1. Introduction
The insemination of sows has become a very useful technique that is widely used in swine production. In addition to the classical productive and reproductive traits of sows and boars, their sperm characteristics and libido also play an important role. An emphasis on improved sperm quality and quantity can be beneficial in terms of the economics of insemination stations [
1], as well as in the context of the profitability of integrated swine-production systems [
2]. Therefore, the improvement of such functional traits could influence the swine sector with regard to its complexity.
Sperm characteristics and libido form the mainstay of the reproductive evaluation of breeding boars. According to Knecht et al. [
3], the main parameters responsible for boar AI culling include a low semen value (23.7%) and reduced demand for semen from the given boar (22.5%). However, leg problems resulted in a 14.9% culling rate, and a low or lack of libido represented only a 9.3% culling rate. It was reported that almost half of the selected young breeding boars were culled due to low-sperm-quality parameters [
4]. Similarly, the authors of [
5] identified genetic improvements, poor semen quality, and foot and leg issues as the major reasons for boar replacement. Therefore, the reproductive abilities of boars should be considered together with their productive and other functional traits when constructing comprehensive selection criteria for breeding boars. The effect of inbreeding depression on the genetic evaluation of various reproductive traits in boars of different populations has also been studied in the literature [
6,
7]. To achieve the highest possible efficiency in the production of insemination doses, pressure is put on the whole process. However, this is largely dependent on the reproductive cycle of the boar. The sexual maturity in domestic pigs occurs between seven and nine months under the influence of genetic, social, and environmental factors [
8]. There are many papers oriented on analyses of factors affecting the reproduction cycle of boars [
5,
9,
10].
The estimation of the breeding values of sperm characteristics and libido is intended to reflect the genetic potential of animals through these traits. The estimated parameters can provide useful information for the establishment of a comprehensive breeding scheme in purebred sire [
11,
12] and dam [
10,
12,
13] breeds and in various pig lines, i.e., crossbreeds [
14,
15]. Studies focused on genetic evaluation have varied in their range and in the way semen production and quality are expressed. The traits that are measured directly are represented by the semen volume, the sperm concentration, which is log-transformed [
14] and expressed as a percentage [
16], sperm motility (e.g., in the last two cited studies), and sperm progressive motility [
17]. Further, the total number [
17] and percentage of abnormal sperm [
10,
14], the proportion of morphologically normal sperm (an alternative to [
11]), and various characteristics that define abnormalities in sperm morphology [
18] have been examined. The traits derived from direct measurements include the total number of sperm in the ejaculate [
10,
11], the number of functional spermatozoa, and the number of insemination doses [
15]. Studies evaluating the libido (expressing the willingness of boars to mount) are rare (e.g., [
12]); they indicated genetic parameters that were variable among dam and sire breeds and evaluated their traits (reproductive, growth, locomotion, exterior, and feeding traits).
A general task at present is the implementation of molecular information into routine breeding processes to enhance the genetic progress of such traits. The detection and application of novel candidate genes for sperm characteristics through marker-assisted selection is generally considered [
13] to provide information for boar selection at an earlier age. Molecular techniques open the possibility for a deeper study and understanding of the genetic mechanisms, pathways, and complex processes underlying the traits of semen production and quality (e.g., [
11]), even when considering the transcriptome and the traits’ seasonal variability in animals [
19]. The authors of [
20] reported that the use of genomic data was successful enough to achieve a 50% greater genetic gain in traits of interest.
Even though semen traits have not yet taken a place among the breeding goals for the Czech pig population [
21], they have been partially prioritized by local breeders [
22], and their designation as local breeding goals (especially for sire breeds) is desired with respect to their direct economic importance [
2]. A previous genetic evaluation [
10] also pointed out that AI centers should place an optimal emphasis on the associated breeding values. Therefore, the routine estimation of the breeding values of semen traits has been provided in the local pig population for more than a decade (based mainly on [
10,
15]). Recently, a simultaneous assessment of SNP data and pedigree data from an endangered Czech pig breed was carried out in a pilot study [
23] based on the ongoing genotyping of the local pig population. The aim of the present work was to provide a comprehensive evaluation of the genetic parameters and genomic estimates of semen traits in the commercial Czech pig population to be further applied in animal selection. The estimates for the libido trait were provided for this local pig population for the first time.
