Notch Signaling Regulates Mouse Perivascular Adipose Tissue Function via Mitochondrial Pathways
Abstract
:1. Introduction
2. Materials and Methods
3. Results
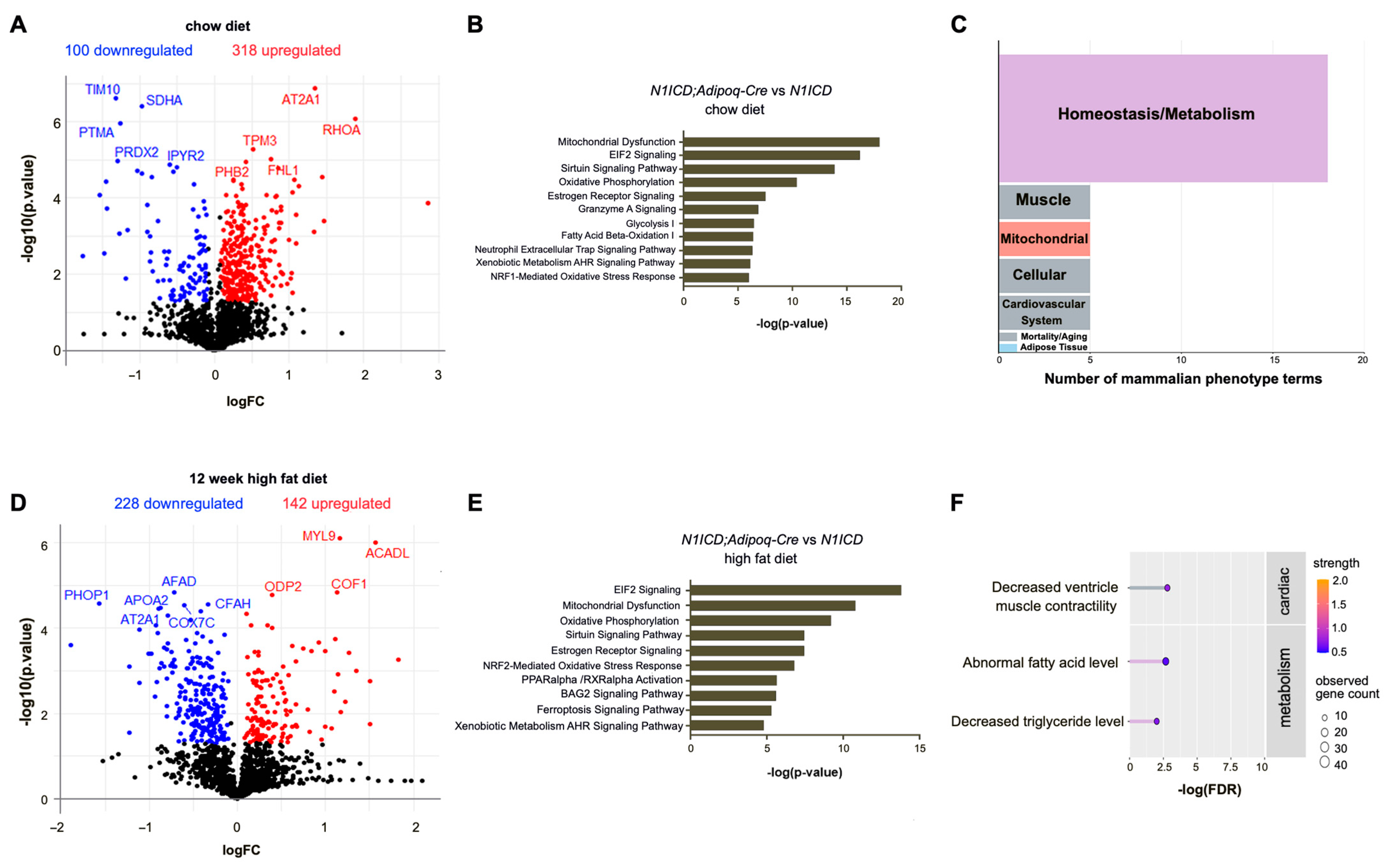
3.1. Notch Activation Regulates Metabolism-Related Pathways in Perivascular Adipose Tissue In Vivo
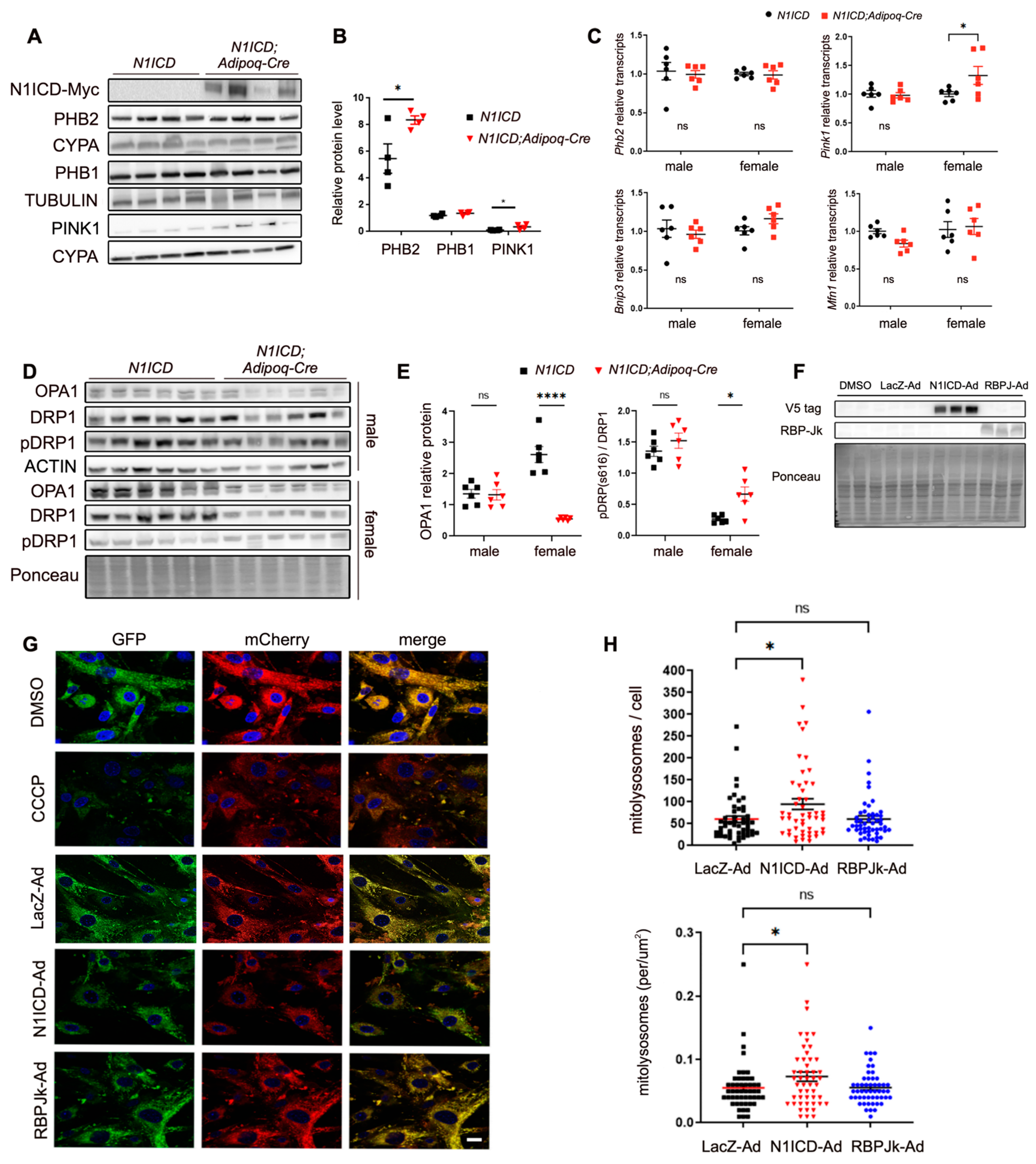
3.2. Activation of Notch Signaling Alters Mitochondria Biogenesis and Metabolism Gene Expression in Differentiated PVAT SVFs
3.3. Notch Signaling Regulates Mitochondrial Dynamics in PVAT-SVF
3.4. Notch Signaling Regulates Ferroptosis Pathways in PVAT-SVF Differentiated Adipocytes In Vitro
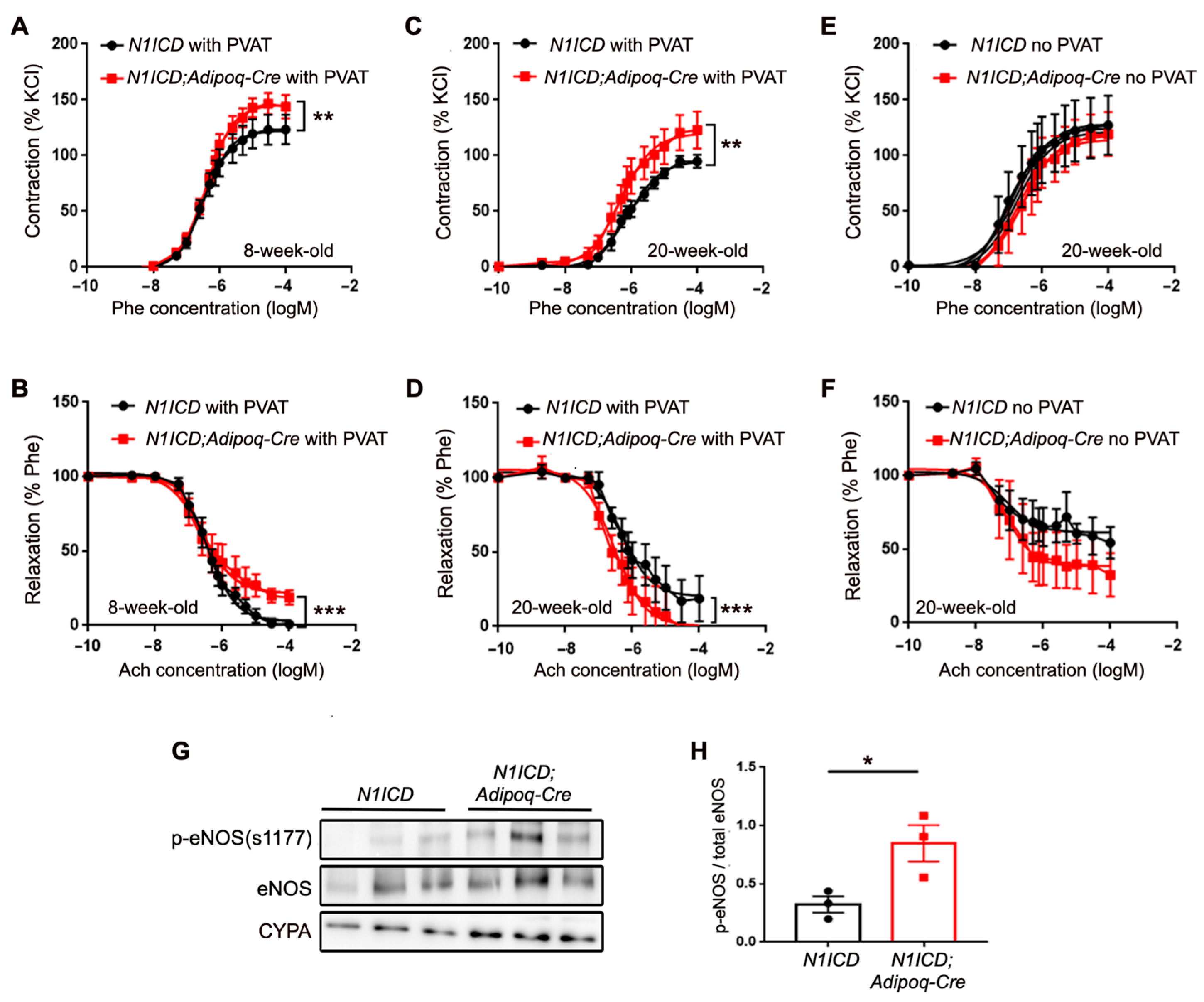
3.5. Activation of Notch in PVAT Leads to Altered Vascular Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Major Resources Tables
| Strain | Source | Background | Sex |
|---|---|---|---|
| Mus musculus B6.FVB-Tg(Adipoqcre)1Evdr/J | Jackson Laboratory Stock 028020 | C57BL/6;FVB | Male and Female |
| FVB-Tg(CAG-GFP;N1ICD) | Liaw Laboratory | FVB | Male and Female |
| Gt(ROSA)26Sortm1(CAG-mCherry/GFP)Ganl (mito-QC) | The Ganeley Lab | C57BL/6 | Male |
| Target Antigen | Vendor | Product Number | Working Concentration |
|---|---|---|---|
| β-ACTIN | Cell Signaling | 3700 | 0.84 mg/mL |
| α-TUBULIN | Cell Signaling | 2144 | 0.035 mg/mL |
| COX IV | Cell Signaling | 4850 | 0.358 mg/mL |
| DRP-1 | Cell Signaling | 5391 | 0.099 μg/mL |
| p-DRP1(s616) | Cell Signaling | 3455 | 0.12 mg/mL |
| GPX4 | Cell Signaling | 52455 | 0.095 mg/mL |
| MYC tag | Cell Signaling | 2278 | 0.013 mg/mL |
| OPA1 | BD Biosciences | 612606 | 0.25 mg/mL |
| PHB1 | Cell Signaling | 2426 | 0.025 μg/mL |
| PHB2 | Cell Signaling | 14805 | 0.05 mg/mL |
| PINK1 | Novus Biologicals | BC100-494 | 1 mg/mL |
| RBPJ-k | Cell Signaling | 5313 | 0.025 mg/mL |
