MLH1 Promoter Methylation Could Be the Second Hit in Lynch Syndrome Carcinogenesis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients Cohort
2.2. DNA Extraction and MSI Analysis
2.3. MLH1 Methylation Analysis
2.4. Germline MMR Analysis
2.5. Statistical Analysis
3. Results
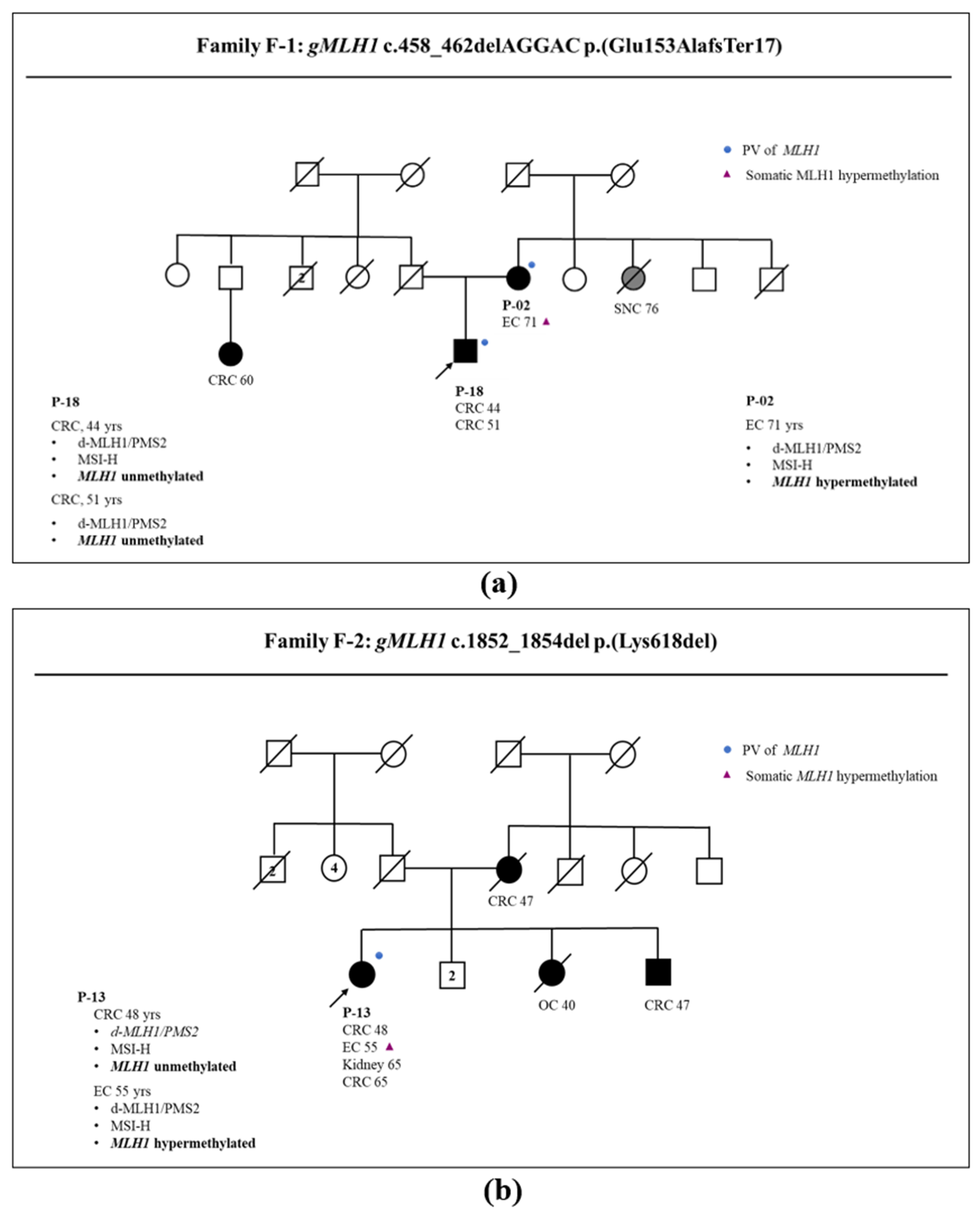
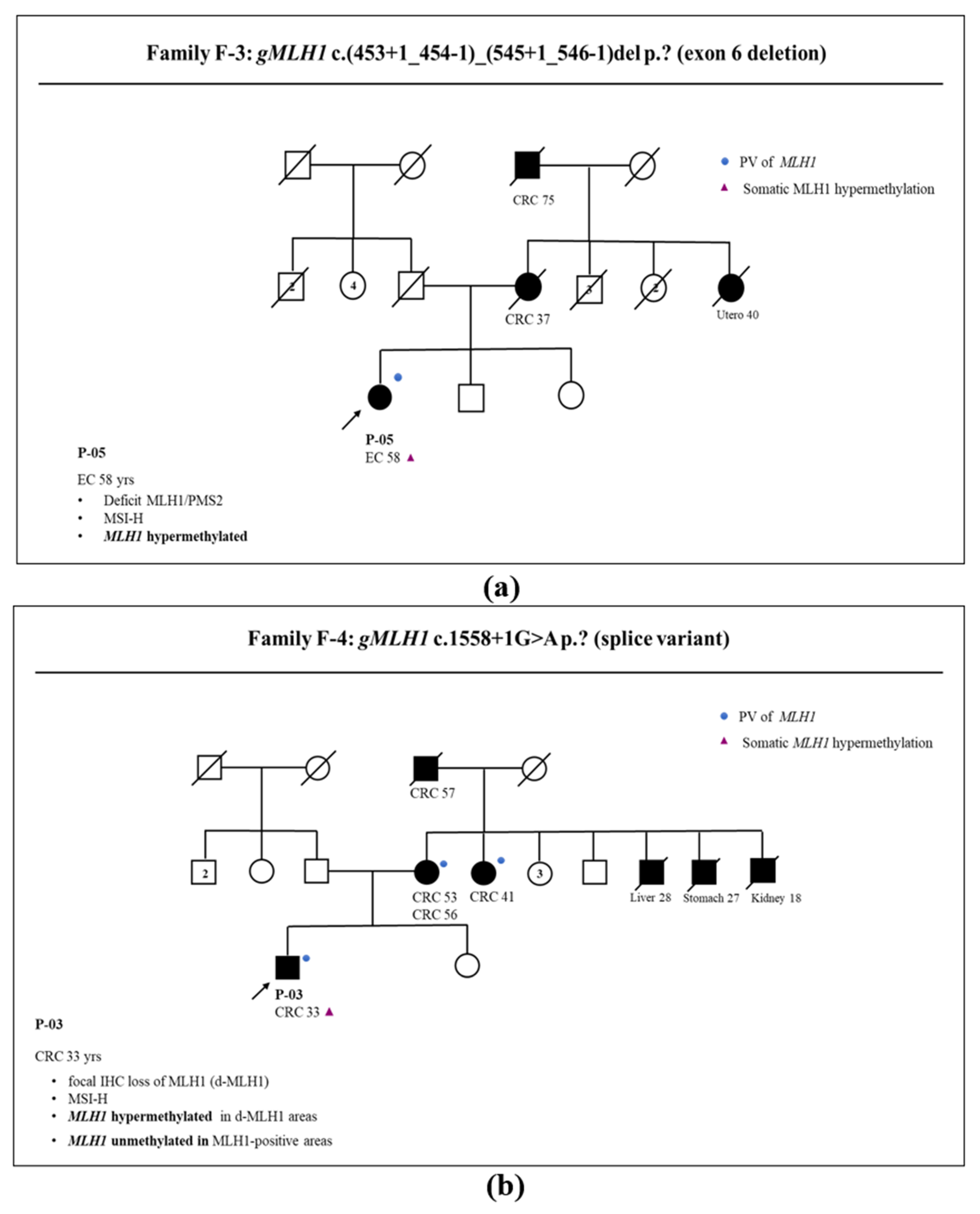
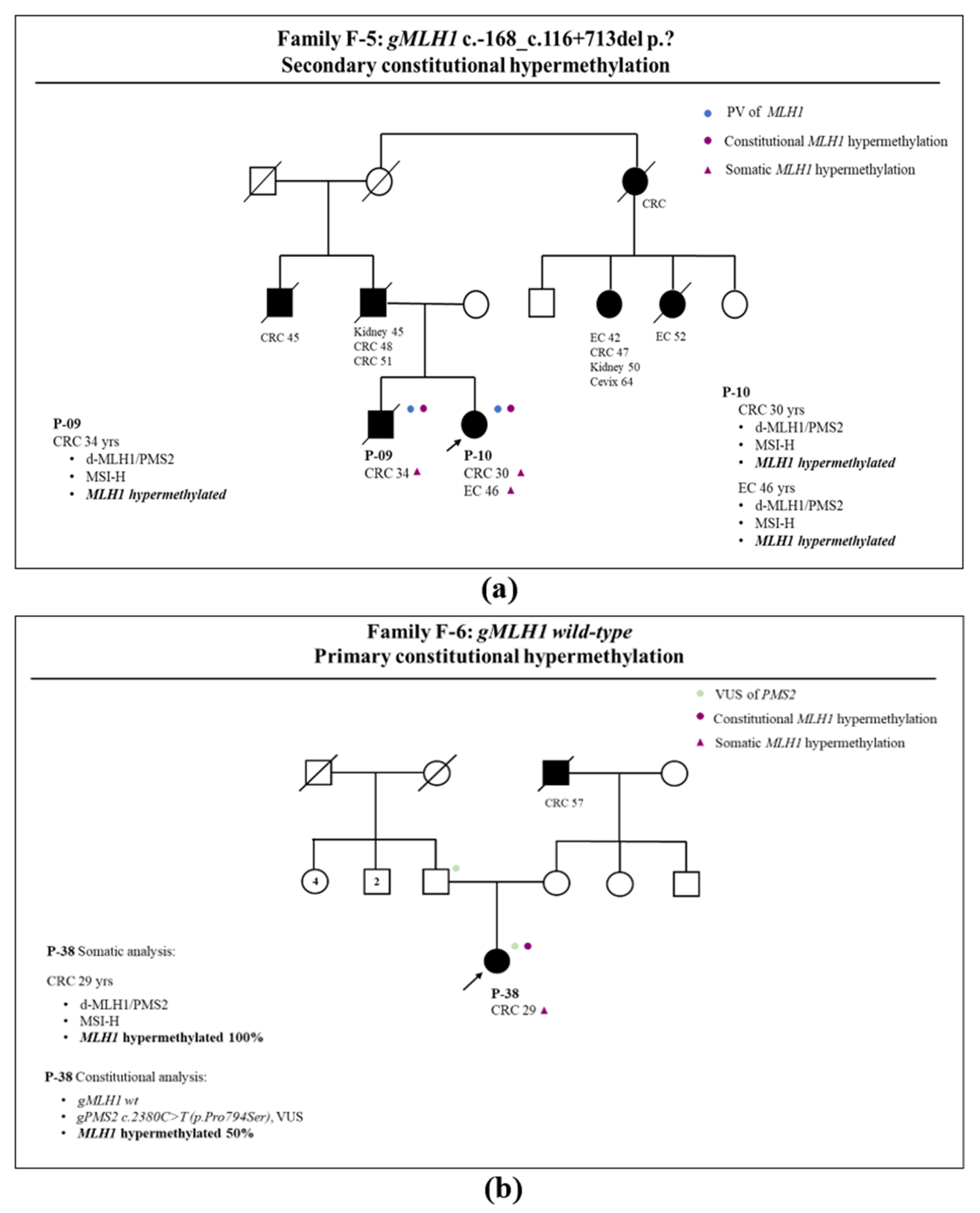
3.1. Somatic and Germline Analysis
3.2. Correlation between Somatic Methylation and Germline Variants of MLH1 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists (ACOG) Committee. Hereditary Cancer Syndromes and Risk Assessment: ACOG Committee Opinion Summary, Number 793. Obstet. Gynecol. 2019, 134, 1366–1367. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Eisenberg, R.; Vnencak-Jones, C.L. Differences in Microsatellite Instability Profiles between Endometrioid and Colorectal Cancers: A Potential Cause for False-Negative Results? J. Mol. Diagn. 2017, 19, 57–64. [Google Scholar] [CrossRef]
- Deng, G.; Bell, I.; Crawley, S.; Gum, J.; Terdiman, J.P.; Allen, B.A.; Truta, B.; Sleisenger, M.H.; Kim, Y.S. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin. Cancer Res. 2004, 10, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Tibiletti, M.G.; Carnevali, I.; Calò, V.; Cini, G.; Lucci Cordisco, E.; Remo, A.; Urso, E.; Oliani, C.; Ranzani, G.N. Universal testing for MSI/MMR status in colorectal and endometrial cancers to identify Lynch syndrome cases: State of the art in Italy and consensus recommendations from the Italian Association for the Study of Familial Gastrointestinal Tumors (A.I.F.E.G.). Eur. J. Cancer Prev. 2022, 31, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Ryan, N.A.J.