Lung Inflammatory Genes in Cystic Fibrosis and Their Relevance to Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapies
Abstract
1. Introduction
1.1. Immunoinflammation in CFLD
1.2. CFTR Modulators: A New Paradigm in CF Treatment
2. Immune Gene Variants Involved in CFLD Pathogenesis and Progression
2.1. Collectins
2.2. Cellular Receptors
2.3. Inflammasome
2.4. Cytokines
| Gene | Variant | Associated with | Reference |
|---|---|---|---|
| Soluble Mediators | |||
| MBL2 | Y0/Y0, X0/Y0, and XA/Y0 diplotypes | MBL2 deficiency, which was associated with more rapid decline of pulmonary function and enhanced by high-producing TGFB1 gene variants | Dorfman et al., 2008 [87] |
| SFTPA1, SFTA2, SFTB, SFTPC, SFTPD | SFTPB SNP (rs7316) | Mild lung disease | Lin et al., 2018 [89] |
| Intergenic interactions between SFTPB and SFTPD/SFTA1+SFTPA2 | Mild lung disease | ||
| Intergenic interactions between SFTPB and SFTPD/SFTA1+SFTPA2 | Moderate/severe lung disease | ||
| Intergenic interactions between SFTPA1 and SFTPA2 | |||
| Cellular Receptors | |||
| TLR5 | c.1174C>T (rs5744168) | Improvements in lung function associated with the T allele were not statistically significant | Blohmke et al., 2010 [92] |
| TLR5 | rs5744174 | Extreme fast FEV1 decline | Haerynck et al., 2013 [91] |
| TLR1 | Homozygous for rs5743551 | Faster decline of FEV1 compared to heterozygous genotype | Haerynck et al., 2013 [91] |
| TLR2 | rs1898830, rs5743708, and rs3804100 | Lung disease severity | Haerynck et al., 2013 [91] |
| AGER | Variant −429T/C | More severe CFLD | Beucher et al., 2012 [93] |
| Inflammasome | |||
| NLRP3 | p.(Q705K) | A higher rate of P. aeruginosa colonization and worsened lung function | Graustein et al., 2021 [96] |
| NLRC4 | p.(A929S) | A lower rate of P. aeruginosa colonization and protection from lung function decline | |
| Cytokines | |||
| IL8 | rs4073 | Protective | Hillian et al., 2008 [98] |
| rs2227306 and rs2227307 | Severe lung disease | ||
| IL8 | rs4073, rs2227306, and rs2227307 | Markers of severe lung disease | Furlan et al., 2016 [107] |
| IL8 | rs4073 | More severe lung disease | de Vries et al., 2014 [99] |
| IL1B | Severe lung disease | Stanke et al., 2011 [100] | |
| IL1B | rs3917356 and rs4848306 | Severe lung disease | Labenski et al., 2011 [101] |
| IL1B | rs16944 | More severe lung disease | de Vries et al., 2014 [99] |
| TNFA | genotype TNF-α–308GA | Higher neutrophil elastase activity in sputum | Shmarina et al., 2013 [103] |
| TNFA | −857C/T polymorphism | Severe pulmonary phenotype | Hassanzad et al. [108] |
| TNFR2 | +587T/G polymorphism | Severe pulmonary phenotype | Hassanzad et al. [108] |
| IL10 | rs1800896 | More severe lung disease | de Vries et al., 2014 [99] |
| LTA | +252GG polymorphism | Higher neutrophil elastase activity in sputum | Shmarina et al., 2013 [103] |
| TGFB1 | +869CT | Less-pronounced rate of decline in FEV1 | Corvol et al., 2008 [105] |
| Homozygous TT genotype | High levels of sputum TNF-α | Trojan et al., 2022 [106] |
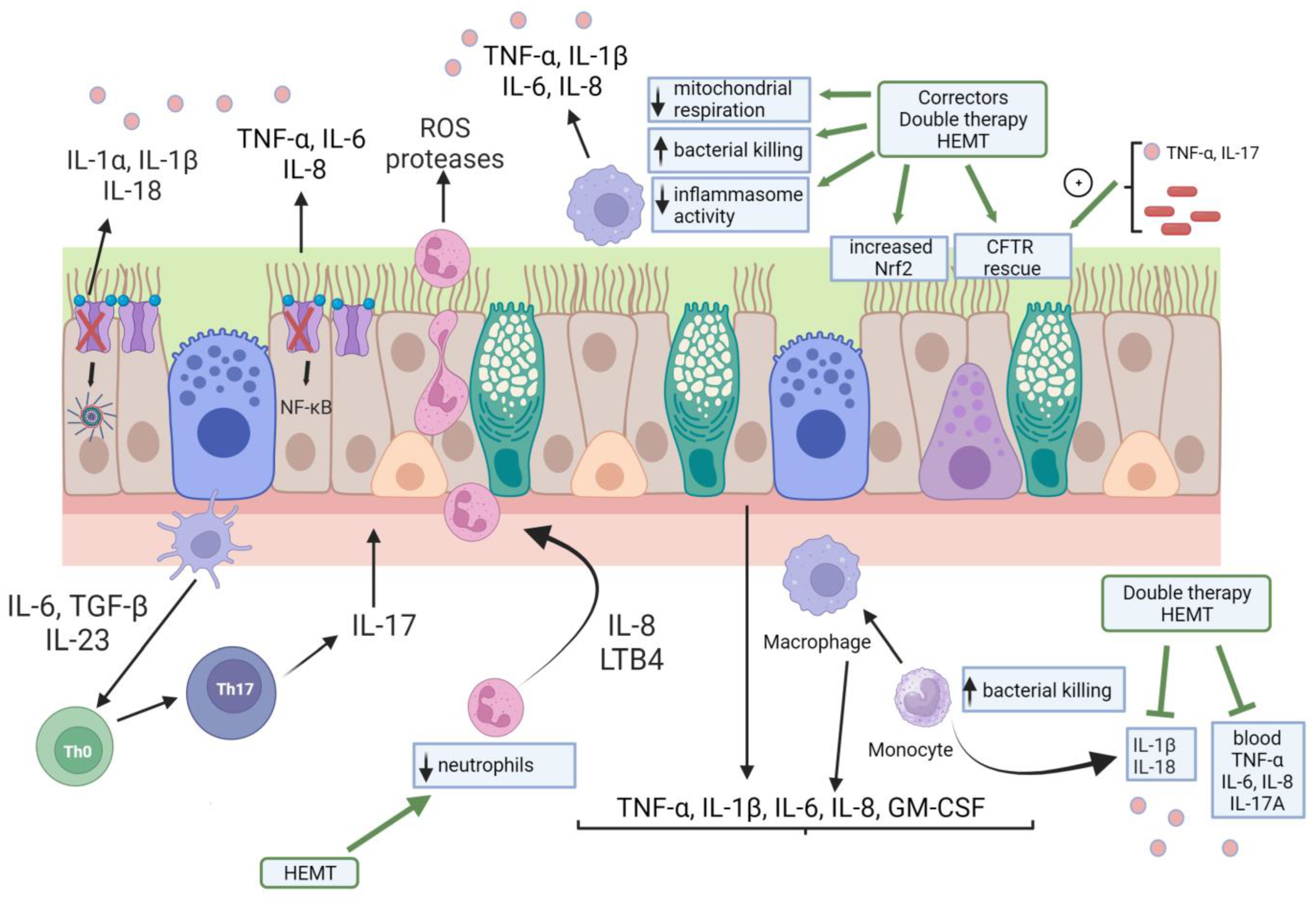
3. Effects of CFTR Modulator Therapies on Inflammation/Immunity
3.1. Effects of CFTR Modulators on CF Airway Epithelial Cells
3.2. Effects of CFTR Modulators on CF Monocytes/Macrophages
3.3. Effects of CFTR Modulators on Neutrophils
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Rowe, S.M.; Miller, S.; Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 2005, 352, 1992–2001. [Google Scholar] [PubMed]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Prim. 2015, 1, 15010. [Google Scholar] [CrossRef]
- Giacalone, V.D.; Dobosh, B.S.; Gaggar, A.; Tirouvanziam, R.; Margaroli, C. Immunomodulation in Cystic Fibrosis: Why and How? Int. J. Mol. Sci. 2020, 21, 3331. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef]
- Bruscia, E.M.; Bonfield, T.L. Update on Innate and Adaptive Immunity in Cystic Fibrosis. Clin. Chest Med. 2022, 43, 603–615. [Google Scholar] [CrossRef]
- The Cystic Fibrosis Centre at the Hospital for Sick Children. Cystic Fibrosis Mutation Database; The Cystic Fibrosis Centre at the Hospital for Sick Children: Toronto, ON, Canada, 2011. [Google Scholar]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef]
- Boucher, R.C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.S.; Parkins, M.D. Microbial Epidemiology of the Cystic Fibrosis Airways: Past, Present, and Future. Semin. Respir. Crit. Care Med. 2023, 44, 269–286. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Tang, X.X.; Hoegger, M.J.; Abou Alaiwa, M.H.; Ramachandran, S.; Moninger, T.O.; Karp, P.H.; Wohlford-Lenane, C.L.; Haagsman, H.P.; van Eijk, M.; et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012, 487, 109–113. [Google Scholar] [CrossRef]
- Tang, X.X.; Ostedgaard, L.S.; Hoegger, M.J.; Moninger, T.O.; Karp, P.H.; McMenimen, J.D.; Choudhury, B.; Varki, A.; Stoltz, D.A.; Welsh, M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Investig. 2016, 126, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwa, M.H.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA 2014, 111, 18703–18708. [Google Scholar] [CrossRef]
