Recent Progress on Genetically Modified Animal Models for Membrane Skeletal Proteins: The 4.1 and MPP Families
Abstract
1. Protein 4.1 Family
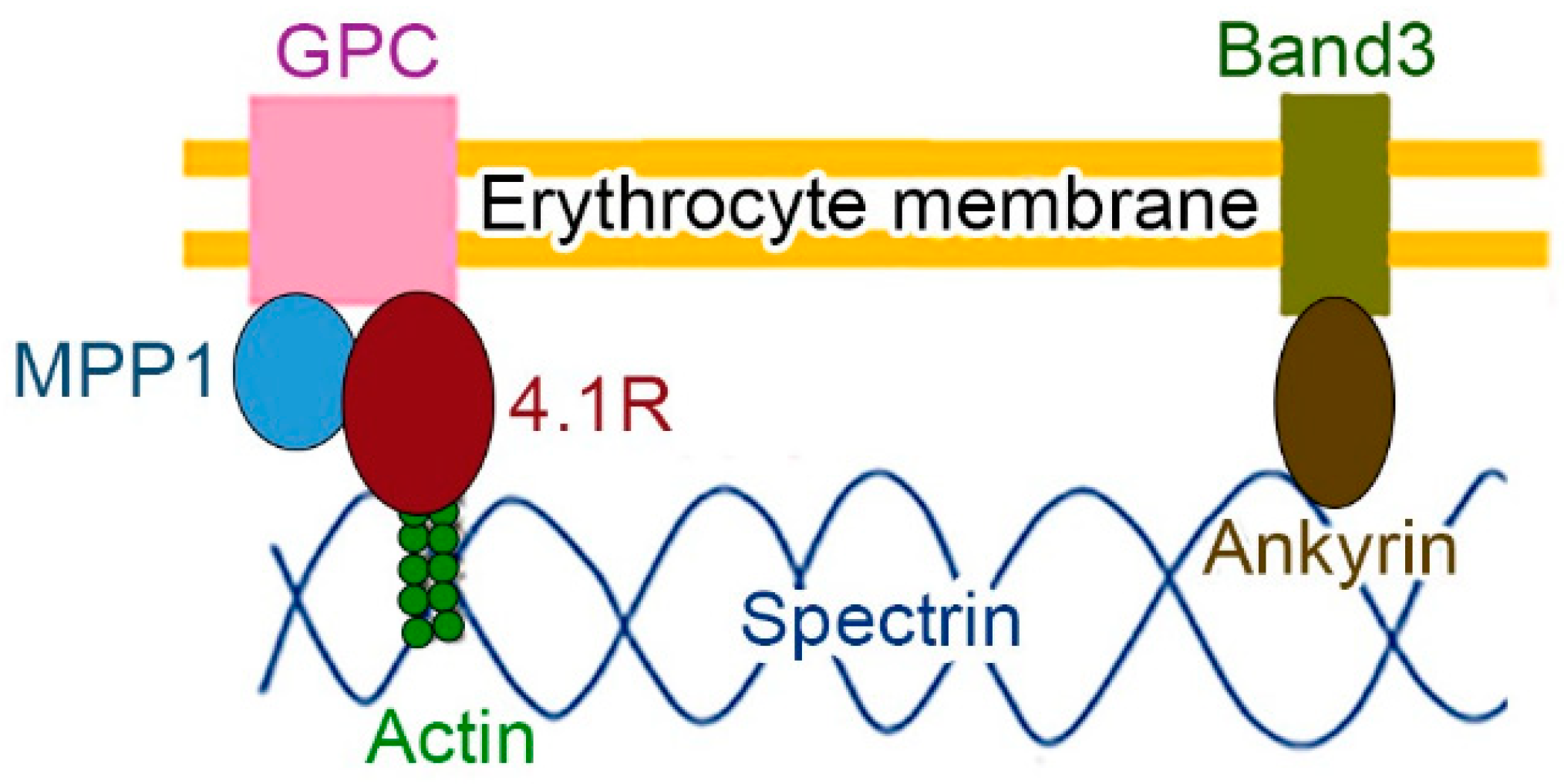
1.1. Protein 4.1 in the Membrane Skeleton
1.2. Protein 4.1G in PNS
1.3. Protein 4.1G in Bone Formation
2. MPP Family
2.1. MPP in Membrane Skeleton
2.2. MPP6 in PNS
2.3. MPPs and Lin7
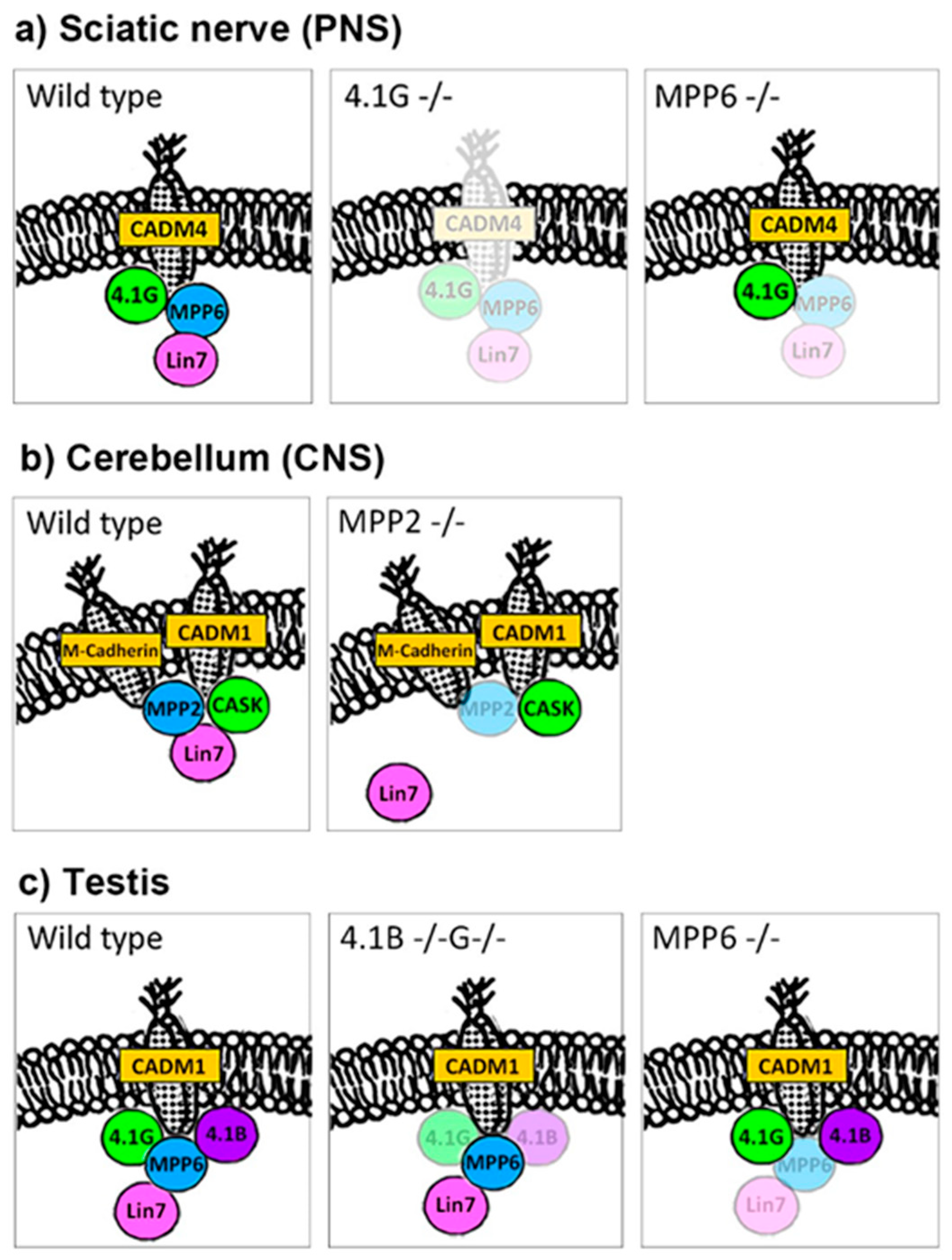
2.3.1. Lin7 in PNS (Figure 2a)
2.3.2. Lin7 in the CNS (Figure 2b)
2.3.3. Lin7 in Testis (Figure 2c)
2.3.4. Proteins Interact with Lin7
| Protein Name | Category | Tissues and Cells | Method | Related Proteins | Functional Consideration | References |
|---|---|---|---|---|---|---|
| AQP1 | 2 | Human melanoma WM115 and endothelial HMEC1 cell lines | IP, KD | β-catenin | AQP1-KD affects Lin7/β-catenin expression | [81] |
| BLT2 (Leukotriene B4 receptor) | 5 | MDCK cell line | PD, KD | CASK (Lin2) Mint (Lin10) | Transportation from the Golgi apparatus to the plasma membrane | [95] |
| BGT-1 (GABA transporter) | 4 | Recombinant Lin-7 and BGT-1 (PDZ target motif) | BC | Localization of transporter to plasma membranes | [82] | |
| CASK (Lin2) | 1 | Recombinant CASK, Velis proteins, rat brain Mouse brain | IHC, YTH, IP | Mint (Lin10) | Synaptic plasma membranes, synaptic vesicle exocytosis to cell adhesion | [69] |
| CASK | 1 | Mouse brain | BC, PD | Mint (Lin10) KIF17 | NR2B sorting vesicle carried by KIF17–Lin10 complex | [87] |
| Crumbs (Drosophila) | 1 | Drosophila eye under Lin7 mutation | IHC, PD | Stardust-PATJ | Light-dependent degeneration of photoreceptors | [96] |
| β-catenin | 2 | Recombinant β-catenin and Lin7a, MDCK cell line and rat brain lysate | BC, IP | E-cadherin | Cadherin–β-catenin adhesion complex | [82] |
| GluN2B (NMDA receptor) | 4 | Rat cerebral cortex, transfected NR2B or MALS | IP, PD | PSD95 | MALS2 directly binds to NR2B | [88] |
| Grindelwald (Drosophila; TNF receptor) | 5 | Transfection of mutated Lin7 | IHC | Stardust-PATJ-Crumbs | Transport of TNF (tumor necrosis factor) receptor | [90] |
| IRSp53 | 3 | Rat brain, MDCK cell line | YTH, IP | SAP102 | Formation or maintenance of the adhesion structure of epithelium | [97] |
| LET-23 (C. elegans; EGF receptor) | 5 | Transfection of mutated Lin-7 | IHC, YTH | CASK (Lin2) | Vulval induction | [98] |
| LET-23 | 5 | Transfection of mutated Lin-7 | IHC | Lin2-Lin10 complex | Transport of LET-23 from the Golgi apparatus to the cell membrane | [89] |
| Mint (Lin10) | 1 | Rat homolog of the C. elegans Lin10 | Cloning, IHC | CASK (Lin2) | Distributed in the membrane fraction in rat brain | [99] |
| MPP4 | 1 | Porcine retinal membranes Transfection of bovine MPP4 L27C or L27N + C domain | IP, PD | MPP5 | Veli3 and MPP4 most intense staining in photoreceptor terminals of the outer plexiform layer (OPL) | [72] |