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, M.B.; Waring, P.M.; Tan, A.; Trivett, M.; Kovalenko, S.; Beshay, V.; Young, M.A.; McArthur, G.; Boussioutas, A.; Dobrovic, A. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam. Cancer 2007, 6, 301–310. [Google Scholar] [CrossRef]
- Weissman, S.M.; Burt, R.; Church, J.; Erdman, S.; Hampel, H.; Holter, S.; Jasperson, K.; Kalady, M.F.; Haidle, J.L.; Lynch, H.T.; et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J. Genet. Couns. 2012, 21, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Newland, A.; National Institute for Health and Care Excellence (NICE) Committiee. Molecular Testing Strategies for Lynch Syndrome in People with Colorectal Cancer (DG27). Available online: https://www.nice.org.uk/guidance/dg27 (accessed on 2 November 2023).
- Yokoyama, T.; Takehara, K.; Sugimoto, N.; Kaneko, K.; Fujimoto, E.; Okazawa-Sakai, M.; Okame, S.; Shiroyama, Y.; Teramoto, N.; Ohsumi, S.; et al. Lynch syndrome-associated endometrial carcinoma with MLH1 germline mutation and MLH1 promoter hypermethylation: A case report and literature review. BMC Cancer 2018, 18, 576. [Google Scholar] [CrossRef]
