Abstract
Familial thoracic aortic aneurysms and dissections may occur as an isolated hereditary trait or as part of connective tissue disorders with Mendelian inheritance, but severe cardiovascular disease in pediatric patients is extremely rare. There is growing knowledge on pathogenic variants causing the disease; however, much of the phenotypic variability and gene–gene interactions remain to be discovered. We present a case report of a 5.5-year-old girl with an aortic aneurysm and concomitant polycystic kidney disease. Whole exome sequencing was performed, followed by family screening by amplicon deep sequencing and diagnostic imaging studies. In the proband, two pathogenic variants were identified: p.Tyr257Ter in the LOX gene inherited from her mother, and p.Thr2977Ile in the PKD1 gene inherited from her father. All adult carriers of either of these variants showed symptoms of aortic disease. We conclude that the coexistence of two independent genetic variants in the proband may be the reason for an early onset of disease.
1. Introduction
Thoracic aortic aneurysms and dissections (TAAD) are one of the major causes of death in developed countries, affecting 1% of the general population [1,2]. There is growing recognition of genetic predispositions to TAAD [3]. To date, at least 37 TAAD-causing genes have been identified, and up to 25–30% of individuals with TAAD harbor an underlying Mendelian pathogenic variant in one of these genes [1,4,5]. Three major categories of gene alterations have been identified. The first relates to mutations in gene coding for various elements of the transforming growth factor β signaling pathway (e.g., TGFBR1, TGFBR2, TGFB2, SMAD3), which together are called TGF-β vasculopathies, the second group relates to smooth muscle contraction vasculopathies (ACTA2, MYH11, MYLK and PRKG1) and the third one comprises genes encoding for extracellular matrix proteins (e.g., COL3A1, LOX, EFEMP2) [1,3,4,6].
Familial TAAD may occur as an isolated hereditary trait or as part of connective tissue disorders, e.g., Marfan syndrome (FBN1), Loyes–Dietz (TGFBR1, TGFBR2, TGFB2, TGFB3, SMAD3) or Ehlers-Danlos IV (COL3A1), which affect ocular, skeletal and vascular systems [2].
A thoracic aortic aneurysm occurs most often in people aged 65 and older and is uncommon among nonsyndromic pediatric patients. A severe cardiovascular disease in pediatric patients is usually limited to autosomal recessive cardiomyopathies [7,8] and uncommon in TAAD, even in syndromic forms [9,10].
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, affecting 1 in 400 to 1 in 1000 individuals in the United States [11]. Its primary manifestation is the development of cysts in renal parenchyma, causing kidneys to enlarge and lose function over time. Its complications include hypertension, frequent cyst infections, haematuria and nephrolithiasis. Cysts may affect other organs, mostly the liver; however, vascular abnormalities are the most life-threatening ADPKD effects, the majority of them being: left ventricular hypertrophy, cardiac valvular defects, intra- or extracranial aneurysms, arterial rupture or dissection [12]. The frequency of an intracranial aneurysm in patients with ADPKD has been reported to be up to 4–11% [13,14]. It has also been suggested that the presence of ADPKD is a significant risk factor for aortic aneurysms (OR = 4.18) and for aortic dissections (OR = 9.08) [15]. However, the genes encoding polycystines are absent from clinical guidelines for the diagnosis and management of aortic disease [16] or from most commercially offered next-generation diagnostic panels targeted at aortopathy.
Here we present a case report of a 5.5-year-old girl with a diagnosis of polycystic kidney disease who was admitted to the Department of Pediatric Cardiology because of a suspicion of an aortic aneurysm.
2. Materials and Methods
Whole exome sequencing (WES) was performed for the proband and her father using a SureSelectXT Human All Exon v7 library preparation kit (Agilent Technologies, Santa Clara, CA, USA) and HiSeq 1500 sequencing platform (Illumina, San Diego, CA, USA). Reads were controlled for quality, trimmed, aligned to the hg38 reference genome and annotated, as described previously [17].
For family screening, amplicon deep sequencing was performed using the Nextera XT Kit (Illumina) and sequenced on the HiSeq 1500 sequencing platform.