| MPP5 (Pals1) | 1 | Cloning of Lin-7 binding partners | PD | MPP6 CASK (Lin2) | Localize to the lateral membrane | [76] |
| MPP6 (VAM1, Pals2) | 1 | Cloning of Lin7 binding partners | Cloning, PD | MPP5 CASK (Lin2) | Localize to the lateral membrane | [76] |
| MPP6 | 1 | Transfection of human Veli1 binds to VAM1 | PD | MPP6 does not bind to 4.1R | [100] | |
| MPP7 | 1 | Transfected human MPP7 L27C domain | PD | Dlg1 | Enhanced localization of Dlg1 to cell junction | [71] |
| Rhotekin | 2 | COS7 cells and rat brain | YTH | PIST | Trafficking of protein in synapses | [101] |
| Stardust (Drosophila; Pals1) | 1 | Transfection of mutated Lin7 | IHC | Crumbs | Transport of Grindelwalt (homologous to TNFR) | [90] |
2.4. MPPs and CADMs
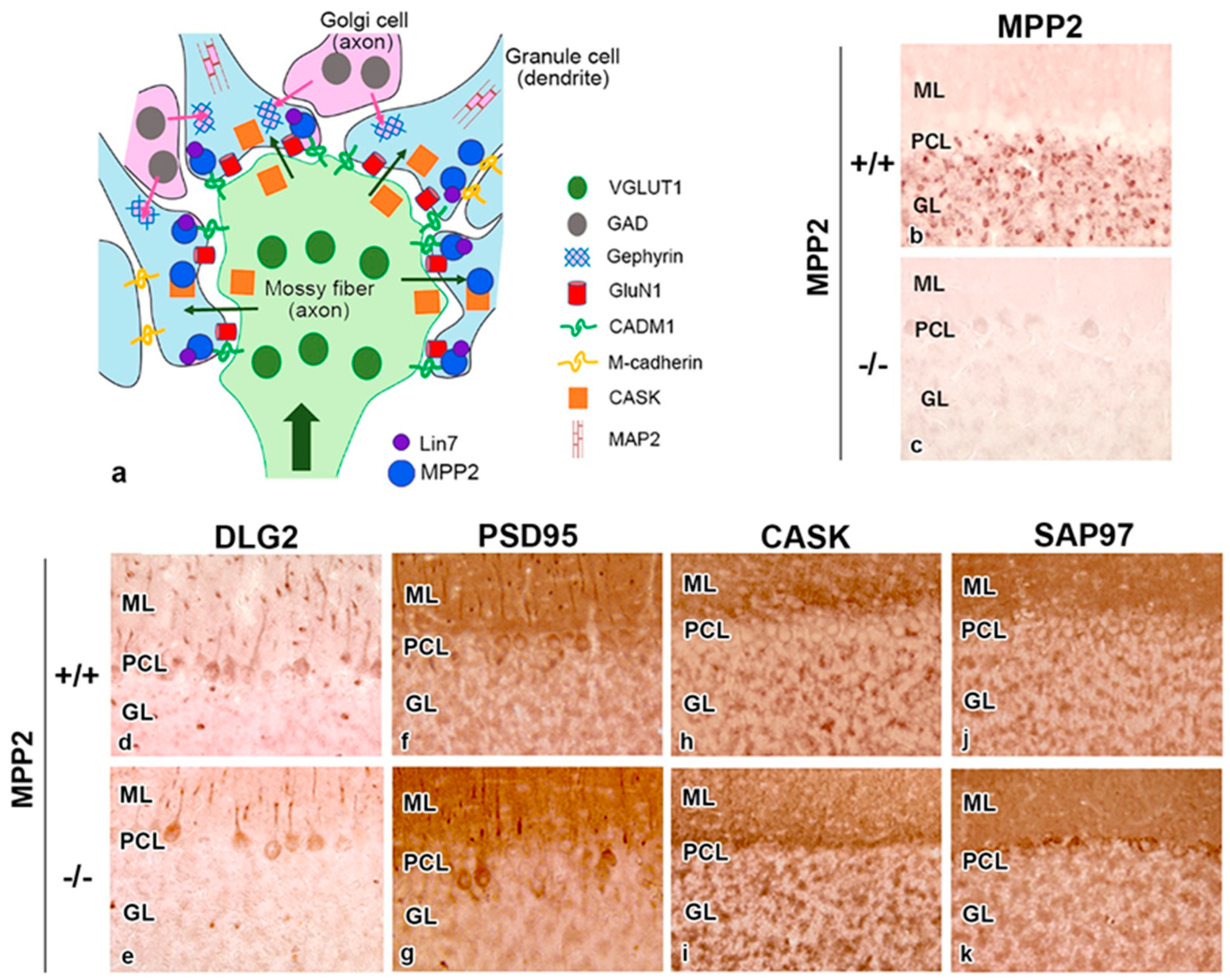
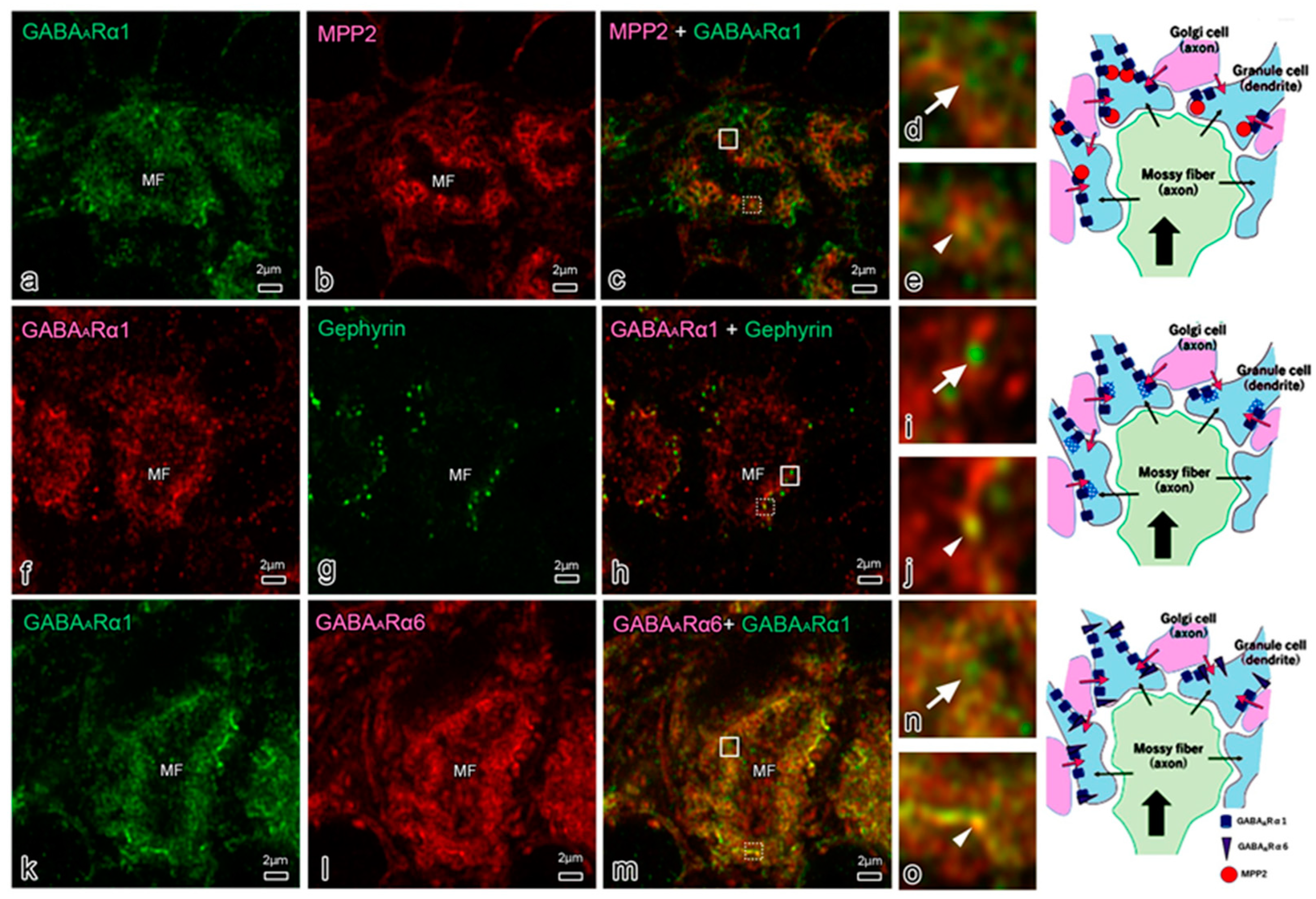
2.5. MPP and Neurotransmitters
2.6. MPP Families in Synapses
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lux, S.E., IV. Anatomy of the red cell membrane skeleton: Unanswered questions. Blood 2016, 127, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.J.; Lu, H.C.; Bennett, P.M. The Protein 4.1 family: Hub proteins in animals for organizing membrane proteins. Biochim. Biophys. Acta 2014, 1838, 605–619. [Google Scholar] [CrossRef]
- Baines, A.J. Link up and fold up-templating the formation of spectrin tetramers. J. Mol. Biol. 2014, 426, 7–10. [Google Scholar] [CrossRef]
- Lux, S.E.; Wolfe, L.C.; Pease, B.; Tomaselli, M.B.; John, K.M.; Bernstein, S.E. Hemolytic anemias due to abnormalities in red cell spectrin: A brief review. Prog. Clin. Biol. Res. 1981, 45, 159–168. [Google Scholar]
- Walensky, L.D.; Gascard, P.; Fields, M.E.; Blackshaw, S.; Conboy, J.G.; Mohandas, N.; Snyder, S.H. The 13-kD FK506 binding protein, FKBP13, interacts with a novel homologue of the erythrocyte membrane cytoskeletal protein 4.1. J. Cell Biol. 1998, 141, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Terada, N.; Yamakawa, H.; Komada, M.; Ohara, O.; Trapp, B.D.; Ohno, S. Expression of protein 4.1G in Schwann cells of the peripheral nervous system. J. Neurosci. Res. 2006, 84, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, A.; Horresh, I.; Golan, N.; Spiegel, I.; Sabanay, H.; Frechter, S.; Ohno, S.; Terada, N.; Mobius, W.; Rosenbluth, J.; et al. The cytoskeletal adapter protein 4.1G organizes the internodes in peripheral myelinated nerves. J. Cell Biol. 2012, 196, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Saitoh, Y.; Ohno, N.; Komada, M.; Saitoh, S.; Peles, E.; Ohno, S. Essential function of protein 4.1G in targeting of membrane protein palmitoylated 6 into Schmidt-Lanterman incisures in myelinated nerves. Mol. Cell Biol. 2012, 32, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Saitoh, Y.; Kamijo, A.; Ohno, S.; Ohno, N. Involvement of membrane skeletal molecules in the Schmidt-Lanterman incisure in Schwann cells. Med. Mol. Morphol. 2016, 49, 5–10. [Google Scholar] [CrossRef]
