Generation of Mutants from the 57B Region of Drosophila melanogaster
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strains
- y[1] w[*]; P{w[+mC]=Act5C-GAL4}17bFO1/TM6B, Tb[1] (BL 3954);
- y[1] w[1118]; P{ry[+t7.2]=70FLP}23 P{v[+t1.8]=70I-SceI}4A/TM3, Sb[1] Ser[1] (BL 6935);
- w|1118]; Df(2R)Exel6072, P{w[+mC]=XP-U}Exel6073/CyO (BL 7554);
- w[1118]; Df(2R)Exel7166, P+PBac{XP5.WH5}Exel7166/CyO (BL 7998);
- w[1118]; wg[SP-1]/CyO; sens[Ly-1]/TM6B, Tb[1] (BL 8136);
- P{y[+t7.7]=nos-phiC31\int.NLS}X, y[1] sc[1] v[1] sev[21]; P{y[+t7.7]=CaryP}attP2 (BL 25710);
- y [1] M{w[+mC]=nos-Cas9.P}ZH-2A w[*] (BL 54591);
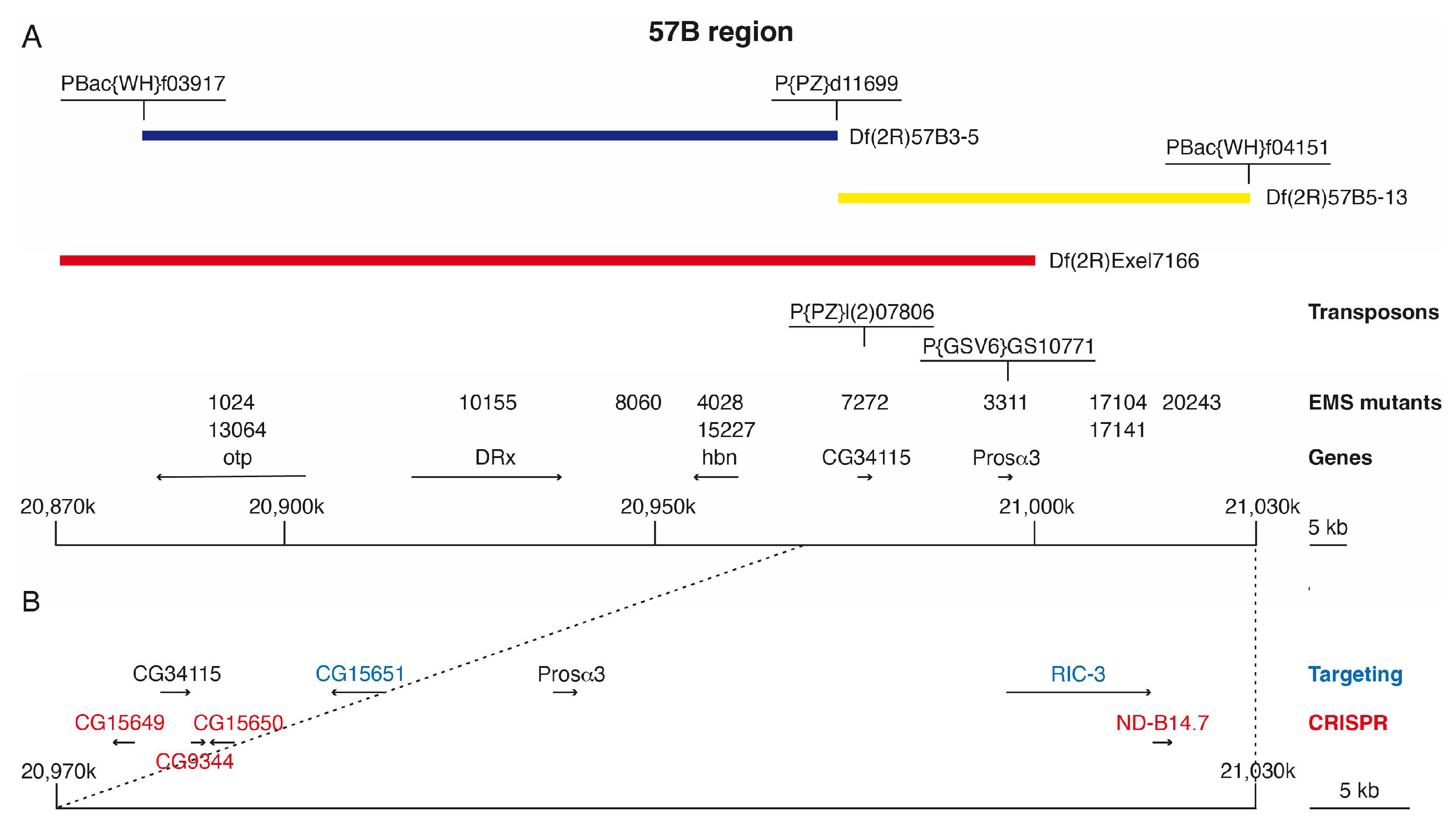
- P{PZ}l(2)07206 (21,057,826) [48];
- P{PZ}l(2)07806 (20,975,585) (BL 12342);
- P{GSV6}GS10771 (20,995,922) (No 202-812 Drosophila Genetic Resource Center, Kyoto);
- PBac{WH}f03917 (20,882,698),
- P{PZ}d11699 (20,975,379),
- PBac{WH}f04151 (21,030,314) (Exelixis Drosophila stock collection, Harvard Medical School).