3. Results
3.1. Analyses of Phenotypes
Collection was started at 313 days of age on average for boars of dam breeds; 54.79 collections were made over a period of 412 days, and the boars produced sperm in 1.15 insemination stations on average. The sire-breed boars were characterized as males that started production at an average age of 338 days and stayed at an insemination station for 489.31 days on average. During this period, an average of 65.00 collections were made, and 1.10 inseminations were performed. The greatest number of collections took place in the months of March and April for both groups of boars. The shortest intervals between collections were also recorded in March and April, again for both groups of boars. Basic information on the evaluated datasets and traits is summarized in
Table 1. Differences in mean values between datasets were evident for almost all traits. The dam-breed boars had a greater sperm volume and slightly higher motility and proportion of abnormal sperm. On the other hand, the boars of sire breeds had a higher sperm concentration and, as a result, achieved a higher total sperm number and number of functional sperm. The average value for libido was the same for both datasets. The average lengths of stay at an insemination station were 15.6 months and 19.0 months for boars of dam and sire breeds, respectively.
The average monthly trends for the evaluated traits in this recent period (2010–2022) are summarized in
Supplementary Figure S1. Over the years, there was a decrease in SV from January to May in both datasets (D—dam and S—sire breeds), followed by a period of increasing SV until December. Similar trends were also observed for TNS and NFS. The libido of the boars of sire breeds reached a higher value during the year than that of the boars of dam breeds. Differences between the boars of dam breeds and the rest of the boars were also noted in the trends of libido during the year.
Supplementary Figure S2 shows the effect of the boar’s age at ejaculation (in months) on each evaluated trait. The sperm volume increased in all boars until the age of approximately 41 months. After this age, there was a decrease in semen volume. The “ideal” age of a boar for sperm production was considered to be in the interval between 18 and 28 months in terms of most of the observed traits. There was a noticeable increase in the value of only the AB trait for the boars of dam breeds. The optimal interval between two consecutive ejaculations appeared to be six days. A shorter interval generally had a negative effect on the traits that were evaluated. While prolonging the interval led to a partial improvement of some traits (SC, TNS), it also led to a deterioration of sperm motility and libido (confirmed by local breeders through personal communication). The effects of the interval between subsequent ejaculations are summarized in
Supplementary Figure S3.
Table 2 and
Table 3 show the variance ratios calculated for the factors affecting the evaluated traits. The proportion of explained variability captured by the model varied among the traits and datasets and could be simply derived from the residual variability. The highest variance was achieved for the motility trait (MO) in both datasets (higher than 60%). However, the lowest values were observed for AB in sire boars (less than 15%). Generally, the available effects captured greater proportions of variability in dam boars (except for SV) than in the sire boars. The greatest part of the variability was explained by the combined effects of the insemination station and the year of sperm collection. In addition, it must be mentioned that all effects in each model were highly statistically significant.
3.2. Genetic and Genomic Parameters
The coefficients of heritability (on the diagonal) and the genetic (above the diagonal) and phenotypic (below the diagonal) correlations between the evaluated traits in the five-trait ssGBLUP animal models are summarized in
Table 4 and
Table 5 for the dam and sire breeds, respectively. The genetic parameters for the derived reproductive traits (i.e., TNS and NFS) are shown in
Table 6.