- Terada, N.; Ohno, N.; Saitoh, S.; Saitoh, Y.; Komada, M.; Kubota, H.; Ohno, S. Involvement of a membrane skeletal protein, 4.1G, for Sertoli/germ cell interaction. Reproduction 2010, 139, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Ohno, N.; Yamauchi, J.; Sakamoto, T.; Terada, N. Deficiency of a membrane skeletal protein, 4.1G, results in myelin abnormalities in the peripheral nervous system. Histochem. Cell Biol. 2017, 148, 597–606. [Google Scholar] [CrossRef]
- Van Pomeren, M.; Selles, R.W.; van Ginneken, B.T.; Schreuders, T.A.; Janssen, W.G.; Stam, H.J. The hypothesis of overwork weakness in Charcot-Marie-Tooth: A critical evaluation. J. Rehabil. Med. 2009, 41, 32–34. [Google Scholar] [CrossRef]
- Vinci, P.; Perelli, S.L.; Gargiulo, P. About the hypothesis of overwork weakness in Charcot-Marie-Tooth disease. J. Rehabil. Med. 2009, 41, 778. [Google Scholar] [CrossRef]
- Kamijo, A.; Saitoh, Y.; Ohno, N.; Ohno, S.; Terada, N. Immunohistochemical study of mouse sciatic nerves under various stretching conditions with “in vivo cryotechnique”. J. Neurosci. Methods 2014, 227, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Golan, N.; Kartvelishvily, E.; Spiegel, I.; Salomon, D.; Sabanay, H.; Rechav, K.; Vainshtein, A.; Frechter, S.; Maik-Rachline, G.; Eshed-Eisenbach, Y.; et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J. Neurosci. 2013, 33, 10950–10961. [Google Scholar] [CrossRef]
- Spiegel, I.; Adamsky, K.; Eshed, Y.; Milo, R.; Sabanay, H.; Sarig-Nadir, O.; Horresh, I.; Scherer, S.S.; Rasband, M.N.; Peles, E. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat. Neurosci. 2007, 10, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Ikeda, W.; Kishimoto, M.; Ogita, H.; Takai, Y. Silencing of ErbB3/ErbB2 signaling by immunoglobulin-like Necl-2. J. Biol. Chem. 2009, 284, 23793–23805. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Kedashiro, S.; Maruoka, M.; Ueda, Y.; Takai, Y. Nectin-like molecule-4/cell adhesion molecule 4 inhibits the ligand-induced dimerization of ErbB3 with ErbB2. Sci. Rep. 2017, 7, 11375. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Azizan, E.A.B.; Goodchild, E.; Garg, S.; Hagiyama, M.; Cabrera, C.P.; Fernandes-Rosa, F.L.; Boulkroun, S.; Kuan, J.L.; Tiang, Z.; et al. Somatic mutations of CADM1 in aldosterone-producing adenomas and gap junction-dependent regulation of aldosterone production. Nat. Genet. 2023, 55, 1009–1021. [Google Scholar] [CrossRef]