All the data not included in the current article are available from the corresponding authors on reasonable request.
The study was approved by the Bioethics Committee in the National Institute of Cardiology, ref. no. IK.NPIA.0021.64.1880/20.
3. Results
The girl was born at 41 weeks of gestation to a primiparous mother by caesarean section (birth weight 3010 g, Apgar score 10). Her cognitive and motor development were considered normal. She had been achieving developmental milestones and educational progress according to her age. She had been in the follow-up of the out-patient pediatric cardiology clinic since the age of 4 years due to a heart murmur and increased aortic root dimensions found in echocardiographic examinations. Additionally, she was in nephrological care due to kidney cysts and neurological care due to periodic visual disturbances. She did not suffer from any chronic diseases or allergies, nor did she take medications on a regular basis. Parents did not report on any disturbing symptoms. She was vaccinated according to the current immunization schedule.
Upon admission to the Pediatric Cardiology Department, the girl’s anthropometric parameters were as follows: body weight, 23 kg (85–90th prc.); height, 1.23 m (>97th prc.); and BMI, 15.2 kg/m2 (25–50th prc.).
Upon physical examination, the girl presented with stable vital parameters (blood oxygen saturation, >95%; resting heart rate, 90 bpm; arterial blood pressure, 101/70 mmHg). On auscultation, her heart sounds were normal, and a systolic murmur grade 2/6 (Levine scale) was audible in the aortic area. No other abnormalities were observed upon physical examination. The electrocardiogram was normal. In the echocardiographic examination, the systolic and diastolic functions and the dimensions of the atria and ventricles were normal, with no pericardial effusion. The diameters of the aorta were as follows: aortic valve, 15 mm (Z-score +0.08/−0.82); sinus of Valsalva, 27 mm (Z-score +2.65/+4.47); and sinotubular (ST) junction, 21 mm (Z-score +1.93/+3.00). The Z-scores were based on the Detroit [18] and Wessex [19] Z-scores for healthy children and are shown, respectively (Table 1). Thus, the echocardiographic study showed an increased aortic root size along with a mild enlargement of the ST junction. The computed tomography angiography (CTA) confirmed the presence of an enlarged aortic root with a mildly enlarged ascending aorta and normal-sized sequential aortic segments (Figure 1). The CTA determined that the diameters of the aorta were as follows: aortic root, 30 × 30 mm (Z-score +5.39); ascending aorta, 24 × 24 mm (Z-score +3.46); ascending aorta proximal to the brachiocephalic trunk, 17 × 18 mm; aortic arch between the left common carotid artery and left subclavian artery, 12 × 13 mm (Z-score −0.52); aortic isthmus, 12 × 13 mm (Z-score +0.23); and descending aorta, 12 × 12 mm (descending aorta at diaphragm level 11 × 11 mm). The CT scan also revealed that the brachiocephalic trunk was curved to the right at 90 degrees and that it arose in close proximity to the left common carotid artery. Additionally, magnetic resonance imaging of the cervical area was performed, which revealed a kinking of the right internal carotid artery and looping of the left internal carotid artery; no other abnormalities were observed. In the ultrasound examination of the abdomen, no aneurysm of the abdominal aorta was reported, and the diameters were 8 mm in diastole and 7 mm in systole (measured 20 mm below the superior mesenteric artery).

Table 1.
Clinical characteristics of examined family members.
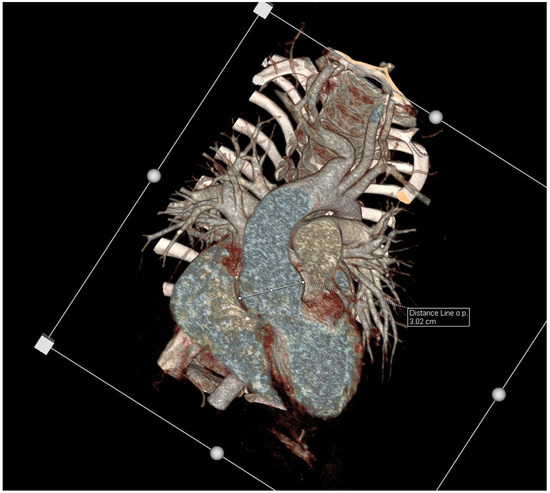
Figure 1.