- CG15649:
- w[1118]; P{GD15200}v42358/TM3 (VDRC 42358)
- P{KK113097}VIE-260B (VDRC 103443)
- CG15650:
- y[1]v[1]; P{TRIP.HMJ22721}attP40 (BL 60430)
- w[1118]; P{GD5930}v13872 (VDRC 13872)
- P{KK107072}VIE-260B (VDRC 101188)
- CG15651:
- y[1]v[1]; P{TRIP.JF03295}attP2 (BL 29616)
- y[1]v[1]; P{TRIP.HMC03463}attP40 (BL 51889)
- w[1118]; P{GD2117}v37206
- P{KK101592}VIE-260B (VDRC 105421)
- ND-B14.7:
- w[1118]; P{GD4517}v31562
- P{KK109518}VIE-260B (VDRC 110295)
- CG9344:
- y[1]v[1]; P{TRIP.HM05211}attP2 (BL 29532)
- y[1]v[1]; P{TRIP.HMJ21492}attP40 (BL 54798)
- w[1118]; P{GD13733}v23689 (VDRC 23689)
2.2. Generation of 57B Deletion Strains
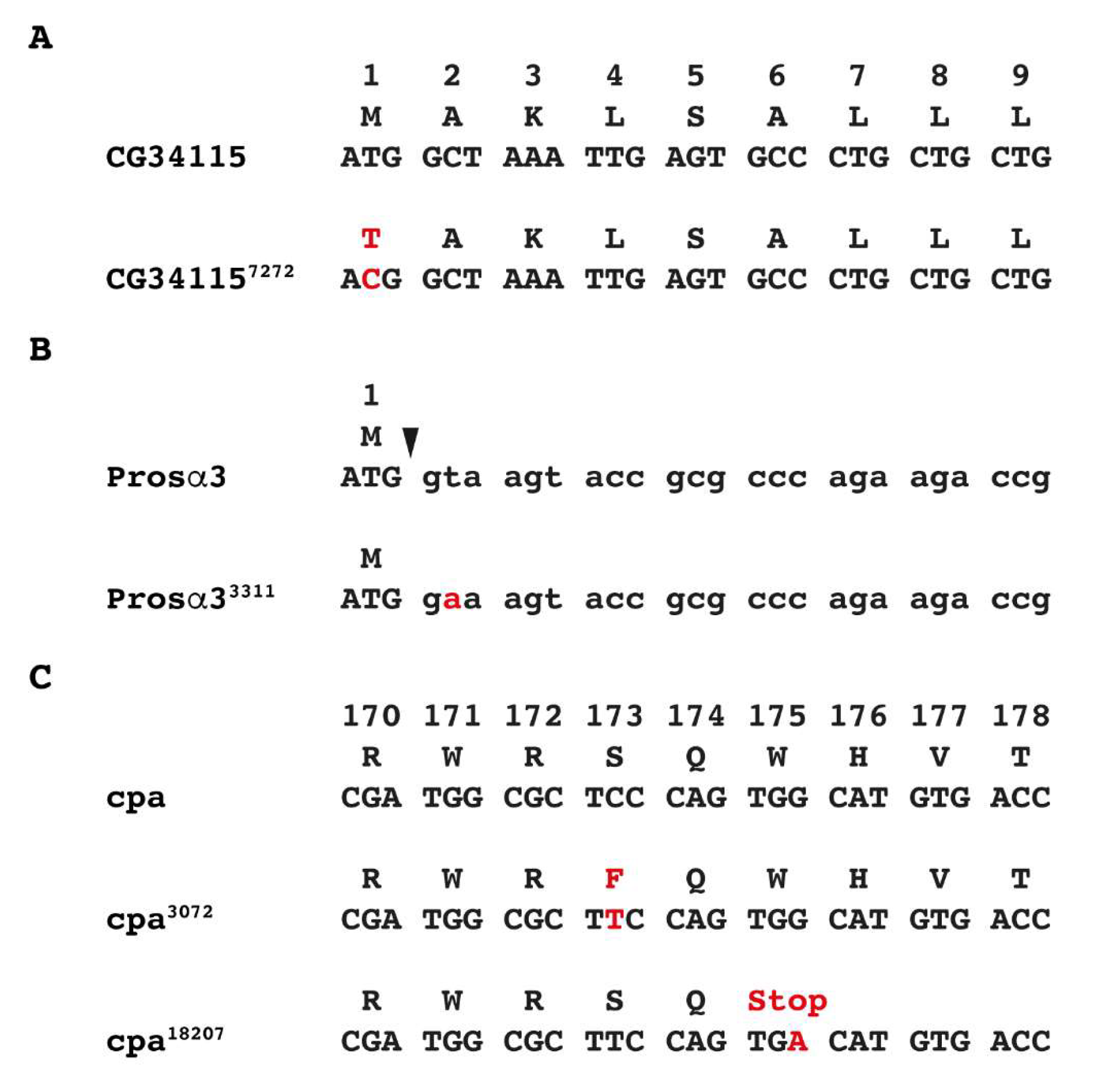
2.3. Sequence Analysis of Mutant Strains with Point Mutations
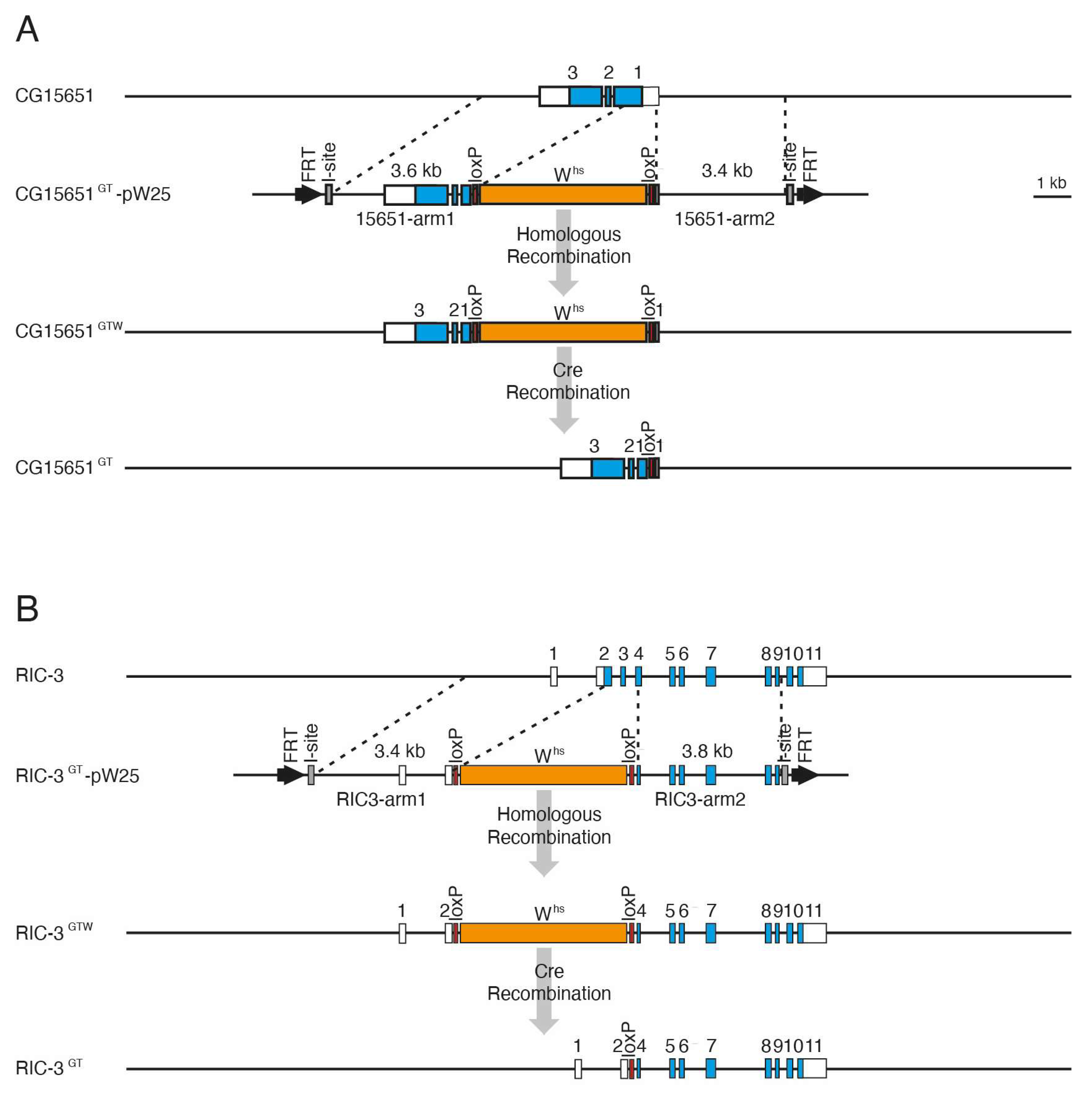
2.4. Generation of CG15651 and RIC-3 Mutants by Ends-Out Homologous Recombination
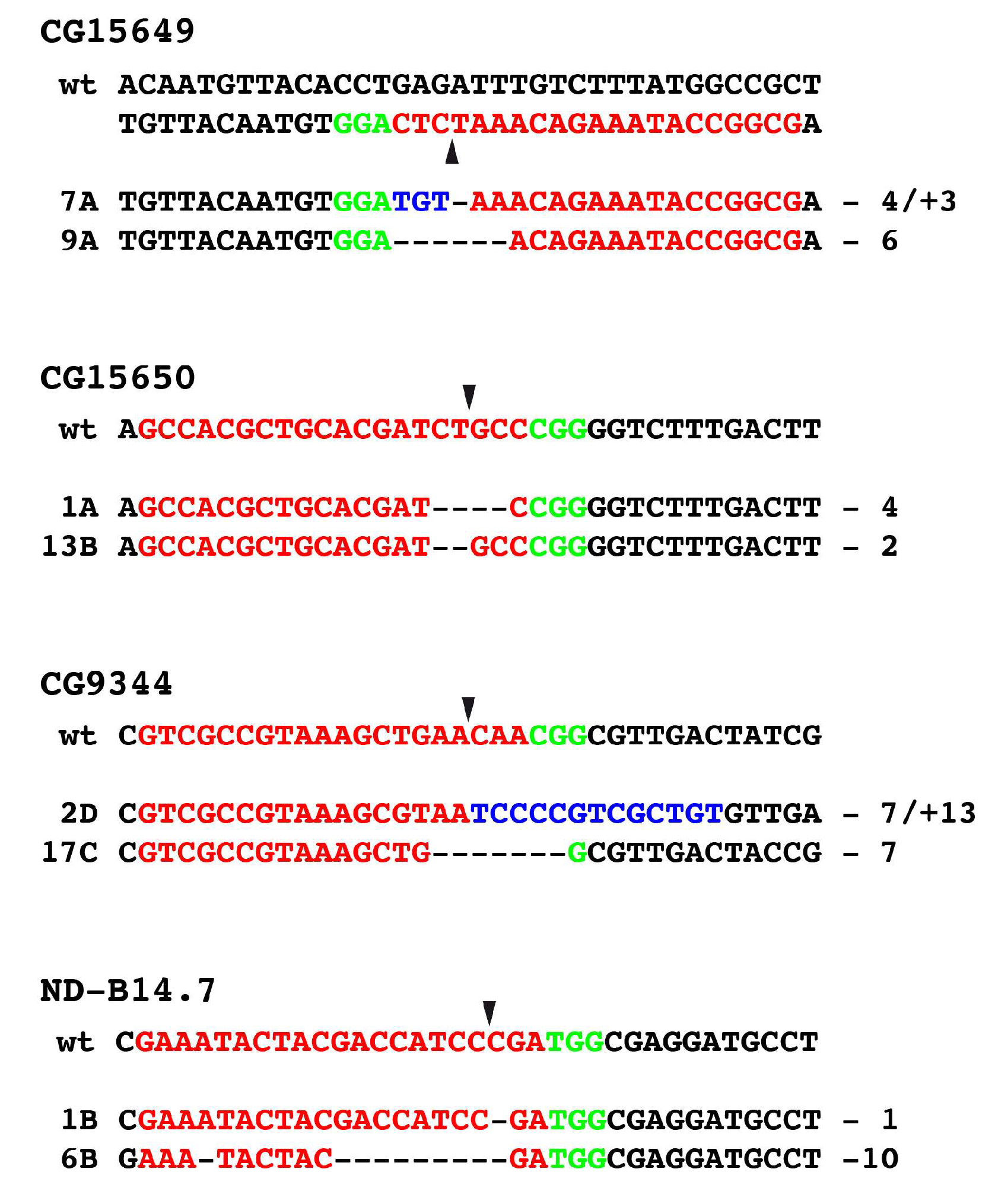
2.5. gRNA Design, Cloning of CRISPR Constructs, and Analysis of CRISPR/Cas9-Induced Mutations
3. Results
3.1. Isolation of 57B Mutants and Rough Mapping Using Newly Generated 57B Deletions
3.2. Identification of EMS-Induced Mutants from the 57B Region
3.3. Generation of CG15651 and RIC-3 Mutants by Gene Targeting
3.4. Generation of Mutants Using CRISPR/Cas9
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDPD | Berkeley Drosophila Genome Project |
| cDNA | complementary desoxyribonucleic acid |
| VDRC | Vienna Drosophila Resource Center |
References
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Celniker, S.E.; Wheeler, D.A.; Kronmiller, B.; Carlson, J.W.; Halpern, A.; Patel, S.; Adams, M.; Champe, M.; Dugan, S.P.; Frise, E.; et al. Finishing a whole-genome shotgun: Release 3 of the Drosophila melanogaster euchromatic genome sequence. Genome Biol. 2002, 3, research0079.1. [Google Scholar] [CrossRef] [PubMed]
- Drosophila 12 Genomes Consortium. Evolution of genes and genomes on the Drosophila phylogeny. Nature 2007, 450, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Dietzl, G.; Chen, D.; Schnorrer, F.; Su, K.C.; Barinova, Y.; Fellner, M.; Gasser, B.; Kinsey, K.; Oppel, S.; Scheiblauer, S.; et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448, 151–156. [Google Scholar] [CrossRef]
- Perkins, L.A.; Holderbaum, L.; Tao, R.; Hu, Y.; Sopko, R.; McCall, K.; Yang-Zhou, D.; Flockhart, I.; Binari, R.; Shim, H.S.; et al. The transgenic RNAi project at Harvard medical school: Resources and validation. Genetics 2015, 201, 843–852. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Pfeiffer, B.D.; Jenett, A.; Hammonds, A.S.; Ngo, T.T.; Misra, S.; Murphy, C.; Scully, A.; Carlson, J.W.; Wan, K.H.; Laverty, T.R.; et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 9715–9720. [Google Scholar] [CrossRef]
- Jenett, A.; Rubin, G.M.; Ngo, T.-T.B.; Shepherd, D.; Murphy, C.; Dionne, H.; Pfeiffer, D.B.; Cavallaro, A.; Hall, D.; Jeter, J.; et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012, 2, 991–1001. [Google Scholar] [CrossRef]
- Jory, A.; Estella, C.; Giorgianni, M.W.; Slattery, M.; Laverty, T.R.; Rubin, G.M.; Mann, R.S. A survey of 6,300 genomic fragments for cis-regulatory activity in the imaginal discs of Drosophila melanogaster. Cell Rep. 2012, 2, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.; Heckscher, E.S.; Purice, M.D.; Roberts, J.; Bennett, A.L.; Kroll, J.R.; Pollard, J.L.; Strader, M.E.; Lupton, J.R.; Dyukareva, A.V.; et al. A resource for manipulating gene expression and analyzing cis-regulatory modules in the Drosophila CNS. Cell Rep. 2012, 2, 1002–1013. [Google Scholar] [CrossRef]
- Kvon, E.Z.; Kazmar, T.; Stampfel, G.; Yánez-Cuna, J.O.; Pagani, M.; Schernhuber, K.; Dickson, B.J.; Stark, A. Genome-scale functional characterization of Drosophila developmental enhancers in vivo. Nature 2014, 512, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Callaerts, P.; Gehring, W.J. New perspectives on eye evolution. Curr. Opin. Genet. Dev. 1995, 5, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.; Björklund, M.; Furger, E.; Schertel, C.; Taipale, J.; Basler, K. A versatile platform for creating a comprehensive UAS-ORFeome library in Drosophila. Development 2013, 140, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.; Sheils, E.M.; Björklund, M.; Basler, K. Generation of a transgenic ORFeome library in Drosophila. Nat. Protoc. 2014, 9, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.B.; Bacher, F. Methods of feeding ethyl methane sulfonate (EMS) to Drosophila males. Drosoph. Inf. Serv. 1968, 43, 193. [Google Scholar]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef]
- Jürgens, G.; Wieschaus, E.; Nüsslein-Volhard, C.; Kluding, H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster: II. Zygotic loci on the third chromosome. Wilhelm Roux Arch. Dev. Biol. 1984, 193, 283–285. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Wieschaus, E.; Kluding, H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilhelm Roux Arch. Dev. Biol. 1984, 193, 267–282. [Google Scholar] [CrossRef]
- Wieschaus, E.; Nüsslein-Volhard, C.; Jürgens, G. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. III. Zygotic loci on the X-chromosome and fourth chromosome. Wilhelm Roux Arch. Dev. Biol. 1984, 193, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Roote, J.; Russell, S. Toward a complete Drosophila deficiency kit. Genome Biol. 2012, 13, 149. [Google Scholar] [CrossRef]
- Parks, A.L.; Cook, K.R.; Belvin, M.; Dompe, N.A.; Fawcett, R.; Huppert, K.; Tan, L.R.; Winter, C.G.; Bogart, K.P.; Deal, J.E.; et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 2004, 36, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Thibault, S.T.; Singer, M.A.; Miyazaki, W.Y.; Milash, B.; Dompe, N.A.; Singh, C.M.; Buchholz, R.; Demsky, M.; Fawcett, R.; Francis-Lang, H.L.; et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 2004, 36, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Spradling, A.C. Insertional mutagenesis of Drosophila heterochromatin with single P elements. Proc. Natl. Acad. Sci. USA 1994, 91, 3539–3543. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Gerber, A.S.; Hartl, D.L. Genetic applications of an inverse polymerase chain reaction. Genetics 1988, 120, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, M.R. The origin and evolution of gene targeting. Dev. Biol. 2022, 481, 179–187. [Google Scholar] [CrossRef]
- Thomas, K.R.; Capecchi, M.R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 1990, 346, 847–850. [Google Scholar] [CrossRef]
- Leighton, P.A.; Saam, J.R.; Ingram, R.S.; Stewart, C.L.; Tilghman, S.M. An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 1995, 9, 2079–2089. [Google Scholar] [CrossRef]
- Rong, Y.S.; Golic, K.G. Gene targeting by homologous recombination in Drosophila. Science 2000, 288, 2013–2018. [Google Scholar] [CrossRef]
- Rong, Y.S.; Golic, K.G. A targeted gene knockout in Drosophila. Genetics 2001, 157, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.J.; Golic, K.G. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA 2003, 100, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Tibbit, C.; Ponting, C.P.; Liu, J.L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013, 4, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Ueda, R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 2013, 195, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ren, M.; Wang, Z.; Zhang, B.; Rong, Y.S.; Jiao, R.; Gao, G. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics 2013, 195, 289–291. [Google Scholar] [CrossRef]
- Simeone, A.; D’Apice, M.R.; Nigro, V.; Casanova, J.; Graziani, F.; Acampora, D.; Avantaggiato, V. Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron 1994, 13, 83–101. [Google Scholar] [CrossRef]
- Hildebrandt, K.; Bach, N.; Kolb, D.; Walldorf, U. The homeodomain transcription factor Orthopedia is involved in development of the Drosophila hindgut. Hereditas 2020, 157, 46. [Google Scholar] [CrossRef]
- Eggert, T.; Hauck, B.; Hildebrandt, N.; Gehring, W.J.; Walldorf, U. Isolation of a Drosophila homolog of the vertebrate homeobox gene Rx and its possible role in brain and eye development. Proc. Natl. Acad. Sci. USA 1998, 95, 2343–2348. [Google Scholar] [CrossRef]
- Davis, R.J.; Tavsanli, B.C.; Dittrich, C.; Walldorf, U.; Mardon, G. Drosophila retinal homeobox (drx) is not required for establishment of the visual system, but is required for brain and clypeus development. Dev. Biol. 2003, 259, 272–287. [Google Scholar] [CrossRef]
- Walldorf, U.; Kiewe, A.; Wickert, M.; Ronshaugen, M.; McGinnis, W. Homeobrain, a novel paired-like homeobox gene is expressed in the Drosophila brain. Mech. Dev. 2000, 96, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Kolb, D.; Kaspar, P.; Klöppel, C.; Walldorf, U. The Drosophila homeodomain transcription factor Homeobrain is involved in the formation of the embryonic protocerebrum and the supraesophageal brain commissure. Cells Dev. 2021, 165, 203657. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, C.; Hildebrandt, K.; Kolb, D.; Fürst, N.; Bley, I.; Karlowatz, R.J.; Walldorf, U. Functional analysis of enhancer elements regulating the expression of the Drosophila homeodomain transcription factor DRx by gene targeting. Hereditas 2021, 158, 42. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, K.; Kolb, D.; Klöppel, C.; Kaspar, P.; Wittling, F.; Hartwig, O.; Federspiel, J.; Findji, I.; Walldorf, U. Regulatory modules mediating the complex neural expression patterns of the homeobrain gene during Drosophila brain development. Hereditas 2022, 159, 2. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, K.; Klöppel, C.; Gogel, J.; Hartenstein, V.; Walldorf, U. Orthopedia expression during Drosophila nervous system development and its regulation by micro-RNA-252. Dev. Biol. 2022, 492, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.F.; Massey, E.M.; Kolb, D.; Walldorf, U.; Urbach, R. Retinal homeobox promotes cell growth, proliferation and survival of mushroom body neuroblasts in the Drosophila brain. Mech. Dev. 2016, 142, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Spradling, A.C.; Stern, D.; Beaton, A.; Rhem, E.J.; Laverty, T.; Mozden, N.; Misra, S.; Rubin, G.M. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 1999, 153, 135–177. [Google Scholar] [CrossRef]
- Venken, K.J.T.; Carlson, J.W.; Schulze, K.L.; Pan, H.; He, Y.; Spokony, R.; Wan, K.H.; Koriabine, M.; de Jong, P.J.; White, K.P.; et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature Methods 2009, 6, 431–434. [Google Scholar] [CrossRef]
- Rubin, G.M.; Spradling, A.C. Genetic transformation of Drosophila with transposable element vectors. Science 1982, 218, 348–353. [Google Scholar] [CrossRef]
- Gratz, S.J.; Ukken, F.P.; Rubinstein, C.D.; Thiede, G.; Donohue, L.K.; Cummings, A.M.; O’Connor-Giles, K.M. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 2014, 196, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Port, F.; Chen, H.M.; Lee, T.; Bullock, S.L. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, E2967–E2976. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://flycrispr.org/target-finder (accessed on 1 April 2016).
- Bischof, J.; Maeda, R.K.; Hediger, M.; Karch, F.; Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 2007, 104, 3312–3317. [Google Scholar] [CrossRef]
- Uemera, T.; Oda, H.; Kraut, R.; Hayashi, S.; Kotaoka, Y.; Takeichi, M. Zygotic Drosophila E-cadherin expression is required for process of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996, 10, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Hazelrigg, T.; Watkins, W.S.; Marcey, D.; Tu, C.; Karow, M.; Lin, X.R. The exuperantia gene is required for Drosophila spermatogenesis as well as anteroposterior polarity of the developing oocyte, and encodes overlapping sex-specific transcripts. Genetics 1990, 126, 607–617. [Google Scholar] [CrossRef]
- Kraut, R.; Campos Ortega, J.A. inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 1996, 174, 65–81. [Google Scholar] [CrossRef]
- Grigliati, T. Drosophila—A Practical Approach; Rickwood, D., Hames, B.D., Eds.; IRL Press: Oxford, UK, 1986; pp. 39–58. [Google Scholar]
- Tepass, U.; Gruszynski-DeFeo, E.; Haag, T.A.; Omatyar, L.; Török, T.; Hartenstein, V. Shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996, 10, 672–685. [Google Scholar] [CrossRef]
- Haass, C.; Pesold-Hurt, B.; Kloetzel, P.M. The Drosophila PROS-29 gene is a new member of the PROS-gene family. Nucleic Acids Res. 1990, 18, 4018. [Google Scholar] [CrossRef]
- Sheth, N.; Roca, X.; Hastings, M.L.; Roeder, T.; Krainer, A.R.; Sachidanandam, R. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006, 34, 3955–3967. [Google Scholar] [CrossRef]
- Halevi, S.; Yassin, L.; Eshel, M.; Sala, F.; Sala, S.; Treinin, M. Conservation within the RIC-3 Gene Family. J. Biol. Chem. 2003, 278, 34411–34417. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Collins, T.; Yabe, A.; Gee, V.J.; Gibb, A.J.; Millar, N.S. Host-cell specific aspects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologues. J. Neurochem. 2008, 105, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Halevi, S.; McKay, J.; Palfreyman, M.; Yassin, L.; Eshel, M.; Jorgensen, E.; Treinin, M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002, 5, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.M.; van der Zwaag, B.; Jongbloed, J.D.H.; Vogel, M.J.; Brüggenwirth, H.T.; Lekanne Deprez, R.H.; Mook, O.; Ruivenkamp, C.A.L.; van Slegtenhorst, M.A.; van den Wijngaard, A.; et al. Best practice guidelines for the use of next-generation sequencing applications in genome diagnostics: A national collaborative study of Dutch genome diagnostic laboratories. Hum. Mutat. 2013, 34, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Sobol, R.W. Mutation research/fundamental and molecular mechanisms of mutagenesis: Special issue: DNA repair and genetic instability. Mutat. Res. 2013, 743–744, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wieschaus, E.; Nüsslein-Volhard, C. The Heidelberg Screen for Pattern Mutants of Drosophila: A Personal Account. Annu. Rev. Cell Dev. Biol. 2016, 32, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.C. A short history and description of Drosophila melanogaster Classical Genetics: Chromosome Aberrations, Forward Genetic Screens, and the Nature of Mutations. Genetics 2017, 206, 665–689. [Google Scholar] [CrossRef]
- Tamura, T.; Lee, D.H.; Osaka, F.; Fujiwara, T.; Shin, S.; Chung, C.H.; Tanaka, K.; Ichihara, A. Molecular cloning and sequence analysis of cDNAs for five major subunits of human proteasomes (multi-catalytic proteinase complexes). Biochim. Biophys. Acta 1991, 1089, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Coleman, Z.; Curley, M.; Nityanandam, A.; Platt, A.; Robles-Murguia, M.; Jiao, J.; Finkelstein, D.; Wang, Y.-D.; Xu, B. Proteasome stress in skeletal muscle mounts a long-range protective response that delays retinal and brain aging. Cell Metab. 2021, 33, 1137–1154. [Google Scholar] [CrossRef]
- Delalle, I.; Pfleger, C.M.; Buff, E.; Lueras, P.; Hariharan, I. Mutations in the Drosophila orthologs of the F-actin capping portein a- and b-subunits cause actin accumulation and subsequent retinal degeneration. Genetics 2005, 171, 1757–1765. [Google Scholar] [CrossRef]
- Yamashita, A.; Maeda, K.; Maeda, Y. Crystal structure of CapZ: Structural basis for actin filament barbed end capping. EMBO J. 2003, 22, 1529–1538. [Google Scholar] [CrossRef]
- Baena-Lopez, L.A.; Alexandre, C.; Mitchell, A.; Pasakarnis, L.; Vincent, J.-P. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development 2013, 140, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Golden, K.L.; Lee, C.-Y. dFezf/earmuff maintains the restricted developmental potential of intermediate neural progenitors in Drosophila. Dev. Cell 2010, 18, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, K.; Kübel, S.; Minet, M.; Fürst, N.; Klöppel, C.; Steinmetz, E.; Walldorf, U. Enhancer analysis of the Drosophila zinc finger transcription factor Earmuff by gene targeting. Hereditas 2021, 158, 41. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hino, M.; Yoshida, H.; Ichimiya, T.; Maeda, M.; Kimura, Y.; Sasaki, N.; Aoki-Kinoshita, K.F.; Kinoshita-Toyoda, A.; Toyoda, H.; Ueda, R.; et al. Phenotype-based clustering of glycosylation-related genes by RNAi-mediated gene silencing. Genes Cells 2015, 20, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, E.; Gai, Y.; Farkas, C.; Richter, D.; Nicholas, E.; Keleman, K.; Davis, R.L. Identification of genes that promote or inhibit olfactory memory formation in Drosophila. Genetics 2015, 199, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- FlyBase. Computation of D. melanogaster Genes Relevant to Disease Based on Their Orthology to Human ‘Disease Genes’. 2019. Available online: http://flybase.org (accessed on 1 September 2023).
- Ben-David, Y.; Mizrachi, T.; Kagan, S.; Krisher, T.; Cohen, E.; Brenner, T.; Treinin, M. RIC-3 expression and splicing regulate nAChR functional expression. Mol. Brain 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Ito, R.; Yamamoto, H.; Otsubo, S.; Matsumoto, R.; Ojima, H.; Komori, Y.; Matsuda, K.; Ihara, M. Effects of cofactors RIC-3, TMX3 and UNC-50, together with distinct subunit ratios on the agonist actions of imidacloprid on Drosophila melanogaster Da1/Db1 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Pestic. Biochem. Physiol. 2022, 187, 105177. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.M.; Henser-Brownhill, T.; Monserrat, J.; Poetsch, A.R.; Luscombe, N.M.; Scaffidi, P. Target-Specific Precision of CRISPR-Mediated Genome Editing. Mol. Cell 2019, 73, 699–713.e6. [Google Scholar] [CrossRef]
- Neely, G.G.; Hess, A.; Costigan, M.; Keene, A.C.; Goulas, S.; Langeslag, M.; Griffin, R.S.; Belfer, I.; Dai, F.; Smith, S.B.; et al. A genome-wide Drosophila screen for heat nociception identifies a3d3 as an evolutionary conserved pain gene. Cell 2010, 143, 628–638. [Google Scholar] [CrossRef]
- Hosono, C.; Matsuda, R.; Adryan, B.; Samakovlis, C. Transient junction anisotropies orient annular cell polarization in the Drosophila airway tubes. Nat. Cell Biol. 2015, 17, 1569–1576. [Google Scholar] [CrossRef]
- Loop, T.; Leemans, R.; Stiefel, U.; Hermida, L.; Egger, B.; Xie, F.; Primig, F.; Certa, U.; Fischbach, K.F.; Reichert, H.; et al. Transcriptional signature of an adult brain tumor in Drosophila. BMC Genom. 2004, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Mount, S.M.; Salz, H.K. Pre-messenger RNA-processing factors in the Drosophila genome. J. Cell Biol. 2000, 150, F37–F43. [Google Scholar] [CrossRef] [PubMed]
- Guruharsha, K.G.; Rual, J.-F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, D.; Barsanti, P.; Pesole, G.; Caggese, C. The nuclear OXPHOS genes in insecta: A common evolutionary origin, a common cis-regulatory motif, a common destiny for gene duplicates. BMC Evol. Biol. 2007, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Rhooms, S.-K.; Murari, A.; Copuraju, N.S.V.; Vilanueva, M.; Owusu-Ansah, E. Insights from Drosophila on mitochondrial complex I. Cell. Mol. Life Sci. 2020, 77, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Agip, A.-N.A.; Chung, I.; Sanchez-Martinez, A.; Whitworth, A.J.; Hirst, J. Cryo-EM structures of mitochondrial respiratory complex I from Drosophila melanogaster. eLife 2023, 12, e84424. [Google Scholar] [CrossRef]
- Andrews, B.; Carroll, J.; Ding, S.; Walker, J.E. Assembly factors for the membrane arm of human complex I. Proc. Natl. Acad. Sci. USA 2013, 110, 18394–18939. [Google Scholar] [CrossRef]
- Stroud, D.A.; Surgenor, E.E.; Formosa, L.E.; Reljic, B.; Frazier, A.E.; Dibley, M.G.; Osellame, L.D.; Stait, T.; Beilharz, T.H.; Thorburn, D.R.; et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef]
- Port, F.; Strein, C.; Stricker, M.; Rauscher, B.; Heigwer, F.; Zhou, J.; Beyersdörffer, C.; Frei, J.; Hess, A.; Kern, K.; et al. A large-scale resource for tissue-specific CRISPR mutagenesis in Drosophila. eLife 2020, 9, e53865. [Google Scholar] [CrossRef]
- Meltzer, H.; Marom, E.; Alyagor, I.; Mayseless, O.; Berkun, V.; Segal-Gilboa, N.; Unger, T.; Luginbuhl, D.; Schuldiner, O. Tissue-specific (ts)CRISPR as an efficient strategy for in vivo screening in Drosophila. Nat. Commun. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Zirin, J.; Hu, Y.; Liu, L.; Yang-Zhou, D.; Colbeth, R.; Yan, D.; Ewen-Campen, B.; Tao, R.; Vogt, E.; VanNest, S.; et al. Large-Scale transgenic Drosophila resource collections for loss- and Gain-of-Function studies. Genetics 2020, 214, 755–767. [Google Scholar] [CrossRef]
- Port, F.; Starostecka, M.; Boutros, M. Multiplexed conditional genome editing with Cas12a in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 22890–22899. [Google Scholar] [CrossRef]
- Hay, E.A.; Khalaf, A.R.; Marini, P.; Brown, A.; Heath, K.; Sheppard, A.; MacKenzie, A. An analysis of possible off targets following CAS9/CRISPR targeted deletions of neuropeptide gene enhancer from the mouse genome. Neuropeptides 2017, 64, 101–107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinmetz, E.L.; Noh, S.; Klöppel, C.; Fuhr, M.F.; Bach, N.; Raffael, M.E.; Hildebrandt, K.; Wittling, F.; Jann, D.; Walldorf, U. Generation of Mutants from the 57B Region of Drosophila melanogaster. Genes 2023, 14, 2047. https://doi.org/10.3390/genes14112047
Steinmetz EL, Noh S, Klöppel C, Fuhr MF, Bach N, Raffael ME, Hildebrandt K, Wittling F, Jann D, Walldorf U. Generation of Mutants from the 57B Region of Drosophila melanogaster. Genes. 2023; 14(11):2047. https://doi.org/10.3390/genes14112047
Chicago/Turabian StyleSteinmetz, Eva Louise, Sandra Noh, Christine Klöppel, Martin F. Fuhr, Nicole Bach, Mona Evelyn Raffael, Kirsten Hildebrandt, Fabienne Wittling, Doris Jann, and Uwe Walldorf. 2023. "Generation of Mutants from the 57B Region of Drosophila melanogaster" Genes 14, no. 11: 2047. https://doi.org/10.3390/genes14112047
APA StyleSteinmetz, E. L., Noh, S., Klöppel, C., Fuhr, M. F., Bach, N., Raffael, M. E., Hildebrandt, K., Wittling, F., Jann, D., & Walldorf, U. (2023). Generation of Mutants from the 57B Region of Drosophila melanogaster. Genes, 14(11), 2047. https://doi.org/10.3390/genes14112047