- Ito, A.; Ichiyanagi, N.; Ikeda, Y.; Hagiyama, M.; Inoue, T.; Kimura, K.B.; Sakurai, M.A.; Hamaguchi, K.; Murakami, Y. Adhesion molecule CADM1 contributes to gap junctional communication among pancreatic islet α-cells and prevents their excessive secretion of glucagon. Islets 2012, 4, 49–55. [Google Scholar] [CrossRef]
- Cheng, C.L.; Molday, R.S. Interaction of 4.1G and cGMP-gated channels in rod photoreceptor outer segments. J. Cell Sci. 2013, 126, 5725–5734. [Google Scholar] [CrossRef]
- Sanuki, R.; Watanabe, S.; Sugita, Y.; Irie, S.; Kozuka, T.; Shimada, M.; Ueno, S.; Usukura, J.; Furukawa, T. Protein-4.1G-mediated membrane trafficking is essential for correct rod synaptic location in the retina and for normal visual function. Cell Rep. 2015, 10, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wang, Z. 4.1N-mediated interactions and functions in nerve system and cancer. Front. Mol. Biosci. 2021, 8, 711302. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Andrews, S.B.; Wong, A.; O’Connell, M.; Griffin, J.W. Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J. Neurocytol. 1989, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Liang, J.Y.; Vu, L.V.; Yu, F.H.; Ou, A.C.; Ou, J.P.; Zhang, H.S.; Burnett, K.M.; Benz, E.J., Jr. Epithelial-specific isoforms of protein 4.1R promote adherens junction assembly in maturing epithelia. J. Biol. Chem. 2020, 295, 191–211. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Saito, M.; Sugai, M.; Katsushima, Y.; Yanagisawa, T.; Sukegawa, J.; Nakahata, N. Increase in cell-surface localization of parathyroid hormone receptor by cytoskeletal protein 4.1G. Biochem. J. 2005, 392, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Chiba, A.; Sukegawa, J.; Yanagisawa, T.; Saito, M.; Nakahata, N. Suppression of adenylyl cyclase-mediated cAMP production by plasma membrane associated cytoskeletal protein 4.1G. Cell Signal. 2013, 25, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Cui, L.; Hirano, M.; Li, G.; Yanagisawa, T.; Sato, T.; Sukegawa, J. Activity of adenylyl cyclase type 6 is suppressed by direct binding of the cytoskeletal protein 4.1G. Mol. Pharmacol. 2019, 96, 441–451. [Google Scholar] [CrossRef]
- Saito, M.; Hirano, M.; Izumi, T.; Mori, Y.; Ito, K.; Saitoh, Y.; Terada, N.; Sato, T.; Sukegawa, J. Cytoskeletal Protein 4.1G is essential for the primary ciliogenesis and osteoblast differentiation in bone formation. Int. J. Mol. Sci. 2022, 23, 2094. [Google Scholar] [CrossRef]
- Pala, R.; Alomari, N.; Nauli, S.M. Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Therond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.S.; Chang, C.F.; Lin, S.S. Sonic hedgehog signaling in organogenesis, tumors, and tumor microenvironments. Int. J. Mol. Sci. 2020, 21, 758. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I.S.; Kato, Y. Ciliary signaling in stem cells in health and disease: Hedgehog pathway and beyond. Semin. Cell Dev. Biol. 2022, 129, 115–125. [Google Scholar] [CrossRef]