Enhancement CT scan, VRT, coronal views showing a dilated aortic root.
The proband’s DNA sample was sent for genetic testing with the suspicion of Marfan syndrome or collagenopathy. WES identified the presence of two heterozygous variants of interest: NM_002317.7:c.771T>G(p.Tyr257Ter) in the LOX gene and NM_000296.4:c.8930C>T (p.Thr2977Ile) in the PKD1 gene (Table 2). The family screening by amplicon deep sequencing revealed that the LOX variant was inherited from her mother (II:3) and was also present in one of the maternal aunts (II:2) and her son (III:4). The PKD1 variant was identified in the proband’s father (II:4) and brother (III:6) (Figure 2). Subsequently, a WES analysis was performed for the proband’s father (II:4) in order to look for other genetic factors causative for TAAD. Among the analysed variants in 43 TAAD-associated genes of frequency 0.001 and lower in the GnomAD database, no non-benign variants were identified.

Table 2.
Details regarding identified variants.
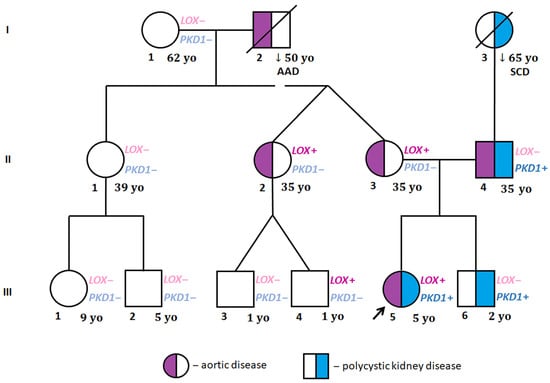
Figure 2.
Family pedigree. I, II, III—generations; AD—aortic dissection, LOX+—LOX NM_002317.7 c.771T>G (p.Tyr257Ter) identified; LOX−—LOX NM_002317.7 c.771T>G (p.Tyr257Ter) ruled out; PKD1+—PKD1 NM_000296.4 c.8930C>T (p.Thr2977Ile) identified; PKD1−—PKD1 NM_000296.4 c.8930C>T (p.Thr2977Ile) ruled out; SCD—sudden cardiac death; yo—years old (age of family members at the beginning of proband’s cardiac evaluation; the arrow indicates proband.
Both the adult LOX variant carriers showed no symptoms of generalized connective tissue disorder, demonstrated mildly increased aortic dimensions and had a positive family history of acute aortic dissection type A (the proband’s maternal grandfather at the age of 50 years, I:2). The proband’s mother (II:3), aged 35 years, asymptomatic, physically fit and with normal blood pressure (ABPM mean values of 107/70 mmHg), was found to have a mildly dilated aortic root z-score of 3.05, with borderline other ascending aortic dimensions of the STJ, 30 mm, and aortic arch, 31 mm, and normal values for the descending aorta, 18 mm, and abdominal aorta of 15 mm; the values have been confirmed in the whole aortic CT scan. Her sister (II:2) complained of an allergy, was treated for hypothyroidism, and had normal blood pressure. On the echocardiogram, her aortic dimensions were mildly increased, with an aortic root of 38 mm, a z-score of 2.58, an ST-J of 27.5 mm, an ascending aorta of 33 mm, an aortic arch of 29.6 mm, a descending aorta of 19 mm, and an abdominal aorta of 16 mm. Her cousin (III:4) is currently a 4-year-old boy who periodically reports stabbing chest pain on exertion, which resolves spontaneously, and impaired exercise tolerance compared to his peers. His blood pressure measurements remained normal. In the echocardiography, the dimensions of the aorta were normal: an aortic root of 19 mm, with a z-score of −0.3, an ST-J of 16 mm, with a z-score of −0.1, and an ascending aorta of 17 mm, with a z-score of +0.4. Parameters were also confirmed in the CTA. The presence of kidney cysts was excluded in all the LOX-carrying family members (II:2, II:3, III:4) by either an ultrasound scan or CTA (Table 1).
All the examined PKD1-carrying family members suffer from kidney cysts and have a positive family history of kidney/liver cysts and sudden cardiac death (proband’s paternal grandmother). The proband’s father (II:4), aged 35 and 1.98 m tall, had a history of hypertension treated with 50 mg losartan. He presented with multiple single cysts in both kidneys (diagnosis made by CTA). He was also complaining of an allergy and has concomitant hyperlipidemia and mild fasting hyperglycemia. He was a long-distance runner training several times a week and below age of 30 years. On the echocardiogram, he was found to have enlarged aortic bulb of 47 mm with a z-score of 4.12, an ST-T junction of 38 mm, an ascending aorta of 36 mm, an aortic arch of 30 mm, and a descending aorta of 24 mm. The proband’s brother (III:6) had a normal echocardiogram; however, an ultrasound scan of his abdomen revealed several cysts in both kidneys (Table 1). None of the examined family members manifested an atypical arrangement of the major arteries within the chest and abdomen.
4. Discussion
Several pathogenic variants in the LOX gene have been associated with aortic aneurysm formation since 2016 [23]. p.Tyr257Ter is a novel truncating variant and is one of two known mechanisms described to be the cause of TAAD associated with the LOX gene, the other being missense variants affecting highly conserved amino acids in the catalytic domain [24]. LOX encodes for lysyl oxidase, an enzyme playing an essential role in the proper formation and maintenance of the extracellular matrix (ECM)—a main component of connective tissue. Lysyl oxidase catalytic activity initiates the cross-linking between two main proteins that form ECM-collagen and elastin. Lysyl oxidase also binds the TGF-β transcription factor involved in the regulatory process controlling the constant remodeling of the ECM [25]. Defects in the functioning of the TGF-β signaling pathway underlie the mechanisms of arterial aneurysm formation in several disorders, including Marfan syndrome and Loeys–Dietz syndrome, and overlapping syndromic features, such as pectus deformities, joint hypermobility and striae, which were reported in family members with LOX variants [23].
p.Thr2977Ile in the PKD1 gene is a missense variant affecting an evolutionary conserved amino acid and is absent from population databases. It was previously reported in a patient with ADPKD [26]. Defects in the polycystine-1-coding PKD1 gene are responsible for 85% of ADPKD cases [27].
Whereas LOX is an established TAAD gene, PKD1 is considered a strong TAAD risk factor, especially among hypertensive ADPKD patients, in a number of reports [28,29,30,31]. So far, our understanding of the pathomechanism behind vascular abnormalities in ADPKD is limited. However, the data indicate that polycystines participate in maintaining the integrity of the arterial wall. Polycystines are expressed in vascular smooth muscle and endothelium and play a significant role in mechano-sensation by modulating the activity of the stretch-activated cation channels and myogenic contraction. There is some evidence that specific defects in the PKD1 gene are more likely to predispose to a vascular phenotype, as observed in unrelated families, but the mechanism behind it remains unclear. Interestingly, the phenotypic features of some patients with ADPKD overlap those characteristic of connective tissue disorders, e.g., tall and slender build resembling Marfan syndrome [30]. In murine models, Pkd1–Fbn1 double heterozygotes display an exacerbation of the typical Fbn1 heterozygous aortic phenotype on the basis of a further upregulation of TGF-β signalling; additionally, Pkd1 haploinsufficiency alone is sufficient to increase responsiveness to TGF-β [32].
Assuming the above data, the history of hypertension and the absence of other apparent TAAD-causing variants, it is possible that the PKD1 variant was sufficient to cause TAA in the proband’s father alone. The coexistence of both variants might explain the atypically early onset of TAA in the proband herself; nevertheless, aortic aneurysms have been reported previously in LOX-variant-carrying individuals as young as 6 [24] and 11 [23] years old. In both cases, these children were the only LOX variant carriers among their family members with such an early disease onset and thus could carry additional yet unidentified genetic phenotype modifiers.