| UCP1 | Cell Signaling | 14670 | 0.4 mg/mL |
| V5 tag | ThermoFisher | R960-25 | 0.204 μg/mL |
| Transcript | Forward: 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| Notch1 | TGCTCCTGTGCCACCTACTC | CCGGAGCTGGACGGTTGAATGC |
| Plin1 | TCAGGATAAGCTCTATGTCTCGTG | CCTGATCTTGAATGTTCTGTGGT |
| Fabp4 | CATGAAAGAAGTGGGAGTGGGC | AAGTACTCTCTGACCGGATGGT |
| Adipoq | TGACGACACCAAAAGCCCTCAG | CAGGATGTCCTGGGATGCCTG |
| Pgc1α | GTCAACAGCAAAAGCCACAA | TCTGGGGTCAGAGGAAGAGA |
| Ucp1 | GGGCCCTTGTAAACAACAAA | GTCGGTCCTTCCTTGGTGTA |
| Cidea | CTCGGCTGTCTCAATGTCAA | GGGATGGCTGCTCTTCTGTA |
| Prdm16 | CCACCAGCGAGGACTTCAC | GGAGGACTCTCGTAGCTCGAA |
| Phb2 | ATCCGTGTTCACCGTGGAAG | CCCGAATGTCATAGATGATGGG |
| Pink1 | GAGGAGCAGACTCCCAGTTC | CAGCCATCTGAGTCCCACTC |
| Bnip3 | TCCTGGGTAGAACTGCACTTC | GCTGGGCATCCAACAGTATTT |
| Mfn1 | ATGGCAGAAACGGTATCTCCA | CTCGGATGCTATTCGATCAAGTT |
| Cox1 | TAGCCCATGCAGGAGCATCA | TGGCTGGGGGTTTCATGTTGA |
| Cox2 | ACCTGGTGAACTACGACTGCT | CCTAGGGAGGGGACTGCTCA |
| Atp6 | TGGCATTAGCAGTCCGGCTT | ATGGTAGCTGTTGGTGGGCT |
| Atp8 | TTCCCACTGGCACCTTCACC | TGTTGGGGTAATGAATGAGGCAA |
| 16s RNA | CCGCAAGGGAAAGATGAAAGAC | TCGTTTGGTTTCGGGGTTTC |
| Actb | CCTGTGCTGCTCACCGAGGC | GACCCCGTCTCCGGAGTCCATC |
| Ppia | CCACCGTGTTCTTCGACAT | CAGTGCTCAGAGCTCGAAAG |
| Component | Stock Concentration | Working Concentration |
|---|---|---|
| DMEM/F-12 (50/50) | 1× | 1× |
| HI-FBS | 10× | 1× |
| Pen Strep or AB/AM | 100× | 1× |
| FGF (mouse) | 100 μg/mL | 0.01 μg/mL |
| Component | Stock Concentration | Working Concentration |
|---|---|---|
| IBMX | 500 mM | 0.5 mM |
| Dexamethasone | 1 mM | 5 μM |
| Indomethacin | 125 mM | 125 μM |
| DMEM/F-12 (50/50) | 1× | 1× |
| HI-FBS | 10× | 1× |
| Insulin | 200 μM | 170 nM |
| T3 | 1 mM | 2 nM |
| Rosiglitazone | 5 mM | 1 μM |
| Pen Strep or AB/AM | 100× | 1× |