- Qiu, N.; Xiao, Z.; Cao, L.; Buechel, M.M.; David, V.; Roan, E.; Quarles, L.D. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J. Cell Sci. 2012, 125, 1945–1957. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, J.; He, X.; Serra, R.; Qu, J.; Cao, X.; Yang, S. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat. Commun. 2016, 7, 11024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Q.; Zhang, H.; Tao, D.; Wang, Y.; Yue, R.; Sun, Y. Lineage tracing of cells expressing the ciliary gene IFT140 during bone development. Dev. Dyn. 2021, 250, 574–583. [Google Scholar] [CrossRef]
- Noda, K.; Kitami, M.; Kitami, K.; Kaku, M.; Komatsu, Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc. Natl. Acad. Sci. USA 2016, 113, E2589–E2597. [Google Scholar] [CrossRef]
- Uihlein, A.V.; Leder, B.Z. Anabolic therapies for osteoporosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 507–525. [Google Scholar] [CrossRef]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Roberson, P.; Parfitt, A.M.; Manolagas, S.C. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Investig. 1999, 104, 439–446. [Google Scholar] [CrossRef]
- Balani, D.H.; Ono, N.; Kronenberg, H.M. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J. Clin. Investig. 2017, 127, 3327–3338. [Google Scholar] [CrossRef]
- Zheng, L.; Cao, Y.; Ni, S.; Qi, H.; Ling, Z.; Xu, X.; Zou, X.; Wu, T.; Deng, R.; Hu, B.; et al. Ciliary parathyroid hormone signaling activates transforming growth factor-β to maintain intervertebral disc homeostasis during aging. Bone Res. 2018, 6, 21. [Google Scholar] [CrossRef]
- Martin-Guerrero, E.; Tirado-Cabrera, I.; Buendia, I.; Alonso, V.; Gortazar, A.R.; Ardura, J.A. Primary cilia mediate parathyroid hormone receptor type 1 osteogenic actions in osteocytes and osteoblasts via Gli activation. J. Cell Physiol. 2020, 235, 7356–7369. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Cabrera, I.; Martin-Guerrero, E.; Heredero-Jimenez, S.; Ardura, J.A.; Gortazar, A.R. PTH1R translocation to primary cilia in mechanically-stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL-6 secretion. J. Cell Physiol. 2022, 237, 3927–3943. [Google Scholar] [CrossRef]
- Nunomura, W.; Takakuwa, Y.; Parra, M.; Conboy, J.; Mohandas, N. Regulation of protein 4.1R, p55, and glycophorin C ternary complex in human erythrocyte membrane. J. Biol. Chem. 2000, 275, 24540–24546. [Google Scholar] [CrossRef]
- Dimitratos, S.D.; Woods, D.F.; Stathakis, D.G.; Bryant, P.J. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. BioEssays News Rev. Mol. Cell. Dev. Biol. 1999, 21, 912–921. [Google Scholar] [CrossRef]