- Birket, S.E.; Davis, J.M.; Fernandez, C.M.; Tuggle, K.L.; Oden, A.M.; Chu, K.K.; Tearney, G.J.; Fanucchi, M.V.; Sorscher, E.J.; Rowe, S.M. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018, 3, e97199. [Google Scholar] [CrossRef]
- Clary-Meinesz, C.; Mouroux, J.; Cosson, J.; Huitorel, P.; Blaive, B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur. Respir. J. 1998, 11, 330–333. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Kerem, E.; Ferkol, T.; Elizur, A. Airway inflammation in cystic fibrosis: Molecular mechanisms and clinical implications. Thorax 2013, 68, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Kappler, M.; Gaggar, A.; Hartl, D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur. Respir. J. 2008, 32, 783–795. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 2008, 40, 1238–1245. [Google Scholar] [CrossRef]
- McKelvey, M.C.; Weldon, S.; McAuley, D.F.; Mall, M.A.; Taggart, C.C. Targeting Proteases in Cystic Fibrosis Lung Disease. Paradigms, Progress, and Potential. Am. J. Respir. Crit. Care Med. 2020, 201, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef]
- Regamey, N.; Jeffery, P.K.; Alton, E.W.; Bush, A.; Davies, J.C. Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax 2011, 66, 624–629. [Google Scholar] [CrossRef]
- Palaniyar, N. Antibody equivalent molecules of the innate immune system: Parallels between innate and adaptive immune proteins. Innate Immun. 2010, 16, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Frevert, C.W. Innate immunity in the lungs. Proc. Am. Thorac. Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Nourkami-Tutdibi, N.; Freitag, K.; Zemlin, M.; Tutdibi, E. Genetic Association with Pseudomonas aeruginosa Acquisition in Cystic Fibrosis: Influence of Surfactant Protein D and Mannose-Binding Lectin. Front. Immunol. 2021, 12, 587313. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.; Stecenko, A.A.; King, G.; Blackwell, T.R.; Brigham, K.L.; Christman, J.W.; Blackwell, T.S. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-β processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2000, 23, 396–403. [Google Scholar] [CrossRef]
- Cabrini, G.; Rimessi, A.; Borgatti, M.; Lampronti, I.; Finotti, A.; Pinton, P.; Gambari, R. Role of Cystic Fibrosis Bronchial Epithelium in Neutrophil Chemotaxis. Front. Immunol. 2020, 11, 1438. [Google Scholar] [CrossRef]
- De Rose, V.; Molloy, K.; Gohy, S.; Pilette, C.; Greene, C.M. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediat. Inflamm. 2018, 2018, 1309746. [Google Scholar] [CrossRef]
- Nichols, D.P.; Chmiel, J.F. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S39–S56. [Google Scholar] [CrossRef] [PubMed]
- Forrest, O.A.; Ingersoll, S.A.; Preininger, M.K.; Laval, J.; Limoli, D.H.; Brown, M.R.; Lee, F.E.; Bedi, B.; Sadikot, R.T.; Goldberg, J.B.; et al. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J. Leukoc. Biol. 2018, 104, 665–675. [Google Scholar] [CrossRef]
- Margaroli, C.; Garratt, L.W.; Horati, H.; Dittrich, A.S.; Rosenow, T.; Montgomery, S.T.; Frey, D.L.; Brown, M.R.; Schultz, C.; Guglani, L.; et al. Elastase Exocytosis by Airway Neutrophils Is Associated with Early Lung Damage in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Turton, K.B.; Ingram, R.J.; Valvano, M.A. Macrophage dysfunction in cystic fibrosis: Nature or nurture? J. Leukoc. Biol. 2021, 109, 573–582. [Google Scholar] [CrossRef]
- Koeppen, K.; Nymon, A.; Barnaby, R.; Li, Z.; Hampton, T.H.; Ashare, A.; Stanton, B.A. CF monocyte-derived macrophages have an attenuated response to extracellular vesicles secreted by airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L530–L544. [Google Scholar] [CrossRef]
- Bruscia, E.M.; Zhang, P.X.; Ferreira, E.; Caputo, C.; Emerson, J.W.; Tuck, D.; Krause, D.S.; Egan, M.E. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am. J. Respir. Cell Mol. Biol. 2009, 40, 295–304. [Google Scholar] [CrossRef]
- Di, A.; Brown, M.E.; Deriy, L.V.; Li, C.; Szeto, F.L.; Chen, Y.; Huang, P.; Tong, J.; Naren, A.P.; Bindokas, V.; et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006, 8, 933–944. [Google Scholar] [CrossRef]
- Averna, M.; Bavestrello, M.; Cresta, F.; Pedrazzi, M.; De Tullio, R.; Minicucci, L.; Sparatore, B.; Salamino, F.; Pontremoli, S.; Melloni, E. Abnormal activation of calpain and protein kinase Calpha promotes a constitutive release of matrix metalloproteinase 9 in peripheral blood mononuclear cells from cystic fibrosis patients. Arch. Biochem. Biophys. 2016, 604, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Haggie, P.M.; Verkman, A.S. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J. Biol. Chem. 2007, 282, 31422–31428. [Google Scholar] [CrossRef]
- Barriere, H.; Bagdany, M.; Bossard, F.; Okiyoneda, T.; Wojewodka, G.; Gruenert, D.; Radzioch, D.; Lukacs, G.L. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol. Biol. Cell 2009, 20, 3125–3141. [Google Scholar] [CrossRef]