The heritability coefficients had lower values for all sperm-quality traits. For the basic traits, the heritability ranged from 0.099 (for SC in dam-breed boars) to 0.280 (for SV in dam-breed boars). Some of the heritability coefficients found for the dam and sire breeds even showed significant differences between the populations. The greatest difference was observed for the SC trait; for dam breeds, it reached a value of 0.099, whereas for sire breeds, it was 0.276. Slightly higher values of the heritability coefficients were obtained in the dam breeds for SV, MO, and AB. The opposite trend was observed for the LI trait (higher heritability in sire breeds). The coefficient of heritability for libido was twice as high for boars of the sire breeds as it was for boars of the dam breeds.
Similarly, for the derived traits, which were evaluated separately with single-trait models, lower heritability coefficients were obtained. There were basically no differences when comparing the heritability values between the evaluated populations. Although a small difference was observed for TNS, where the heritability value for the dam breeds was 0.149, for the sire boar population it was 0.123. The heritability coefficient for NFS reached values of 0.153 and 0.152 for the dam and sire breeds, respectively. The variance caused by the permanent effects of derived traits reached values from 0.235 (for TNS in sire-breed boars) to 0.287 (for TNS in dam-breed boars).
The genetic correlations among the sperm-quality traits ranged widely from moderately negative to moderately positive. According to the results, libido did not seem to have a negligible genetic influence on some of the evaluated reproductive traits. Moderately positive correlations were found among libido, SV, and MO, whereas a moderately negative relationship was detected with AB when boars of dam breeds were evaluated. A weak negative genetic relationship was observed between LI and SC.
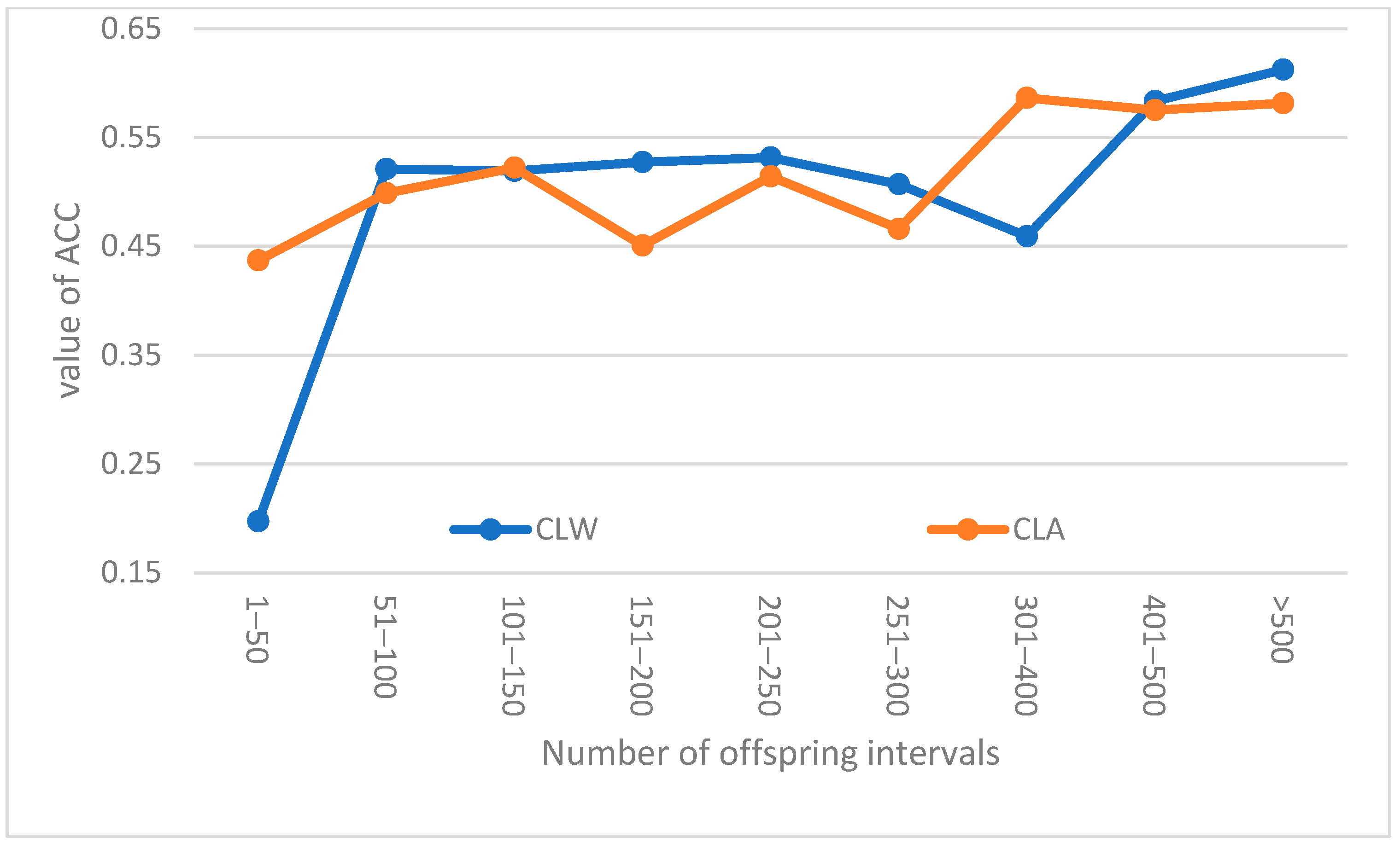
Table 7 contains the basic statistical parameters for the breeding values and their accuracies (in parentheses) expressed for all evaluated breeding boars and their basic sperm quality traits. Further,