- Chytla, A.; Gajdzik-Nowak, W.; Olszewska, P.; Biernatowska, A.; Sikorski, A.F.; Czogalla, A. Not just another scaffolding protein family: The multifaceted MPPs. Molecules 2020, 25, 4954. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.S.; Jeong, J.J.; Zeng, L.; Takoudis, C.G.; Quinn, B.J.; Khan, A.A.; Hanada, T.; Chishti, A.H. Alternatively spliced exon 5 of the FERM domain of protein 4.1R encodes a novel binding site for erythrocyte p55 and is critical for membrane targeting in epithelial cells. Biochim. Biophys. Acta 2009, 1793, 281–289. [Google Scholar] [CrossRef][Green Version]
- Fukata, Y.; Fukata, M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 2010, 11, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Kamijo, A.; Yamauchi, J.; Sakamoto, T.; Terada, N. The membrane palmitoylated protein, MPP6, is involved in myelin formation in the mouse peripheral nervous system. Histochem. Cell Biol. 2019, 151, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bolis, A.; Coviello, S.; Visigalli, I.; Taveggia, C.; Bachi, A.; Chishti, A.H.; Hanada, T.; Quattrini, A.; Previtali, S.C.; Biffi, A.; et al. Dlg1, Sec8, and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J. Neurosci. 2009, 29, 8858–8870. [Google Scholar] [CrossRef] [PubMed]
- Valiente, M.; Andres-Pons, A.; Gomar, B.; Torres, J.; Gil, A.; Tapparel, C.; Antonarakis, S.E.; Pulido, R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J. Biol. Chem. 2005, 280, 28936–28943. [Google Scholar] [CrossRef]
- Cotter, L.; Ozcelik, M.; Jacob, C.; Pereira, J.A.; Locher, V.; Baumann, R.; Relvas, J.B.; Suter, U.; Tricaud, N. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science 2010, 328, 1415–1418. [Google Scholar] [CrossRef]
- Noseda, R.; Belin, S.; Piguet, F.; Vaccari, I.; Scarlino, S.; Brambilla, P.; Martinelli Boneschi, F.; Feltri, M.L.; Wrabetz, L.; Quattrini, A.; et al. DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J. Neurosci. 2013, 33, 15295–15305. [Google Scholar] [CrossRef]
- Domenech-Estevez, E.; Baloui, H.; Meng, X.; Zhang, Y.; Deinhardt, K.; Dupree, J.L.; Einheber, S.; Chrast, R.; Salzer, J.L. Akt regulates axon wrapping and myelin sheath thickness in the PNS. J. Neurosci. 2016, 36, 4506–4521. [Google Scholar] [CrossRef] [PubMed]
- Figlia, G.; Gerber, D.; Suter, U. Myelination and mTOR. Glia 2018, 66, 693–707. [Google Scholar] [CrossRef]
- Norrmen, C.; Suter, U. Akt/mTOR signalling in myelination. Biochem. Soc. Trans. 2013, 41, 944–950. [Google Scholar] [CrossRef]
- Pereira, J.A.; Lebrun-Julien, F.; Suter, U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012, 35, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Goebbels, S.; Oltrogge, J.H.; Wolfer, S.; Wieser, G.L.; Nientiedt, T.; Pieper, A.; Ruhwedel, T.; Groszer, M.; Sereda, M.W.; Nave, K.A. Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy. EMBO Mol. Med. 2012, 4, 486–499. [Google Scholar] [CrossRef]