| TGF-β RI Kinase Inhibitor VI | 10 mM | 5 μM |
| L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate | 50 mg/mL | 50 μg/mL |
| Component | Stock Concentration | Working Concentration |
|---|---|---|
| DMEM/F-12 (50/50) | 1× | 1× |
| HI-FBS | 10× | 1× |
| Insulin | 200 μM | 170 nM |
| T3 | 1 mM | 2 nM |
| Rosiglitazone | 5 mM | 1 μM |
| Pen Strep or AB/AM | 100× | 1× |
| TGF-β RI Kinase Inhibitor VI | 10 mM | 5 μM |
| L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate | 50 mg/mL | 50 μg/mL |
| Component | Vendor | Product Number |
|---|---|---|
| Heparin | Sigma | H3149 |
| Acetylcholine | Sigma | A2661 |
| Phenylephrine | Sigma | P6126 |
| Carbonyl cyanide 3-Chlorophenylhydrazone | Sigma | C2759 |
| BODIPYTM 581/591 C11 | ThermoFisher | D3861 |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Rosell, M.; Kaforou, M.; Frontini, A.; Okolo, A.; Chan, Y.W.; Nikolopoulou, E.; Millership, S.; Fenech, M.E.; MacIntyre, D.; Turner, J.O.; et al. Brown and white adipose tissues: Intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E945–E964. [Google Scholar] [CrossRef]
- Angueira, A.R.; Sakers, A.P.; Holman, C.D.; Cheng, L.; Arbocco, M.N.; Shamsi, F.; Lynes, M.D.; Shrestha, R.; Okada, C.; Batmanov, K.; et al. Defining the lineage of thermogenic perivascular adipose tissue. Nat. Metab. 2021, 3, 469–484. [Google Scholar] [CrossRef]
- Boucher, J.M.; Robich, M.; Scott, S.S.; Yang, X.; Ryzhova, L.; Turner, J.E.; Pinz, I.; Liaw, L. Rab27a Regulates Human Perivascular Adipose Progenitor Cell Differentiation. Cardiovasc. Drugs Ther. 2018, 32, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhang, G.; Liu, H.; Shi, J.; Qiu, H.; Liu, Y.; Han, F.; Hou, N. Relationships Between Perivascular Adipose Tissue and Abdominal Aortic Aneurysms. Front. Endocrinol. 2021, 12, 704845. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.M.; Ryzhova, L.; Harrington, A.; Davis-Knowlton, J.; Turner, J.E.; Cooper, E.; Maridas, D.; Ryzhov, S.; Rosen, C.J.; Vary, C.P.H.; et al. Pathological Conversion of Mouse Perivascular Adipose Tissue by Notch Activation. Arter. Thromb. Vasc. Biol. 2020, 40, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Shan, T.; Liu, W.; Yue, F.; Yang, X.; Liang, X.R.; Wang, J.; Li, J.; Carlesso, N.; Liu, X.; et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat. Med. 2014, 20, 911–918. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K.; Takimoto, E. Perivascular adipose tissue in vascular pathologies-a novel therapeutic target for atherosclerotic disease? Front. Cardiovasc. Med. 2023, 10, 1151717. [Google Scholar] [CrossRef]
- Hillock-Watling, C.; Gotlieb, A.I. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc. Pathol. 2022, 61, 107459. [Google Scholar] [CrossRef] [PubMed]
- Schottl, T.; Kappler, L.; Fromme, T.; Klingenspor, M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol. Metab. 2015, 4, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Mudder, K.; Schlotterer, A.; Fleming, T.; Heidenreich, E.; Qiu, R.; Hammes, H.P.; Nawroth, P.; Muckenthaler, M.U. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab. 2021, 51, 101235. [Google Scholar] [CrossRef]
- Venkatesh, D.A.; Park, K.S.; Harrington, A.; Miceli-Libby, L.; Yoon, J.K.; Liaw, L. Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations. Circ. Res. 2008, 103, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Walden, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- McWilliams, T.G.; Prescott, A.R.; Allen, G.F.; Tamjar, J.; Munson, M.J.; Thomson, C.; Muqit, M.M.; Ganley, I.G. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell. Biol. 2016, 214, 333–345. [Google Scholar] [CrossRef]
- Quiros, P.M.; Goyal, A.; Jha, P.; Auwerx, J. Analysis of mtDNA/nDNA Ratio in Mice. Curr. Protoc. Mouse Biol. 2017, 7, 47–54. [Google Scholar] [CrossRef]
- Sheikh, F.; Raskin, A.; Chu, P.H.; Lange, S.; Domenighetti, A.A.; Zheng, M.; Liang, X.; Zhang, T.; Yajima, T.; Gu, Y.; et al. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J. Clin. Investig. 2008, 118, 3870–3880. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell. Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef]
- Wei, Y.; Chiang, W.C.; Sumpter, R., Jr.; Mishra, P.; Levine, B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 2017, 168, 224–238.e10. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Mahdaviani, K.; Liesa, M.; Sereda, S.B.; Si, Y.; Las, G.; Twig, G.; Petrovic, N.; Zingaretti, C.; Graham, A.; et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 2014, 33, 418–436. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem. Biophys. Res. Commun. 2016, 478, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Q.; Xia, J.; Cheng, S.; Pei, D.; Zhang, X.; Shu, X. The E3 Ligase MIB1 Promotes Proteasomal Degradation of NRF2 and Sensitizes Lung Cancer Cells to Ferroptosis. Mol. Cancer Res. 2022, 20, 253–264. [Google Scholar] [CrossRef]
- Xing, M.; Ma, X.; Wang, X.; Wang, H.; Xie, M.; Zhang, Z.; Zhou, J. Emodin disrupts the Notch1/Nrf2/GPX4 antioxidant system and promotes renal cell ferroptosis. J. Appl. Toxicol. 2023, 43, 1702–1718. [Google Scholar] [CrossRef]
- Liang, X.; Qi, Y.; Dai, F.; Gu, J.; Yao, W. PVAT: An important guardian of the cardiovascular system. Histol. Histopathol. 2020, 35, 779–787. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular Adipose Tissue Oxidative Stress in Obesity. Antioxidants 2023, 12, 1595. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Stieber, C.; Malka, K.; Boucher, J.M.; Liaw, L. Human Perivascular Adipose Tissue as a Regulator of the Vascular Microenvironment and Diseases of the Coronary Artery and Aorta. J. Cardiol. Cardiovasc. Sci. 2019, 3, 10–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, Y.; Peng, T.; Li, L.; Zhu, W.; Liu, F.; Liu, S.; An, X.; Luo, R.; Cheng, J.; et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin. Sci. 2019, 133, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, J.; Liu, M.; Cui, J.; Lian, B.; Sun, Y.; Li, C. Notch3 regulates ferroptosis via ROS-induced lipid peroxidation in NSCLC cells. FEBS Open Bio 2022, 12, 1197–1205. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov. 2022, 8, 394. [Google Scholar] [CrossRef]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Yang, X.; Harrington, A.; Potts, C.; Kaija, A.; Ryzhova, L.; Liaw, L. Notch Signaling Regulates Mouse Perivascular Adipose Tissue Function via Mitochondrial Pathways. Genes 2023, 14, 1964. https://doi.org/10.3390/genes14101964
Yang C, Yang X, Harrington A, Potts C, Kaija A, Ryzhova L, Liaw L. Notch Signaling Regulates Mouse Perivascular Adipose Tissue Function via Mitochondrial Pathways. Genes. 2023; 14(10):1964. https://doi.org/10.3390/genes14101964
Chicago/Turabian StyleYang, Chenhao, Xuehui Yang, Anne Harrington, Christian Potts, Abigail Kaija, Larisa Ryzhova, and Lucy Liaw. 2023. "Notch Signaling Regulates Mouse Perivascular Adipose Tissue Function via Mitochondrial Pathways" Genes 14, no. 10: 1964. https://doi.org/10.3390/genes14101964
APA StyleYang, C., Yang, X., Harrington, A., Potts, C., Kaija, A., Ryzhova, L., & Liaw, L. (2023). Notch Signaling Regulates Mouse Perivascular Adipose Tissue Function via Mitochondrial Pathways. Genes, 14(10), 1964. https://doi.org/10.3390/genes14101964