Considering the available data on the function of polycystine 1 and the significantly higher risk of life-threatening vascular complications among ADPKD patients, it is possible that the pathogenic PKD1 variant acted as a genotype modifier towards the early onset of disease in the proband carrying the LOX variant. However, since Van Gucht et al. [24] reported that the time of onset of an aortic aneurysm in LOX patients is variable but can be as early as 6 years of age, we cannot exclude that the early age of onset in our patient is just one end of the disease spectrum. On the other hand, it cannot be excluded that the early-onset cases, including those described by Van Gucht et al. [24] and Guo et al. [23], are caused by additional genetic defects that have not been found. Indeed, there are reports suggesting the role of digenic/oligogenic/polygenic inheritance in TAA [33,34,35,36].
Author Contributions
Conceptualization, Z.T.B., R.P. and W.B.; methodology, A.P. and G.T.; validation, A.P.; formal analysis, W.B.; investigation, W.P.-P., K.J.-P., I.M. and E.S.; data curation, J.K.P.; writing—original draft preparation, W.B., Z.T.B. and J.K.P.; writing—review and editing, J.K.P. and R.P.; visualization, J.K.P.; supervision, R.P.; project administration, Z.T.B.; funding acquisition, Z.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Cardiology, internal grant number 2.60/VII/20.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of National Institute of Cardiology (protocol code: IK.NPIA.0021.64.1880/20) on 8 December 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the data that supports the findings of this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faggion Vinholo, T.; Brownstein, A.J.; Ziganshin, B.A.; Zafar, M.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2019 Update and Clinical Implications. Aorta 2019, 7, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, A.J.; Kostiuk, V.; Ziganshin, B.A.; Zafar, M.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2018 Update and Clinical Implications. Aorta 2018, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pomianowski, P.; Elefteriades, J.A. The genetics and genomics of thoracic aortic disease. Ann. Cardiothorac. Surg. 2013, 2, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Renard, M.; Francis, C.; Ghosh, R.; Scott, A.F.; Witmer, P.D.; Adès, L.C.; Andelfinger, G.U.; Arnaud, P.; Boileau, C.; Callewaert, B.L.; et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018, 72, 605–615. [Google Scholar] [CrossRef]
- Poninska, J.K.; Bilinska, Z.T.; Franaszczyk, M.; Michalak, E.; Rydzanicz, M.; Szpakowski, E.; Pollak, A.; Milanowska, B.; Truszkowska, G.; Chmielewski, P.; et al. Next-generation sequencing for diagnosis of thoracic aortic aneurysms and dissections: Diagnostic yield, novel mutations and genotype phenotype correlations. J. Transl. Med. 2016, 14, 115. [Google Scholar] [CrossRef]
- Takeda, N.; Komuro, I. Genetic basis of hereditary thoracic aortic aneurysms and dissections. J. Cardiol. 2019, 74, 136–143. [Google Scholar] [CrossRef]
- Salazar-Mendiguchía, J.; Ochoa, J.P.; Palomino-Doza, J.; Domínguez, F.; Díez-López, C.; Akhtar, M.; Ramiro-León, S.; Clemente, M.M.; Pérez-Cejas, A.; Robledo, M.; et al. Mutations in TRIM63 cause an autosomal-recessive form of hypertrophic cardiomyopathy. Heart 2020, 106, 1342–1348. [Google Scholar] [CrossRef]
- Ploski, R.; Rydzanicz, M.; Ksiazczyk, T.M.; Franaszczyk, M.; Pollak, A.; Kosinska, J.; Michalak, E.; Stawinski, P.; Ziolkowska, L.; Bilinska, Z.T.; et al. Evidence for troponin C (TNNC1) as a gene for autosomal recessive restrictive cardiomyopathy with fatal outcome in infancy. Am. J. Med. Genet. A 2016, 170, 3241–3248. [Google Scholar] [CrossRef]
- de Vries, B.B.; Pals, G.; Odink, R.; Hamel, B.C. Homozygosity for a FBN1 missense mutation: Clinical and molecular evidence for recessive Marfan syndrome. Eur. J. Hum. Genet. 2007, 15, 930–935. [Google Scholar] [CrossRef]
- Hilhorst-Hofstee, Y.; Rijlaarsdam, M.E.; Scholte, A.J.; Swart-van den Berg, M.; Versteegh, M.I.; van der Schoot-van Velzen, I.; Schäbitz, H.J.; Bijlsma, E.K.; Baars, M.J.; Kerstjens-Frederikse, W.S.; et al. The clinical spectrum of missense mutations of the first aspartic acid of cbEGF-like domains in fibrillin-1 including a recessive family. Hum. Mutat. 2010, 31, E1915–E1927. [Google Scholar] [CrossRef]
- Iglesias, C.G.; Torres, V.E.; Offord, K.P.; Holley, K.E.; Beard, C.M.; Kurland, L.T. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935-1980. Am. J. Kidney Dis. 1983, 2, 630–639. [Google Scholar] [CrossRef]
- Ecder, T.; Schrier, R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef]
- Chapman, A.B.; Rubinstein, D.; Hughes, R.; Stears, J.C.; Earnest, M.P.; Johnson, A.M.; Gabow, P.A.; Kaehny, W.D. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1992, 327, 916–920. [Google Scholar] [CrossRef]
- Ruggieri, P.M.; Poulos, N.; Masaryk, T.J.; Ross, J.S.; Obuchowski, N.A.; Awad, I.A.; Braun, W.E.; Nally, J.; Lewin, J.S.; Modic, M.T. Occult intracranial aneurysms in polycystic kidney disease: Screening with MR angiography. Radiology 1994, 191, 33–39. [Google Scholar] [CrossRef]
- Nunes, R.; Gouveia, E.M.R.; Almeida, A.G.; de Almeida, E.; Pinto, F.J.; Pedro, L.M.; Caldeira, D. Does autosomal dominant polycystic kidney disease increase the risk of aortic aneurysm or dissection: A point of view based on a systematic review and meta-analysis. J. Nephrol. 2022, 35, 1585–1593. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Śmigiel, R.; Biela, M.; Szmyd, K.; Błoch, M.; Szmida, E.; Skiba, P.; Walczak, A.; Gasperowicz, P.; Kosińska, J.; Rydzanicz, M.; et al. Rapid Whole-Exome Sequencing as a Diagnostic Tool in a Neonatal/Pediatric Intensive Care Unit. J. Clin. Med. 2020, 9, 2220. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef]
- Daubeney, P.E.; Blackstone, E.H.; Weintraub, R.G.; Slavik, Z.; Scanlon, J.; Webber, S.A. Relationship of the dimension of cardiac structures to body size: An echocardiographic study in normal infants and children. Cardiol. Young 1999, 9, 402–410. [Google Scholar] [CrossRef]
- Devereux, R.B.; de Simone, G.; Arnett, D.K.; Best, L.G.; Boerwinkle, E.; Howard, B.V.; Kitzman, D.; Lee, E.T.; Mosley, T.H., Jr.; Weder, A.; et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012, 110, 1189–1194. [Google Scholar] [CrossRef]
- Rozendaal, L.; Groenink, M.; Naeff, M.S.; Hennekam, R.C.; Hart, A.A.; van der Wall, E.E.; Mulder, B.J. Marfan syndrome in children and adolescents: An adjusted nomogram for screening aortic root dilatation. Heart 1998, 79, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.A.; Li, Y.; Rizzo, J.A.; Charilaou, P.; Saeyeldin, A.; Velasquez, C.A.; Mansour, A.M.; Bin Mahmood, S.U.; Ma, W.G.; Brownstein, A.J.; et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2018, 155, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Van Gucht, I.; Krebsova, A.; Diness, B.R.; Laga, S.; Adlam, D.; Kempers, M.; Samani, N.J.; Webb, T.R.; Baranowska, A.A.; Van Den Heuvel, L.; et al. Novel LOX Variants in Five Families with Aortic/Arterial Aneurysm and Dissection with Variable Connective Tissue Findings. Int. J. Mol. Sci. 2021, 22, 7111. [Google Scholar] [CrossRef]
- Atsawasuwan, P.; Mochida, Y.; Katafuchi, M.; Kaku, M.; Fong, K.S.; Csiszar, K.; Yamauchi, M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J. Biol. Chem. 2008, 283, 34229–34240. [Google Scholar] [CrossRef]
- Neumann, H.P.; Jilg, C.; Bacher, J.; Nabulsi, Z.; Malinoc, A.; Hummel, B.; Hoffmann, M.M.; Ortiz-Bruechle, N.; Glasker, S.; Pisarski, P.; et al. Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol. Dial. Transpl. 2013, 28, 1472–1487. [Google Scholar] [CrossRef]
- Thivierge, C.; Kurbegovic, A.; Couillard, M.; Guillaume, R.; Coté, O.; Trudel, M. Overexpression of PKD1 causes polycystic kidney disease. Mol. Cell Biol. 2006, 26, 1538–1548. [Google Scholar] [CrossRef]
- Peczkowska, M.; Januszewicz, A.; Grzeszczak, W.; Moczulski, D.; Janaszek-Sitkowska, H.; Kabat, M.; Biederman, A.; Hendzel, P.; Prejbisz, A.; Cendrowska-Demkow, I.; et al. The coexistence of acute aortic dissection with autosomal dominant polycystic kidney disease--description of two hypertensive patients. Blood Press. 2004, 13, 283–286. [Google Scholar] [CrossRef]
- Sung, P.H.; Yang, Y.H.; Chiang, H.J.; Chiang, J.Y.; Chen, C.J.; Liu, C.T.; Yu, C.M.; Yip, H.K. Risk of aortic aneurysm and dissection in patients with autosomal-dominant polycystic kidney disease: A nationwide population-based cohort study. Oncotarget 2017, 8, 57594–57604. [Google Scholar] [CrossRef]
- Perrone, R.D.; Malek, A.M.; Watnick, T. Vascular complications in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 589–598. [Google Scholar] [CrossRef]
- Spinelli, L.; Giugliano, G.; Esposito, G. Cardiac Involvement in Autosomal Dominant Polycystic Kidney Disease. Cardiogenetics 2021, 11, 39–49. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.J.; Judge, D.P.; Halushka, M.K.; Ni, J.; Habashi, J.P.; Moslehi, J.; Bedja, D.; Gabrielson, K.L.; Xu, H.; et al. A Pkd1-Fbn1 genetic interaction implicates TGF-β signaling in the pathogenesis of vascular complications in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2014, 25, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kwartler, C.S.; Gong, L.; Chen, J.; Wang, S.; Kulmacz, R.; Duan, X.Y.; Janda, A.; Huang, J.; Kamm, K.E.; Stull, J.T.; et al. Variants of Unknown Significance in Genes Associated with Heritable Thoracic Aortic Disease Can Be Low Penetrant “Risk Variants”. Am. J. Hum. Genet. 2018, 103, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Tcheandjieu, C.; Xiao, K.; Tejeda, H.; Lynch, J.A.; Ruotsalainen, S.; Bellomo, T.; Palnati, M.; Judy, R.; Klarin, D.; Kember, R.L.; et al. High heritability of ascending aortic diameter and trans-ancestry prediction of thoracic aortic disease. Nat. Genet. 2022, 54, 772–782. [Google Scholar] [CrossRef]
- Disha, K.; Schulz, S.; Mierzwa, M.; Owais, T.; Girdauskas, E.; Kuntze, T. Double-Hit Mutations in Bicuspid Aortic Valve and Blunt Traumatic Acute Aortic Dissection. Ann. Thorac. Surg. 2021, 111, e5–e6. [Google Scholar] [CrossRef]
- Klarin, D.; Devineni, P.; Sendamarai, A.K.; Angueira, A.R.; Graham, S.E.; Shen, Y.H.; Levin, M.G.; Pirruccello, J.P.; Surakka, I.; Karnam, P.R.; et al. Genome-wide association study of thoracic aortic aneurysm and dissection in the Million Veteran Program. Nat. Genet. 2023, 55, 1106–1115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).