- Bolino, A.; Bolis, A.; Previtali, S.C.; Dina, G.; Bussini, S.; Dati, G.; Amadio, S.; Del Carro, U.; Mruk, D.D.; Feltri, M.L.; et al. Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J. Cell Biol. 2004, 167, 711–721. [Google Scholar] [CrossRef]
- Guerrero-Valero, M.; Grandi, F.; Cipriani, S.; Alberizzi, V.; Di Guardo, R.; Chicanne, G.; Sawade, L.; Bianchi, F.; Del Carro, U.; De Curtis, I.; et al. Dysregulation of myelin synthesis and actomyosin function underlies aberrant myelin in CMT4B1 neuropathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2009469118. [Google Scholar] [CrossRef] [PubMed]
- Saarikangas, J.; Zhao, H.; Lappalainen, P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010, 90, 259–289. [Google Scholar] [CrossRef]
- Goebbels, S.; Oltrogge, J.H.; Kemper, R.; Heilmann, I.; Bormuth, I.; Wolfer, S.; Wichert, S.P.; Mobius, W.; Liu, X.; Lappe-Siefke, C.; et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 2010, 30, 8953–8964. [Google Scholar] [CrossRef]
- Quinn, B.J.; Welch, E.J.; Kim, A.C.; Lokuta, M.A.; Huttenlocher, A.; Khan, A.A.; Kuchay, S.M.; Chishti, A.H. Erythrocyte scaffolding protein p55/MPP1 functions as an essential regulator of neutrophil polarity. Proc. Natl. Acad. Sci. USA 2009, 106, 19842–19847. [Google Scholar] [CrossRef]
- Baumgartner, M.; Weiss, A.; Fritzius, T.; Heinrich, J.; Moelling, K. The PDZ protein MPP2 interacts with c-Src in epithelial cells. Exp. Cell Res. 2009, 315, 2888–2898. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Saitoh, Y.; Ohno, N.; Komada, M.; Yamauchi, J.; Ohno, S. Involvement of Src in the membrane skeletal complex, MPP6-4.1G, in Schmidt-Lanterman incisures of mouse myelinated nerve fibers in PNS. Histochem. Cell Biol. 2013, 140, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Poliak, S.; Matlis, S.; Ullmer, C.; Scherer, S.S.; Peles, E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J. Cell Biol. 2002, 159, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Butz, S.; Okamoto, M.; Sudhof, T.C. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 1998, 94, 773–782. [Google Scholar] [CrossRef]
- Aartsen, W.M.; Kantardzhieva, A.; Klooster, J.; van Rossum, A.G.; van de Pavert, S.A.; Versteeg, I.; Cardozo, B.N.; Tonagel, F.; Beck, S.C.; Tanimoto, N.; et al. Mpp4 recruits Psd95 and Veli3 towards the photoreceptor synapse. Hum. Mol. Genet. 2006, 15, 1291–1302. [Google Scholar] [CrossRef]
- Bohl, J.; Brimer, N.; Lyons, C.; Vande Pol, S.B. The stardust family protein MPP7 forms a tripartite complex with LIN7 and DLG1 that regulates the stability and localization of DLG1 to cell junctions. J. Biol. Chem. 2007, 282, 9392–9400. [Google Scholar] [CrossRef] [PubMed]