- Law, S.M.; Stanfield, S.J.; Hardisty, G.R.; Dransfield, I.; Campbell, C.J.; Gray, R.D. Human cystic fibrosis monocyte derived macrophages display no defect in acidification of phagolysosomes when measured by optical nanosensors. J. Cyst. Fibros. 2020, 19, 203–210. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Zaslona, Z.; Becker-Flegler, K.; Palsson-McDermott, E.M.; Boland, F.; Gunaratnam, C.; Gulbins, E.; O’Neill, L.A.; Reeves, E.P.; McElvaney, N.G. Specific Inhibition of the NLRP3 Inflammasome as an Antiinflammatory Strategy in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.C.; Khanal, S.; Gomez, J.L.; Sauler, M.; Adams, T.S.; Chupp, G.L.; Yan, X.; Poli, S.; Zhao, Y.; Montgomery, R.R.; et al. Single-Cell Transcriptional Archetypes of Airway Inflammation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Vencken, S.F.; Greene, C.M. Toll-Like Receptors in Cystic Fibrosis: Impact of Dysfunctional microRNA on Innate Immune Responses in the Cystic Fibrosis Lung. J. Innate Immun. 2016, 8, 541–549. [Google Scholar] [CrossRef]
- Foell, D.; Seeliger, S.; Vogl, T.; Koch, H.G.; Maschek, H.; Harms, E.; Sorg, C.; Roth, J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax 2003, 58, 613–617. [Google Scholar] [CrossRef]
- Entezari, M.; Weiss, D.J.; Sitapara, R.; Whittaker, L.; Wargo, M.J.; Li, J.; Wang, H.; Yang, H.; Sharma, L.; Phan, B.D.; et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 2012, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Jackson, P.L.; Liu, G.; Hardison, M.; Livraghi, A.; Solomon, G.M.; McQuaid, D.B.; Noerager, B.D.; Gaggar, A.; Clancy, J.P.; et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am. J. Respir. Crit. Care Med. 2008, 178, 822–831. [Google Scholar] [CrossRef]
- Hunt, W.R.; Helfman, B.R.; McCarty, N.A.; Hansen, J.M. Advanced glycation end products are elevated in cystic fibrosis-related diabetes and correlate with worse lung function. J. Cyst. Fibros. 2016, 15, 681–688. [Google Scholar] [CrossRef]
- Lara-Reyna, S.; Holbrook, J.; Jarosz-Griffiths, H.H.; Peckham, D.; McDermott, M.F. Dysregulated signalling pathways in innate immune cells with cystic fibrosis mutations. Cell. Mol. Life Sci. 2020, 77, 4485–4503. [Google Scholar] [CrossRef] [PubMed]
- Scambler, T.; Jarosz-Griffiths, H.H.; Lara-Reyna, S.; Pathak, S.; Wong, C.; Holbrook, J.; Martinon, F.; Savic, S.; Peckham, D.; McDermott, M.F. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. eLife 2019, 8, e49248. [Google Scholar] [CrossRef]
- Balazs, A.; Mall, M.A. Mucus obstruction and inflammation in early cystic fibrosis lung disease: Emerging role of the IL-1 signaling pathway. Pediatr. Pulmonol. 2019, 54 (Suppl. S3), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Lara-Reyna, S.; Scambler, T.; Holbrook, J.; Wong, C.; Jarosz-Griffiths, H.H.; Martinon, F.; Savic, S.; Peckham, D.; McDermott, M.F. Metabolic Reprograming of Cystic Fibrosis Macrophages via the IRE1alpha Arm of the Unfolded Protein Response Results in Exacerbated Inflammation. Front. Immunol. 2019, 10, 1789. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Dittrich, A.S.; Garratt, L.W.; Turkovic, L.; Frey, D.L.; Stick, S.M.; Mall, M.A.; Kicic, A.; Arest, C.F. Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 715–722. [Google Scholar] [CrossRef]
- Rao, S.; Grigg, J. New insights into pulmonary inflammation in cystic fibrosis. Arch. Dis. Child. 2006, 91, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.T.; Mall, M.A.; Kicic, A.; Stick, S.M.; Arest, C.F. Hypoxia and sterile inflammation in cystic fibrosis airways: Mechanisms and potential therapies. Eur. Respir. J. 2017, 49, 1600903. [Google Scholar] [CrossRef]
- Keiser, N.W.; Birket, S.E.; Evans, I.A.; Tyler, S.R.; Crooke, A.K.; Sun, X.; Zhou, W.; Nellis, J.R.; Stroebele, E.K.; Chu, K.K.; et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am. J. Respir. Cell Mol. Biol. 2015, 52, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, T.; Mok, L.C.; Turkovic, L.; Berry, L.J.; Sly, P.D.; Ranganathan, S.; Tiddens, H.; Stick, S.M. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur. Respir. J. 2019, 54, 1801771. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Lu, B.; Quintero, J.R.; Vargas, S.O.; Patel, A.S.; Owen, C.A.; Gerard, N.P.; Gerard, C.; Cernadas, M. CFTR regulates B cell activation and lymphoid follicle development. Respir. Res. 2019, 20, 133. [Google Scholar] [CrossRef]
- Hector, A.; Schafer, H.; Poschel, S.; Fischer, A.; Fritzsching, B.; Ralhan, A.; Carevic, M.; Oz, H.; Zundel, S.; Hogardt, M.; et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am. J. Respir. Crit. Care Med. 2015, 191, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Regamey, N.; Brown, S.; Bush, A.; Lloyd, C.M.; Davies, J.C. The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2011, 184, 252–258. [Google Scholar] [CrossRef]
- Dubin, P.J.; Kolls, J.K. IL-17 in cystic fibrosis: More than just Th17 cells. Am. J. Respir. Crit. Care Med. 2011, 184, 155–157. [Google Scholar] [CrossRef]