Figure 1 presents some trends of the estimated accuracies of the sperm-volume values for breeding boars of the CLA and CLW breeds in their dependence on their numbers of offspring. A positive trend in accuracy could be seen in both breeds. A number of offspring greater than 301 did not affect the average accuracy of the breeding value for the CLA breed, whereas in the CLW breed, the average accuracy increased continuously.
4. Discussion
In our study, unlike many with a similar focus [
11,
12,
18,
30], we estimated the genetic parameters for multiple breeds of the same group (dam and sire breeds) in a single estimate. From our previous study on the evaluation of productive or reproductive traits [
31], as well as from a detailed economic evaluation of production systems [
2] in the local pig population, it was found that there were no remarkable differences in the parameters achieved for individual dam breeds raised in the Czech Republic. The use of the breed’s effect sufficiently (and significantly) captured the different levels of variability in the two evaluated dam breeds. Moreover, for the sire breeds, the joint evaluation was a practical solution, especially considering the lower number of boars of sire breeds that were evaluated.
The heritability coefficients for the sperm characteristics found in the present study ranged from 0.099 to 0.342. Comparable results were attained by the authors of the studies summarized in
Table 8. The statistical data presented there contain only estimates based on single- or multi-trait animal models when the type of breed could be identified. Wolf [
32,
33] analyzed the sperm characteristics of boars bred in the Czech Republic. These analyses were carried out on the same breeds as those in our current study, but they were conducted more than 20 years ago. There is no direct intersection of data between the previous and the current study (i.e., the authors of [
33] used data from 1995 to 2008), but a partial influence through the offspring of the previously evaluated boars could be assumed. The coefficients of heritability presented in Wolf’s studies on dam breeds (Czech Large White and Czech Landrace) reached values ranging from 0.17 for SV to 0.37 for AB. Likewise, in the framework of [
32], the heritability coefficients of the sperm characteristics estimated for dam and sire breeds reached similar values to those presented in the present study. A slight difference between the breed groups was found only in the heritability recorded for two semen traits (AB and SC).
The genetic potential of the evaluated sperm characteristics appeared to be similar for the sire and dam breeds. The heritability coefficients summarized in
Table 8, together with the numbers of estimates and sources, showed minimal differences in the mean values between breed groups (except for the proportion of abnormal sperm), whereas some differences are visible when comparing values between studies. This corresponds to the different variabilities in the values in the selected studies. In addition, in their publication, the authors of [
34] presented average values of the heritability of semen quality traits regardless of the breed group. The average heritability coefficient was 0.19 for both SV and SC. MO and AB had average values of 0.11 and 0.10, respectively. These heritability values were generally lower than or in the bottom range of the intervals presented for the semen traits in
Table 8.
In contrast to our findings, the authors of [
15] obtained higher values of the heritability coefficients for both basic and derived boar-semen characteristics. With the use of different model equations, the heritability coefficients presented in their study reached values of 0.58, 0.49, 0.38, and 0.42 for SV, SC, MO, and TNS, respectively. However, their study was designed with data from boars of nine breeds and ten groups of crossbreeds. In addition, the trait values were designed as arithmetic averages over all samplings for a given boar. The higher estimates obtained could be related to not only additive genetic variance but also a part of the permanent environment of the boar which could have a cumulative effect on the average values for each boar. Most studies focused on the estimation of genetic parameters with single- or multi-trait animal models. Ref. [
35] estimated the genetic parameters of the total sperm count for three breeds bred on two farms using a random regression model. The authors found a gradual increase in heritability from approximately 33 weeks of age to 153 weeks of age ranging from 0.27 to 0.48, respectively.
Table 8.
Average heritability coefficients for sperm characteristics of dam and sire breeds reviewed in different studies.
Table 8.
Average heritability coefficients for sperm characteristics of dam and sire breeds reviewed in different studies.
| | Mean for Dam Breeds (n) 6 | Range 7 | Source | Mean for Sire Breeds (n) 6 | Range 7 | Source |
|---|
| SV 1 | 0.22 (6) | 0.17–0.24 | [30,32,33] | 0.25 (5) | 0.21–0.29 | [6,12,32] |
| SC 2 | 0.21 (7) | 0.18–0.25 | [12,30,32,33] | 0.21 (7) | 0.05–0.34 | [6,11,12,30,32] |
| MO 3 | 0.18 (11) | 0.07–0.31 | [12,14,30,32,33] | 0.21 (12) | 0.12–0.42 | [6,12,14,30,32,36,37] |
| AB 4 | 0.30 (8) | 0.15–0.39 | [14,30,32,33] | 0.24 (7) | 0.16–0.35) | [14,30,32,36,37] |
| TNS 5 | 0.18 (5) | 0.12–0.26 | [12,14,33] | 0.21 (7) | 0.17–0.30 | [6,12,14,32,36,37] |
The genetic relationships between traits, which were expressed as correlation coefficients, reached low to intermediate values in both the positive and negative directions in our study. The strongest appeared to be a relationship between SV and SC (−0.701 and −0.580 for sire and dam boars, respectively). This negative relationship has also been well documented in other studies [
11,
32]. Li et al. [
30] found a moderately negative correlation between SV and SC only for the Duroc breed, whereas for the Landrace and Yorkshire breeds, the correlation was close to zero. Generally, the estimated genetic correlations had values for sperm quality traits that were different from those reported in previous analyses [
11,
12,
14,
18,
30,
32,
33]. The genetic correlation between libido and sperm motility had moderately positive values in our study (0.430 and 0.525 in the D and S datasets, respectively) and was close to zero (0.05 for Duroc) or moderately negative (−0.41 for the Yorkshire breed) according to the findings of [
6]. When interpreting correlation coefficients with libido, the phenotypic expression of the trait could be considered. This trait is evaluated subjectively—usually according to a set scale that is similar to those for exterior traits. The scale was set so that the most desirable performance was associated with the highest value (5) in our study, but it was associated with the lowest value (1) in [
12]. Generally, this genetic correlation revealed that selection for libido would bring the most advantageous improvements in motility and the proportion of abnormal spermatozoa and vice versa.