- Stohr, H.; Molday, L.L.; Molday, R.S.; Weber, B.H.; Biedermann, B.; Reichenbach, A.; Kramer, F. Membrane-associated guanylate kinase proteins MPP4 and MPP5 associate with Veli3 at distinct intercellular junctions of the neurosensory retina. J. Comp. Neurol. 2005, 481, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Long, J.F.; Fan, J.S.; Suetake, T.; Zhang, M. The tetrameric L27 domain complex as an organization platform for supramolecular assemblies. Nat. Struct. Mol. Biol. 2004, 11, 475–480. [Google Scholar] [CrossRef]
- Harris, B.Z.; Venkatasubrahmanyam, S.; Lim, W.A. Coordinated folding and association of the LIN-2, -7 (L27) domain. An obligate heterodimerization involved in assembly of signaling and cell polarity complexes. J. Biol. Chem. 2002, 277, 34902–34908. [Google Scholar] [CrossRef]
- Petrosky, K.Y.; Ou, H.D.; Lohr, F.; Dotsch, V.; Lim, W.A. A general model for preferential hetero-oligomerization of LIN-2/7 domains: Mechanism underlying directed assembly of supramolecular signaling complexes. J. Biol. Chem. 2005, 280, 38528–38536. [Google Scholar] [CrossRef] [PubMed]
- Kamberov, E.; Makarova, O.; Roh, M.; Liu, A.; Karnak, D.; Straight, S.; Margolis, B. Molecular cloning and characterization of Pals, proteins associated with mLin-7. J. Biol. Chem. 2000, 275, 11425–11431. [Google Scholar] [CrossRef]
- Ozcelik, M.; Cotter, L.; Jacob, C.; Pereira, J.A.; Relvas, J.B.; Suter, U.; Tricaud, N. Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves. J. Neurosci. 2010, 30, 4120–4131. [Google Scholar] [CrossRef]
- Zollinger, D.R.; Chang, K.J.; Baalman, K.; Kim, S.; Rasband, M.N. The polarity protein Pals1 regulates radial sorting of axons. J. Neurosci. 2015, 35, 10474–10484. [Google Scholar] [CrossRef]
- Yamada, T.; Saitoh, Y.; Kametani, K.; Kamijo, A.; Sakamoto, T.; Terada, N. Involvement of membrane palmitoylated protein 2 (MPP2) in the synaptic molecular complex at the mouse cerebellar glomerulus. Histochem. Cell Biol. 2022, 158, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, A.; Saitoh, Y.; Sakamoto, T.; Kubota, H.; Yamauchi, J.; Terada, N. Scaffold protein Lin7 family in membrane skeletal protein complex in mouse seminiferous tubules. Histochem. Cell Biol. 2019, 152, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through Lin7/β-catenin. PLoS ONE 2009, 4, e6167. [Google Scholar] [CrossRef]
- Perego, C.; Vanoni, C.; Massari, S.; Longhi, R.; Pietrini, G. Mammalian LIN-7 PDZ proteins associate with β-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 2000, 19, 3978–3989. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Morishita, R.; Nagata, K.I. Functions of Rhotekin, an effector of Rho GTPase, and its binding partners in mammals. Int. J. Mol. Sci. 2018, 19, 2121. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Perego, C.; Padovano, V.; D’Amico, A.; Raimondi, A.; