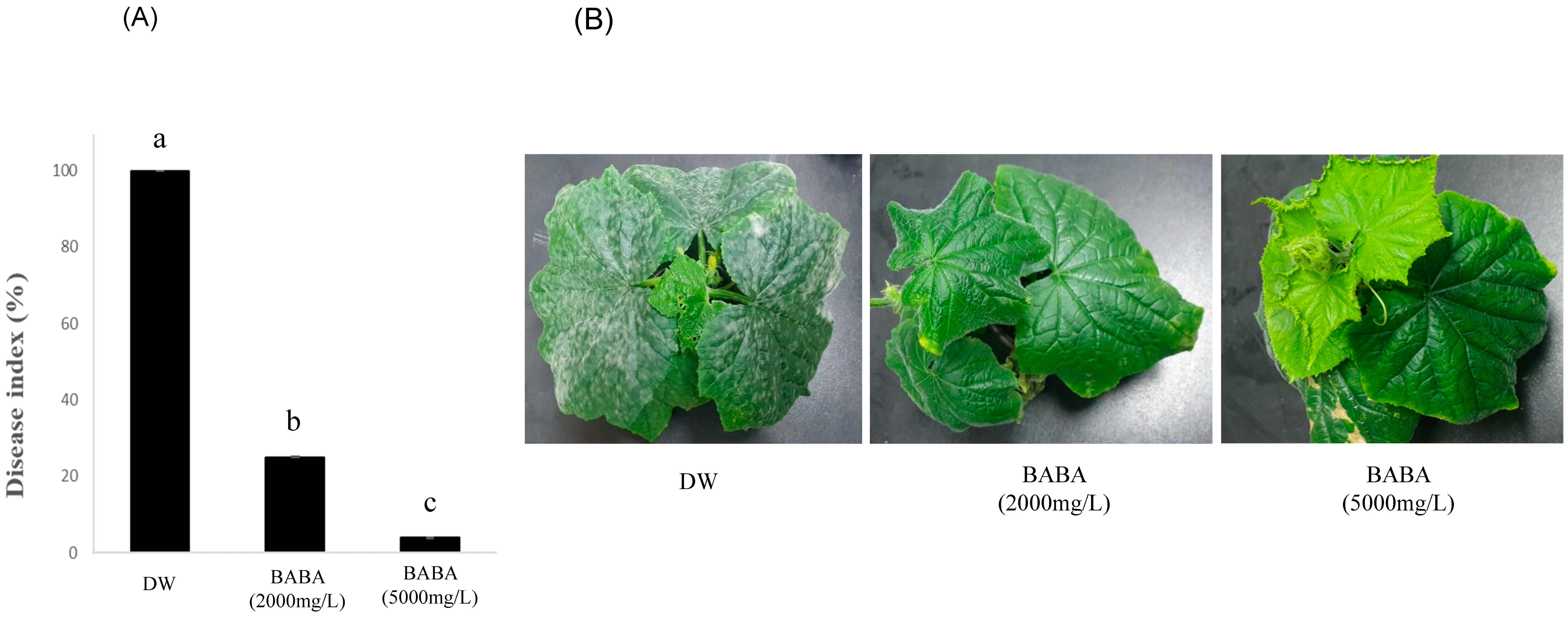
3.1. BABA Inhibits Cucumber PM Disease
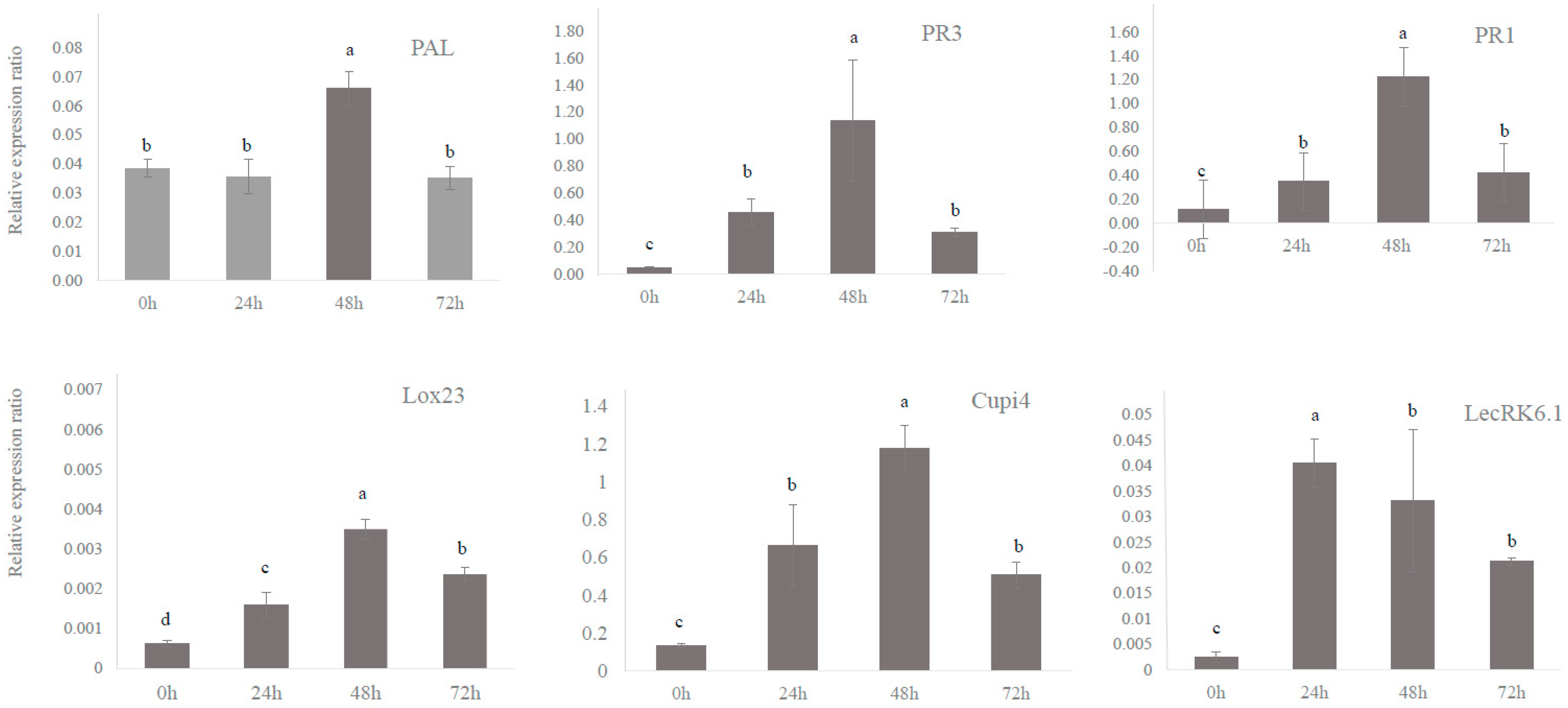
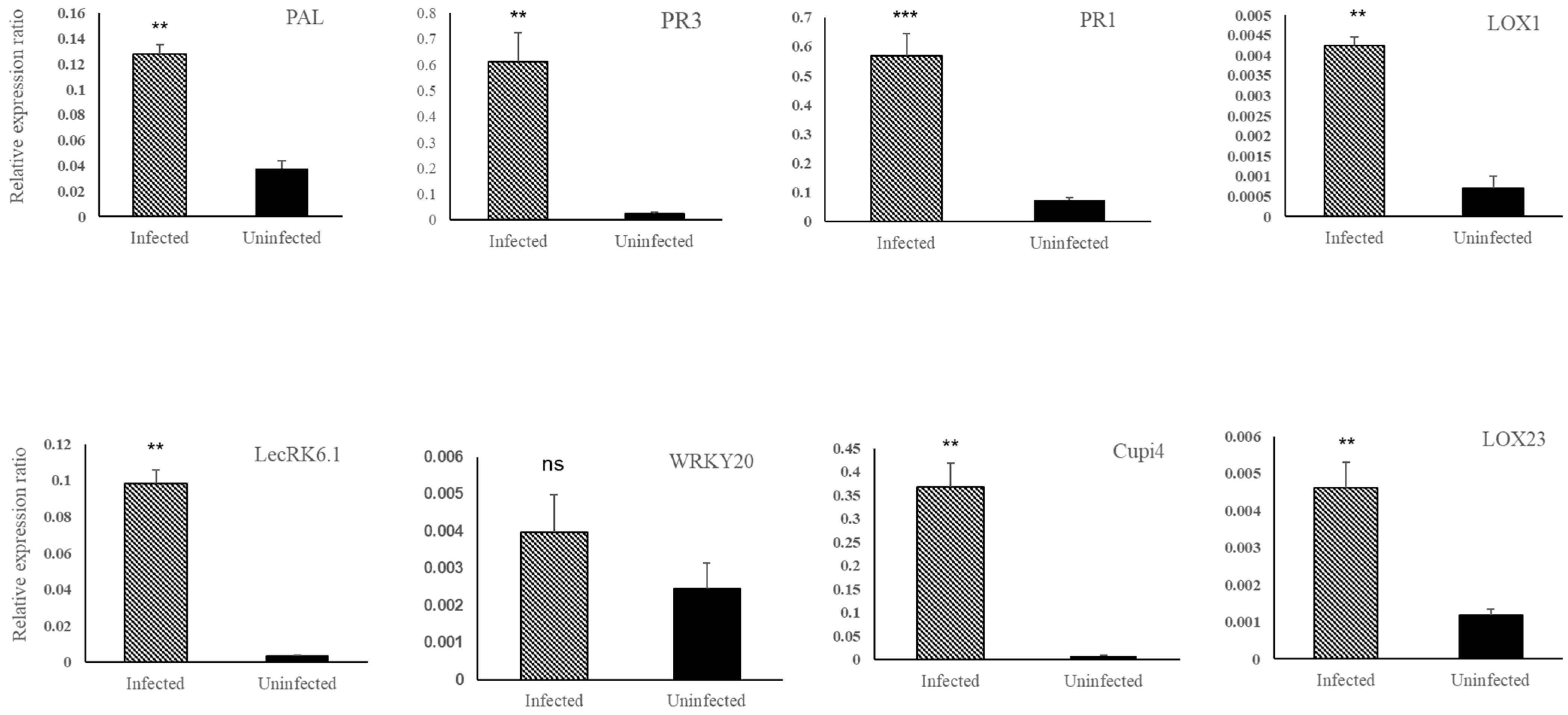
3.2. Defense Genes in Cucumber Were Upregulated by BABA and PM Infection
3.3. BABA Induces SA Accumulation in Cucumber
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reuveni, M.; Agapov, V.; Reuveni, R. A foliar spray of micronutrient solutions induces local and systemic protection against powdery mildew (Sphaerotheca fuliginia) in cucumber plants. Eur. J. Plant Pathol. 1997, 103, 581–588. [Google Scholar] [CrossRef]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Knoth, C.; Ringler, J.; Dangl, J.L.; Eulgem, T. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Mol. Plant-Microbe Interact. 2007, 20, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Xie, B.; Li, P.; Mao, Z.; Ling, J.; Shen, H.; Lin, B. Analysis of the defence-related mechanism in cucumber seedlings in relation to root colonization by nonpathogenic Fusarium oxysporum CS-20. FEMS Microbiol. Lett. 2014, 355, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, L.; Golparvar, A.R.; Nasr-Esfahani, M.; Golabadi, M. Expression analysis of defense-related genes in cucumber (Cucumis sativus L.) against Phytophthora melonis. Mol. Biol. Rep. 2020, 47, 4933–4944. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Fan, A.; Zhao, J.; Yu, Z.; Li, Y.; Wang, X. LecRK-V, an L-type lectin receptor kinase in Haynaldia villosa, plays positive role in resistance to wheat powdery mildew. Plant Biotechnol. J. 2018, 16, 50–62. [Google Scholar] [CrossRef]
- Oh, S.K.; Jang, H.A.; Kim, J.; Choi, D.; Park, Y.I.; Kwon, S.Y. Expression of cucumber LOX genes in response to powdery mildew and defense-related signal molecules. Can. J. Plant Sci. 2014, 94, 845–850. [Google Scholar] [CrossRef]
- Kang, D.S.; Min, K.J.; Kwak, A.M.; Lee, S.Y.; Kang, H.W. Defense response and suppression of Phytophthora blight disease of pepper by water extract from spent mushroom substrate of Lentinula edodes. Plant Pathol. J. 2017, 33, 264. [Google Scholar] [CrossRef]
- Stamler, R.A.; Holguin, O.; Dungan, B.; Schaub, T.; Sanogo, S.; Goldberg, N.; Randall, J.J. BABA and Phytophthora nicotianae induce resistance to Phytophthora capsici in chile pepper (Capsicum annuum). PLoS ONE 2015, 10, e0128327. [Google Scholar] [CrossRef]
- Jakab, G.; Cottier, V.; Toquin, V.; Rigoli, G.; Zimmerli, L.; Métraux, J.P.; Mauch-Mani, B. β-Aminobutyric acid-induced resistance in plants. Eur. J. Plant Pathol. 2001, 107, 29–37. [Google Scholar] [CrossRef]
- Zeighaminejad, R.; Sharifi-Sirchi, G.R.; Mohammadi, H.; Aminai, M.M. Induction of resistance against powdery mildew by β aminobutyric acid in squash. J. Appl. Bot. Food Qual. 2016, 89, 176–182. [Google Scholar]
- Cameron, R.K.; Paiva, N.L.; Lamb, C.J.; Dixon, R.A. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol. Mol. Plant Pathol. 1999, 55, 121–130. [Google Scholar] [CrossRef]
- George, N. Agrios, Plant Pathology, 5th ed.; Elsevier Academic Press: Cambridge, MA, USA, 2005; p. 448. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Pajot, E.; Le Corre, D.; Silué, D. Phytogard® and DL-β-amino Butyric Acid (BABA) Induce Resistance to Downy Mildew (Bremia lactucae) in Lettuce (Lactuca sativa L). Eur. J. Plant Pathol. 2001, 107, 861–869. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Y.; Zhou, S. Comparative analysis of powdery mildew resistant and susceptible cultivated cucumber (Cucumis sativus L.) varieties to reveal the metabolic responses to Sphaerotheca fuliginea infection. BMC Plant Biol. 2021, 21, 24. [Google Scholar]
- Kavroulakis, N.; Papadopoulou, K.K.; Ntougias, S.; Zervakis, G.I.; Ehaliotis, C. Cytological and other aspects of pathogenesis-related gene expression in tomato plants grown on a suppressive compost. Ann. Bot. 2006, 98, 555–564. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Rawat, S.; Ali, S.; Mittra, B.; Grover, A. Expression analysis of chitinase upon challenge inoculation to Alternaria wounding and defense inducers in Brassica juncea. Biotechnol. Rep. 2017, 13, 72–79. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jiang, W.J.; Yu, H.J. The expression profiling of the lipoxygenase (LOX) family genes during fruit development, abiotic stress and hormonal treatments in cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2012, 13, 2481–2500. [Google Scholar] [CrossRef]
- Kawatra, A.; Dhankhar, R.; Mohanty, A.; Gulati, P. Biomedical applications of microbial phenylalanine ammonia lyase: Current status and future prospects. Biochimie 2020, 177, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, F.; Tian, W.; Guo, Z.; Chen, X.; Xu, X.; Hu, X. The wheat WRKY transcription factors TaWRKY49 and TaWRKY62 confer differential high-temperature seedling-plant resistance to Puccinia striiformis f. sp. tritici. PLoS ONE 2017, 12, e0181963. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Wu, X.; Zhang, L.; Xiao, J.; Li, T.; Jia, M. Molecular Cloning and Characterization of WRKY12, A Pathogen Induced WRKY Transcription Factor from Akebia trifoliata. Genes 2023, 14, 1015. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Guo, J.; Qiao, Q.; Guo, X.; Ma, Y. The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Ortic. Res. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Phuntumart, V.; Marro, P.; Metraux, J.-P.; Sticher, L. A novel cucumber gene associated with systemic acquired resistance. Plant Sci. 2006, 171, 555–564. [Google Scholar] [CrossRef]
| Genes | Primer Sequence |
|---|---|
| CsActin | F: 5′-TCG TGC TGG ATT CTG GTG-3′ |
| R: 5′-GGC AGT GGT GGT GAA CAT-3′ | |
| CsPAL | F: 5′-AAA CAC GTC GGA TAA ATA TGG CTT-3′ |
| R: 5′-CAT CCA TTC AGG CGT TCC AG-3′ | |
| CsPR3 | F: 5′-CAC TGC AAC CCT GAC AAC AAC G-3′ |
| R:5′-AAG TGG CCT GGA ATC CGA CTG-3′ | |
| CsPR1 | F: 5′-CTC AAG ACT TCG TCG GTG TCC A-3′ |
| R: CGC CAG AGT TCA CTA GCC TAC | |
| CsLOX1 | F: 5′-TCT TTG CTT CAG GGT ATC AC-3′ |
| R: 5′-GCA AAT TCT TCA TCA CTA CTC C-3′ | |
| LOX23 | F: 5′-TGC CTC CAA CAC CTT CTT CAA-3′ |
| R: 5′-CTT CCA TAT CAA ATC GCC ACA-3′ | |
| CsLecRK6.1 | F: 5′-CGA CCA CAA CGA AAT GTC ACA C-3′ |
| R: 5′-TTT CTT CCA CAC GCC ACT TCC-3′ | |
| CsWRKY20 | F: 5′-GAA ATA ACG TAC AGA GGG AAG C-3′ |
| R: 5′-CAG GTG CTG TTT GTT GGT TAT G-3′ | |
| Cupi4 | F: 5′-TCA CTG TGG TGT GTG CTC TC-3′ |
| R:—ACT CAA GCC ATT GCC TTC CA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kang, H.-W. β-Aminobutyric Acid and Powdery Mildew Infection Enhanced the Activation of Defense-Related Genes and Salicylic Acid in Cucumber (Cucumis sativus L.). Genes 2023, 14, 2087. https://doi.org/10.3390/genes14112087
Kim J-Y, Kang H-W. β-Aminobutyric Acid and Powdery Mildew Infection Enhanced the Activation of Defense-Related Genes and Salicylic Acid in Cucumber (Cucumis sativus L.). Genes. 2023; 14(11):2087. https://doi.org/10.3390/genes14112087
Chicago/Turabian StyleKim, Ja-Yoon, and Hee-Wan Kang. 2023. "β-Aminobutyric Acid and Powdery Mildew Infection Enhanced the Activation of Defense-Related Genes and Salicylic Acid in Cucumber (Cucumis sativus L.)" Genes 14, no. 11: 2087. https://doi.org/10.3390/genes14112087
APA StyleKim, J.-Y., & Kang, H.-W. (2023). β-Aminobutyric Acid and Powdery Mildew Infection Enhanced the Activation of Defense-Related Genes and Salicylic Acid in Cucumber (Cucumis sativus L.). Genes, 14(11), 2087. https://doi.org/10.3390/genes14112087