- Hagen, C.E.; Lefferts, J.; Hornick, J.L.; Srivastava, A. “Null pattern” of immunoreactivity in a Lynch syndrome-associated colon cancer due to germline MSH2 mutation and somatic MLH1 hypermethylation. Am. J. Surg. Pathol. 2011, 35, 1902–1905. [Google Scholar] [CrossRef]
- Rahner, N.; Friedrichs, N.; Steinke, V.; Aretz, S.; Friedl, W.; Buettner, R.; Mangold, E.; Propping, P.; Walldorf, C. Coexisting somatic promoter hypermethylation and pathogenic MLH1 germline mutation in Lynch syndrome. J. Pathol. 2008, 214, 10–16. [Google Scholar] [CrossRef]
- Peltomäki, P. Update on Lynch syndrome genomics. Fam. Cancer 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Hitchins, M.P. The role of epigenetics in Lynch syndrome. Fam. Cancer 2013, 12, 189–205. [Google Scholar] [CrossRef]
- Ward, R.L.; Dobbins, T.; Lindor, N.M.; Rapkins, R.W.; Hitchins, M.P. Identification of constitutional MLH1 epimutations and promoter variants in colorectal cancer patients from the Colon Cancer Family Registry. Genet. Med. 2013, 15, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, J.; Flament, C.; Lovecchio, T.; Delattre, L.; Ait Yahya, E.; Baert-Desurmont, S.; Burnichon, N.; Bronner, M.; Cabaret, O.; Lejeune, S.; et al. Diversity of genetic events associated with MLH1 promoter methylation in Lynch syndrome families with heritable constitutional epimutation. Genet. Med. 2018, 20, 1589–1599. [Google Scholar] [CrossRef]
- Moreira, L.; Muñoz, J.; Cuatrecasas, M.; Quintanilla, I.; Leoz, M.L.; Carballal, S.; Ocaña, T.; López-Cerón, M.; Pellise, M.; Castellví-Bel, S.; et al. Prevalence of somatic mutl homolog 1 promoter hypermethylation in Lynch syndrome colorectal cancer. Cancer 2015, 121, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Gupta, S.; Burke, C.A.; Axell, L.; Chen, L.M.; Chung, D.C.; Clayback, K.M.; Dallas, S.; Felder, S.; Gbolahan, O.; et al. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 1122–1132. [Google Scholar] [CrossRef]
- Balmaña, J.; Balaguer, F.; Cervantes, A.; Arnold, D.; Group, E.G.W. Familial risk-colorectal cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2013, 24 (Suppl. S6), vi73–vi80. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Pothuri, B.; Arend, R.C.; Backes, F.J.; Gehrig, P.A.; Soliman, P.T.; Thompson, J.S.; Urban, R.R.; Burke, W.M. Endometrial cancer: A society of gynecologic oncology evidence-based review and recommendations. Gynecol. Oncol. 2021, 160, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Cini, G.; Carnevali, I.; Quaia, M.; Chiaravalli, A.M.; Sala, P.; Giacomini, E.; Maestro, R.; Tibiletti, M.G.; Viel, A. Concomitant mutation and epimutation of the MLH1 gene in a Lynch syndrome family. Carcinogenesis 2015, 36, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Committee, I.V.I. InSiGHT. Available online: www.insight-group.org (accessed on 2 November 2023).
- Nakad Borrego, S.; Kurnit, K.; Turner, L.J.; Broaddus, R.R. Context-dependent environmental associations with endometrial cancer histotype and genotype. Int. J. Gynecol. Cancer 2023, 33, 1215–1221. [Google Scholar] [CrossRef]
- Laskov, I.; Zilberman, A.; Maltz-Yacobi, L.; Peleg Hasson, S.; Cohen, A.; Safra, T.; Grisaru, D.; Michaan, N. Effect of BMI change on recurrence risk in patients with endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 713–718. [Google Scholar] [CrossRef]
- Vasen, H.F.; Blanco, I.; Aktan-Collan, K.; Gopie, J.P.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Burn, J.; Capella, G.; et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut 2013, 62, 812–823. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnevali, I.W.; Cini, G.; Libera, L.; Sahnane, N.; Facchi, S.; Viel, A.; Sessa, F.; Tibiletti, M.G. MLH1 Promoter Methylation Could Be the Second Hit in Lynch Syndrome Carcinogenesis. Genes 2023, 14, 2060. https://doi.org/10.3390/genes14112060
Carnevali IW, Cini G, Libera L, Sahnane N, Facchi S, Viel A, Sessa F, Tibiletti MG. MLH1 Promoter Methylation Could Be the Second Hit in Lynch Syndrome Carcinogenesis. Genes. 2023; 14(11):2060. https://doi.org/10.3390/genes14112060
Chicago/Turabian StyleCarnevali, Ileana Wanda, Giulia Cini, Laura Libera, Nora Sahnane, Sofia Facchi, Alessandra Viel, Fausto Sessa, and Maria Grazia Tibiletti. 2023. "MLH1 Promoter Methylation Could Be the Second Hit in Lynch Syndrome Carcinogenesis" Genes 14, no. 11: 2060. https://doi.org/10.3390/genes14112060
APA StyleCarnevali, I. W., Cini, G., Libera, L., Sahnane, N., Facchi, S., Viel, A., Sessa, F., & Tibiletti, M. G. (2023). MLH1 Promoter Methylation Could Be the Second Hit in Lynch Syndrome Carcinogenesis. Genes, 14(11), 2060. https://doi.org/10.3390/genes14112060