- Hsu, D.; Taylor, P.; Fletcher, D.; van Heeckeren, R.; Eastman, J.; van Heeckeren, A.; Davis, P.; Chmiel, J.F.; Pearlman, E.; Bonfield, T.L. Interleukin-17 Pathophysiology and Therapeutic Intervention in Cystic Fibrosis Lung Infection and Inflammation. Infect. Immun. 2016, 84, 2410–2421. [Google Scholar] [CrossRef]
- Decraene, A.; Willems-Widyastuti, A.; Kasran, A.; De Boeck, K.; Bullens, D.M.; Dupont, L.J. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable Cystic Fibrosis patients. Respir. Res. 2010, 11, 177. [Google Scholar] [CrossRef]
- Tan, H.L.; Rosenthal, M. IL-17 in lung disease: Friend or foe? Thorax 2013, 68, 788–790. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Taylor-Cousar, J.L. Cystic Fibrosis Modulator Therapies. Annu. Rev. Med. 2023, 74, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Southern, K.W.; Castellani, C.; Lammertyn, E.; Smyth, A.; VanDevanter, D.; van Koningsbruggen-Rietschel, S.; Barben, J.; Bevan, A.; Brokaar, E.; Collins, S.; et al. Standards of care for CFTR variant-specific therapy (including modulators) for people with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 17–30. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Robinson, P.D.; Shteinberg, M.; Downey, D.G. CFTR modulator therapy: Transforming the landscape of clinical care in cystic fibrosis. Lancet 2023, 402, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.; Krishan, A.; Patel, S.; Sinha, I.P.; Southern, K.W. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database Syst. Rev. 2019, 1, CD009841. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.H.; Pilewski, J.M.; Griese, M.; Cooke, J.; Viswanathan, L.; Tullis, E.; Davies, J.C.; Lekstrom-Himes, J.A.; Wang, L.T.; Group, V.X.S. Tezacaftor/Ivacaftor in Subjects with Cystic Fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am. J. Respir. Crit. Care Med. 2018, 197, 214–224. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Drevinek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Pranke, I.; Golec, A.; Hinzpeter, A.; Edelman, A.; Sermet-Gaudelus, I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front. Pharmacol. 2019, 10, 121. [Google Scholar] [CrossRef]
- Sepahzad, A.; Morris-Rosendahl, D.J.; Davies, J.C. Cystic Fibrosis Lung Disease Modifiers and Their Relevance in the New Era of Precision Medicine. Genes 2021, 12, 562. [Google Scholar] [CrossRef]
- Drumm, M.L.; Konstan, M.W.; Schluchter, M.D.; Handler, A.; Pace, R.; Zou, F.; Zariwala, M.; Fargo, D.; Xu, A.; Dunn, J.M.; et al. Genetic modifiers of lung disease in cystic fibrosis. N. Engl. J. Med. 2005, 353, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Eckford, P.D.; Ramjeesingh, M.; Molinski, S.; Pasyk, S.; Dekkers, J.F.; Li, C.; Ahmadi, S.; Ip, W.; Chung, T.E.; Du, K.; et al. VX-809 and related corrector compounds exhibit secondary activity stabilizing active F508del-CFTR after its partial rescue to the cell surface. Chem. Biol. 2014, 21, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; de Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; van de Graaf, E.A.; Nieuwenhuis, E.E.; Houwen, R.H.; et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 2016, 8, 344ra384. [Google Scholar] [CrossRef]
- Pranke, I.M.; Hatton, A.; Simonin, J.; Jais, J.P.; Le Pimpec-Barthes, F.; Carsin, A.; Bonnette, P.; Fayon, M.; Stremler-Le Bel, N.; Grenet, D.; et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci. Rep. 2017, 7, 7375. [Google Scholar] [CrossRef]
- Bacalhau, M.; Camargo, M.; Magalhaes-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef]
- Mesinele, J.; Ruffin, M.; Guillot, L.; Corvol, H. Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes. Int. J. Mol. Sci. 2022, 23, 14205. [Google Scholar] [CrossRef]
- Slieker, M.G.; Sanders, E.A.; Rijkers, G.T.; Ruven, H.J.; van der Ent, C.K. Disease modifying genes in cystic fibrosis. J. Cyst. Fibros. 2005, 4 (Suppl. S2), 7–13. [Google Scholar] [CrossRef]
- Guillot, L.; Beucher, J.; Tabary, O.; Le Rouzic, P.; Clement, A.; Corvol, H. Lung disease modifier genes in cystic fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 83–93. [Google Scholar] [CrossRef]
- Kuroki, Y.; Takahashi, M.; Nishitani, C. Pulmonary collectins in innate immunity of the lung. Cell. Microbiol. 2007, 9, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Pressler, T.; Madsen, H.O.; Frederiksen, B.; Svejgaard, A.; Hoiby, N.; Schwartz, M.; Koch, C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Investig. 1999, 104, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.L.; Murphy, P.C.; Alink, J.J.; Leigh, M.W.; Hull, W.M.; Stahlman, M.T.; Whitsett, J.A. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, R.; Sandford, A.; Taylor, C.; Huang, B.; Frangolias, D.; Wang, Y.; Sang, R.; Pereira, L.; Sun, L.; Berthiaume, Y.; et al. Complex two-gene modulation of lung disease severity in children with cystic fibrosis. J. Clin. Investig. 2008, 118, 1040–1049. [Google Scholar] [CrossRef]
- Zuo, L.; Wang, K.; Luo, X. Use of diplotypes—matched haplotype pairs from homologous chromosomes—in gene-disease association studies. Shanghai Arch. Psychiatry 2014, 26, 165–170. [Google Scholar]
- Lin, Z.; Thorenoor, N.; Wu, R.; DiAngelo, S.L.; Ye, M.; Thomas, N.J.; Liao, X.; Lin, T.R.; Warren, S.; Floros, J. Genetic Association of Pulmonary Surfactant Protein Genes, SFTPA1, SFTPA2, SFTPB, SFTPC, and SFTPD With Cystic Fibrosis. Front. Immunol. 2018, 9, 2256. [Google Scholar] [CrossRef]
- Greene, C.M.; Carroll, T.P.; Smith, S.G.; Taggart, C.C.; Devaney, J.; Griffin, S.; O’Neill, S.J.; McElvaney, N.G. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 2005, 174, 1638–1646. [Google Scholar] [CrossRef]
- Haerynck, F.; Mahachie John, J.M.; Van Steen, K.; Schelstraete, P.; Van daele, S.; Loeys, B.; Van Thielen, M.; De Canck, I.; Nuytinck, L.; De Baets, F. Genetic variations in toll-like receptor pathway and lung function decline in Cystic fibrosis patients. Hum. Immunol. 2013, 74, 1649–1655. [Google Scholar] [CrossRef]
- Blohmke, C.J.; Park, J.; Hirschfeld, A.F.; Victor, R.E.; Schneiderman, J.; Stefanowicz, D.; Chilvers, M.A.; Durie, P.R.; Corey, M.; Zielenski, J.; et al. TLR5 as an anti-inflammatory target and modifier gene in cystic fibrosis. J. Immunol. 2010, 185, 7731–7738. [Google Scholar] [CrossRef] [PubMed]
- Beucher, J.; Boelle, P.Y.; Busson, P.F.; Muselet-Charlier, C.; Clement, A.; Corvol, H.; The French C F Modifier Gene Study Investigators. AGER−429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS ONE 2012, 7, e41913. [Google Scholar]
- De Torre-Minguela, C.; Mesa Del Castillo, P.; Pelegrin, P. The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Front. Immunol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Atalay, M.; Şen, B.; Dayangaç Erden, D. NLRP3 inflammasome as a novel target for cystic fibrosis treatment. Bull. Natl. Res. Cent. 2023, 47, 29. [Google Scholar] [CrossRef]
- Graustein, A.D.; Berrington, W.R.; Buckingham, K.J.; Nguyen, F.K.; Joudeh, L.L.; Rosenfeld, M.; Bamshad, M.J.; Gibson, R.L.; Hawn, T.R.; Emond, M.J. Inflammasome Genetic Variants, Macrophage Function, and Clinical Outcomes in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 65, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hou, Y.; Sun, F.; Yang, Z.; Li, C. Dysregulated Chemokine Signaling in Cystic Fibrosis Lung Disease: A Potential Therapeutic Target. Curr. Drug Targets 2016, 17, 1535–1544. [Google Scholar] [CrossRef]
- Hillian, A.D.; Londono, D.; Dunn, J.M.; Goddard, K.A.; Pace, R.G.; Knowles, M.R.; Drumm, M.L.; CF Gene Modifier Study Group. Modulation of cystic fibrosis lung disease by variants in interleukin-8. Genes Immun. 2008, 9, 501–508. [Google Scholar] [CrossRef][Green Version]
- De Vries, L.; Griffiths, A.; Armstrong, D.; Robinson, P.J. Cytokine gene polymorphisms and severity of CF lung disease. J. Cyst. Fibros. 2014, 13, 699–705. [Google Scholar] [CrossRef]
- Stanke, F.; Becker, T.; Kumar, V.; Hedtfeld, S.; Becker, C.; Cuppens, H.; Tamm, S.; Yarden, J.; Laabs, U.; Siebert, B.; et al. Genes that determine immunology and inflammation modify the basic defect of impaired ion conductance in cystic fibrosis epithelia. J. Med. Genet. 2011, 48, 24–31. [Google Scholar] [CrossRef]
- Labenski, H.; Hedtfeld, S.; Becker, T.; Tummler, B.; Stanke, F. Initial interrogation, confirmation and fine mapping of modifying genes: STAT3, IL1B and IFNGR1 determine cystic fibrosis disease manifestation. Eur. J. Hum. Genet. 2011, 19, 1281–1288. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Shmarina, G.; Pukhalsky, A.; Petrova, N.; Zakharova, E.; Avakian, L.; Kapranov, N.; Alioshkin, V. TNF gene polymorphisms in cystic fibrosis patients: Contribution to the disease progression. J. Transl. Med. 2013, 11, 19. [Google Scholar] [CrossRef]
- Sagwal, S.; Chauhan, A.; Kaur, J.; Prasad, R.; Singh, M.; Singh, M. Association of Serum TGF-beta1 Levels with Different Clinical Phenotypes of Cystic Fibrosis Exacerbation. Lung 2020, 198, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Corvol, H.; Boelle, P.Y.; Brouard, J.; Knauer, N.; Chadelat, K.; Henrion-Caude, A.; Flamant, C.; Muselet-Charlier, C.; Boule, M.; Fauroux, B.; et al. Genetic variations in inflammatory mediators influence lung disease progression in cystic fibrosis. Pediatr. Pulmonol. 2008, 43, 1224–1232. [Google Scholar] [CrossRef]
- Trojan, T.; Alejandre Alcazar, M.A.; Fink, G.; Thomassen, J.C.; Maessenhausen, M.V.; Rietschel, E.; Schneider, P.M.; van Koningsbruggen-Rietschel, S. The effect of TGF-β(1) polymorphisms on pulmonary disease progression in patients with cystic fibrosis. BMC Pulm. Med. 2022, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.L.; Marson, F.A.; Ribeiro, J.D.; Bertuzzo, C.S.; Salomao Junior, J.B.; Souza, D.R. IL8 gene as modifier of cystic fibrosis: Unraveling the factors which influence clinical variability. Hum. Genet. 2016, 135, 881–894. [Google Scholar] [CrossRef]
- Hassanzad, M.; Farnia, P.; Ghanavi, J.; Parvini, F.; Saif, S.; Velayati, A.A. TNFalpha −857 C/T and TNFR2 +587 T/G polymorphisms are associated with cystic fibrosis in Iranian patients. Eur. J. Med. Genet. 2019, 62, 103584. [Google Scholar] [CrossRef]
- Keown, K.; Brown, R.; Doherty, D.F.; Houston, C.; McKelvey, M.C.; Creane, S.; Linden, D.; McAuley, D.F.; Kidney, J.C.; Weldon, S.; et al. Airway Inflammation and Host Responses in the Era of CFTR Modulators. Int. J. Mol. Sci. 2020, 21, 6379. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Awatade, N.T.; Uliyakina, I.; Farinha, C.M.; Clarke, L.A.; Mendes, K.; Sole, A.; Pastor, J.; Ramos, M.M.; Amaral, M.D. Measurements of Functional Responses in Human Primary Lung Cells as a Basis for Personalized Therapy for Cystic Fibrosis. EBioMedicine 2015, 2, 147–153. [Google Scholar] [CrossRef]
- Awatade, N.T.; Wong, S.L.; Hewson, C.K.; Fawcett, L.K.; Kicic, A.; Jaffe, A.; Waters, S.A. Human Primary Epithelial Cell Models: Promising Tools in the Era of Cystic Fibrosis Personalized Medicine. Front. Pharmacol. 2018, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C.; Moskowitz, S.M.; Brown, C.; Horsley, A.; Mall, M.A.; McKone, E.F.; Plant, B.J.; Prais, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; et al. VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Karp, P.H.; Tan, P.; Goodell, B.J.; Pezzulo, A.A.; Thurman, A.L.; Thornell, I.M.; Durfey, S.L.; Duffey, M.E.; Stoltz, D.A.; et al. Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators. J. Clin. Investig. 2021, 131, e150398. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Heltshe, S.L.; Gonska, T.; Donaldson, S.H.; Borowitz, D.; Gelfond, D.; Sagel, S.D.; Khan, U.; Mayer-Hamblett, N.; Van Dalfsen, J.M.; et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 175–184. [Google Scholar] [CrossRef]
- Hisert, K.B.; Heltshe, S.L.; Pope, C.; Jorth, P.; Wu, X.; Edwards, R.M.; Radey, M.; Accurso, F.J.; Wolter, D.J.; Cooke, G.; et al. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. Am. J. Respir. Crit. Care Med. 2017, 195, 1617–1628. [Google Scholar] [CrossRef]
- Harris, J.K.; Wagner, B.D.; Zemanick, E.T.; Robertson, C.E.; Stevens, M.J.; Heltshe, S.L.; Rowe, S.M.; Sagel, S.D. Changes in Airway Microbiome and Inflammation with Ivacaftor Treatment in Patients with Cystic Fibrosis and the G551D Mutation. Ann. Am. Thorac. Soc. 2020, 17, 212–220. [Google Scholar] [CrossRef]
- McNally, P.; Butler, D.; Karpievitch, Y.V.; Linnane, B.; Ranganathan, S.; Stick, S.M.; Hall, G.L.; Schultz, A. Ivacaftor and Airway Inflammation in Preschool Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 605–608. [Google Scholar] [CrossRef]
- Mainz, J.G.; Arnold, C.; Wittstock, K.; Hipler, U.C.; Lehmann, T.; Zagoya, C.; Duckstein, F.; Ellemunter, H.; Hentschel, J. Ivacaftor Reduces Inflammatory Mediators in Upper Airway Lining Fluid from Cystic Fibrosis Patients with a G551D Mutation: Serial Non-Invasive Home-Based Collection of Upper Airway Lining Fluid. Front. Immunol. 2021, 12, 642180. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Boutin, S.; Wielputz, M.O.; Joachim, C.; Frey, D.L.; Wege, S.; Sommerburg, O.; Kauczor, H.U.; Stahl, M.; Dalpke, A.H.; et al. Effects of Lumacaftor-Ivacaftor on Lung Clearance Index, Magnetic Resonance Imaging, and Airway Microbiome in Phe508del Homozygous Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2021, 18, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Meoli, A.; Eickmeier, O.; Pisi, G.; Fainardi, V.; Zielen, S.; Esposito, S. Impact of CFTR Modulators on the Impaired Function of Phagocytes in Cystic Fibrosis Lung Disease. Int. J. Mol. Sci. 2022, 23, 12421. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Hayes, E.; Keenan, J.; Henry, M.; Meleady, P.; Molloy, K.; Jundi, B.; Bergin, D.A.; McCarthy, C.; McElvaney, O.J.; et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014, 124, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Bratcher, P.E.; Rowe, S.M.; Reeves, G.; Roberts, T.; Szul, T.; Harris, W.T.; Tirouvanziam, R.; Gaggar, A. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J. Cyst. Fibros. 2016, 15, 67–73. [Google Scholar] [CrossRef]
- Hisert, K.B.; Schoenfelt, K.Q.; Cooke, G.; Grogan, B.; Launspach, J.L.; Gallagher, C.G.; Donnelly, S.C.; Welsh, M.J.; Singh, P.K.; McKone, E.F.; et al. Ivacaftor-Induced Proteomic Changes Suggest Monocyte Defects May Contribute to the Pathogenesis of Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 54, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; D’Oria, S.; Favia, M.; Castellani, S.; Santostasi, T.; Polizzi, A.M.; Mariggio, M.A.; Gallo, C.; Casavola, V.; Montemurro, P.; et al. CFTR-dependent chloride efflux in cystic fibrosis mononuclear cells is increased by ivacaftor therapy. Pediatr. Pulmonol. 2017, 52, 900–908. [Google Scholar] [CrossRef]
- White, M.M.; Geraghty, P.; Hayes, E.; Cox, S.; Leitch, W.; Alfawaz, B.; Lavelle, G.M.; McElvaney, O.J.; Flannery, R.; Keenan, J.; et al. Neutrophil Membrane Cholesterol Content is a Key Factor in Cystic Fibrosis Lung Disease. EBioMedicine 2017, 23, 173–184. [Google Scholar] [CrossRef]
- Zhang, S.; Shrestha, C.L.; Kopp, B.T. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci. Rep. 2018, 8, 17066. [Google Scholar] [CrossRef]
- Hisert, K.B.; Birkland, T.P.; Schoenfelt, K.Q.; Long, M.E.; Grogan, B.; Carter, S.; Liles, W.C.; McKone, E.F.; Becker, L.; Manicone, A.M.; et al. CFTR Modulator Therapy Enhances Peripheral Blood Monocyte Contributions to Immune Responses in People with Cystic Fibrosis. Front. Pharmacol. 2020, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzi, M.; Vercellone, S.; Barberis, E.; Capraro, M.; De Tullio, R.; Cresta, F.; Casciaro, R.; Castellani, C.; Patrone, M.; Marengo, E.; et al. Identification of Potential Leukocyte Biomarkers Related to Drug Recovery of CFTR: Clinical Applications in Cystic Fibrosis. Int. J. Mol. Sci. 2021, 22, 3928. [Google Scholar] [CrossRef] [PubMed]
- Hardisty, G.R.; Law, S.M.; Carter, S.; Grogan, B.; Singh, P.K.; McKone, E.F.; Gray, R.D. Ivacaftor modifies cystic fibrosis neutrophil phenotype in subjects with R117H residual function CFTR mutations. Eur. Respir. J. 2021, 57, 2002161. [Google Scholar] [CrossRef]
- Barnaby, R.; Koeppen, K.; Nymon, A.; Hampton, T.H.; Berwin, B.; Ashare, A.; Stanton, B.A. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L432–L438. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.J.; Main, E.T.; Wilson, H.M.; Armstrong-James, D.; Warris, A. CFTR Modulators Dampen Aspergillus-Induced Reactive Oxygen Species Production by Cystic Fibrosis Phagocytes. Front. Cell. Infect. Microbiol. 2020, 10, 372. [Google Scholar] [CrossRef]
- Jarosz-Griffiths, H.H.; Scambler, T.; Wong, C.H.; Lara-Reyna, S.; Holbrook, J.; Martinon, F.; Savic, S.; Whitaker, P.; Etherington, C.; Spoletini, G.; et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. eLife 2020, 9, e54556. [Google Scholar] [CrossRef]
- Shrestha, C.L.; Zhang, S.; Wisniewski, B.; Hafner, S.; Elie, J.; Meijer, L.; Kopp, B.T. (R)-Roscovitine and CFTR modulators enhance killing of multi-drug resistant Burkholderia cenocepacia by cystic fibrosis macrophages. Sci. Rep. 2020, 10, 21700. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; Eltobgy, M.; Krause, K.; Hamilton, K.; Estfanous, S.; Daily, K.P.; Abu Khweek, A.; Hegazi, A.; Anne, M.N.K.; Carafice, C.; et al. CFTR Modulators Restore Acidification of Autophago-Lysosomes and Bacterial Clearance in Cystic Fibrosis Macrophages. Front. Cell. Infect. Microbiol. 2022, 12, 819554. [Google Scholar] [CrossRef]
- Gabillard-Lefort, C.; Casey, M.; Glasgow, A.M.A.; Boland, F.; Kerr, O.; Marron, E.; Lyons, A.M.; Gunaratnam, C.; McElvaney, N.G.; Reeves, E.P. Trikafta Rescues CFTR and Lowers Monocyte P2X7R-induced Inflammasome Activation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 205, 783–794. [Google Scholar] [CrossRef]
- Ruffin, M.; Roussel, L.; Maille, E.; Rousseau, S.; Brochiero, E. Vx-809/Vx-770 treatment reduces inflammatory response to Pseudomonas aeruginosa in primary differentiated cystic fibrosis bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L635–L641. [Google Scholar] [CrossRef]
- Borcherding, D.C.; Siefert, M.E.; Lin, S.; Brewington, J.; Sadek, H.; Clancy, J.P.; Plafker, S.M.; Ziady, A.G. Clinically-approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. J. Clin. Investig. 2019, 129, 3448–3463. [Google Scholar] [CrossRef]
- Ribeiro, C.M.P.; Gentzsch, M. Impact of Airway Inflammation on the Efficacy of CFTR Modulators. Cells 2021, 10, 3260. [Google Scholar] [CrossRef] [PubMed]
- Gentzsch, M.; Cholon, D.M.; Quinney, N.L.; Martino, M.E.B.; Minges, J.T.; Boyles, S.E.; Guhr Lee, T.N.; Esther, C.R., Jr.; Ribeiro, C.M.P. Airway Epithelial Inflammation In Vitro Augments the Rescue of Mutant CFTR by Current CFTR Modulator Therapies. Front. Pharmacol. 2021, 12, 628722. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Welsh, M.J. Inflammation as a Regulator of the Airway Surface Liquid pH in Cystic Fibrosis. Cells 2023, 12, 1104. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Hopkins, B.D.; Wolfe, A.L.; DiMango, E.; Kitur, K.; Parsons, R.; Prince, A. Cystic Fibrosis Transmembrane Conductance Regulator Attaches Tumor Suppressor PTEN to the Membrane and Promotes Anti Pseudomonas aeruginosa Immunity. Immunity 2017, 47, 1169–1181.e1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shrestha, C.L.; Robledo-Avila, F.; Jaganathan, D.; Wisniewski, B.L.; Brown, N.; Pham, H.; Carey, K.; Amer, A.O.; Hall-Stoodley, L.; et al. Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor. Eur. Respir. J. 2023, 61, 2102861. [Google Scholar] [CrossRef]
- Aridgides, D.S.; Mellinger, D.L.; Gwilt, L.L.; Hampton, T.H.; Mould, D.L.; Hogan, D.A.; Ashare, A. Comparative effects of CFTR modulators on phagocytic, metabolic and inflammatory profiles of CF and nonCF macrophages. Sci. Rep. 2023, 13, 11995. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Hopfer, L.M.; Wohlgemuth, L.; Knapp, C.L.; Mohamed, A.O.K.; Stukan, L.; Munnich, F.; Husken, D.; Koller, A.S.; Stratmann, A.E.P.; et al. Multimodal analysis of granulocytes, monocytes, and platelets in patients with cystic fibrosis before and after Elexacaftor-Tezacaftor-Ivacaftor treatment. Front. Immunol. 2023, 14, 1180282. [Google Scholar] [CrossRef]
- Sheikh, S.; Britt, R.D., Jr.; Ryan-Wenger, N.A.; Khan, A.Q.; Lewis, B.W.; Gushue, C.; Ozuna, H.; Jaganathan, D.; McCoy, K.; Kopp, B.T. Impact of elexacaftor-tezacaftor-ivacaftor on bacterial colonization and inflammatory responses in cystic fibrosis. Pediatr. Pulmonol. 2023, 58, 825–833. [Google Scholar] [CrossRef]
- Dhote, T.; Martin, C.; Regard, L.; Pesenti, L.; Kanaan, R.; Carlier, N.; Honore, I.; Da Silva, J.; Witko-Sarsat, V.; Burgel, P.R. Normalisation of circulating neutrophil counts after 12 months of elexacaftor-tezacaftor-ivacaftor in patients with advanced cystic fibrosis. Eur. Respir. J. 2023, 61, 2202096. [Google Scholar] [CrossRef]
- Boyle, M.P.; Bell, S.C.; Konstan, M.W.; McColley, S.A.; Rowe, S.M.; Rietschel, E.; Huang, X.; Waltz, D.; Patel, N.R.; Rodman, D. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: A phase 2 randomised controlled trial. Lancet Respir. Med. 2014, 2, 527–538. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef]
- Hampton, T.H.; Thomas, D.; van der Gast, C.; O’Toole, G.A.; Stanton, B.A. Mild Cystic Fibrosis Lung Disease Is Associated with Bacterial Community Stability. Microbiol. Spectr. 2021, 9, e0002921. [Google Scholar] [CrossRef] [PubMed]
- Polineni, D.; Dang, H.; Gallins, P.J.; Jones, L.C.; Pace, R.G.; Stonebraker, J.R.; Commander, L.A.; Krenicky, J.E.; Zhou, Y.H.; Corvol, H.; et al. Airway Mucosal Host Defense Is Key to Genomic Regulation of Cystic Fibrosis Lung Disease Severity. Am. J. Respir. Crit. Care Med. 2018, 197, 79–93. [Google Scholar] [CrossRef]
- Sergeev, V.; Chou, F.Y.; Lam, G.Y.; Hamilton, C.M.; Wilcox, P.G.; Quon, B.S. The Extrapulmonary Effects of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 147–154. [Google Scholar] [CrossRef]
- Hey, J.; Paulsen, M.; Toth, R.; Weichenhan, D.; Butz, S.; Schatterny, J.; Liebers, R.; Lutsik, P.; Plass, C.; Mall, M.A. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat. Commun. 2021, 12, 6520. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, L.I.; Tarca, E.; Cojocaru, E.; Rusu, C.; Moisa, S.M.; Leon Constantin, M.M.; Gorduza, E.V.; Trandafir, L.M. Genetic Modifying Factors of Cystic Fibrosis Phenotype: A Challenge for Modern Medicine. J. Clin. Med. 2021, 10, 5821. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Russo, L.; Madsen, A.; Webster, J.; Becnel, L. Use of Real-World Evidence to Drive Drug Development Strategy and Inform Clinical Trial Design. Clin. Pharmacol. Ther. 2022, 111, 77–89. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Bomberger, J.M.; Ye, S.; Maceachran, D.P.; Koeppen, K.; Barnaby, R.L.; O’Toole, G.A.; Stanton, B.A. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011, 7, e1001325. [Google Scholar] [CrossRef]
- Stanton, B.A. Effects of Pseudomonas aeruginosa on CFTR chloride secretion and the host immune response. Am. J. Physiol. Cell Physiol. 2017, 312, C357–C366. [Google Scholar] [CrossRef]
- Joynt, A.T.; Cutting, G.R.; Sharma, N. Genetics of Cystic Fibrosis: Clinical Implications. Clin. Chest Med. 2022, 43, 591–602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Mutation Class | Mechanism | Examples | CFTR Modulator Therapy |
|---|---|---|---|
| I | Production of a truncated CFTR mRNA → truncated protein and RNA decay | G542X, R553X, R1162X, W1282X | Read-through agents, nonsense-mediated decay pathway inhibitors, gene therapy (in development) |
| II | Defect in CFTR protein trafficking to the PM | G85E, I507del, F508del, N1303K | Dual combination for F508del homozygous or heterozygous for F508del and G551D: - potentiator (ivacaftor)/corrector (lumacaftor (Orkambi) or tezacaftor (Symdeko)) Triple combination for F508del heterozygous: - potentiator (ivacaftor)/correctors (tezacaftor + elexacaftor) (tradename EU: Kaftrio, tradename USA: Trikafta) |
| III | Channel gating defect | S549R, G551D, G1349D | Potentiator, e.g., ivacaftor |
| IV | Defect in channel conductance | R117H, R334W, D1152H | Potentiator, e.g., ivacaftor |
| V | Alternative splicing → reduction in mRNA levels | A455E, 2789+5G>A, 3849+10kbC>T | Amplifier (none in clinic) |
| VI | Lack of CFTR protein recycling → decrease PM levels | F508del, Q1411X | Stabilizer (none in clinic) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, A.; Vitullo, P.; Di Gioia, S.; Conese, M. Lung Inflammatory Genes in Cystic Fibrosis and Their Relevance to Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapies. Genes 2023, 14, 1966. https://doi.org/10.3390/genes14101966
Carbone A, Vitullo P, Di Gioia S, Conese M. Lung Inflammatory Genes in Cystic Fibrosis and Their Relevance to Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapies. Genes. 2023; 14(10):1966. https://doi.org/10.3390/genes14101966
Chicago/Turabian StyleCarbone, Annalucia, Pamela Vitullo, Sante Di Gioia, and Massimo Conese. 2023. "Lung Inflammatory Genes in Cystic Fibrosis and Their Relevance to Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapies" Genes 14, no. 10: 1966. https://doi.org/10.3390/genes14101966
APA StyleCarbone, A., Vitullo, P., Di Gioia, S., & Conese, M. (2023). Lung Inflammatory Genes in Cystic Fibrosis and Their Relevance to Cystic Fibrosis Transmembrane Conductance Regulator Modulator Therapies. Genes, 14(10), 1966. https://doi.org/10.3390/genes14101966







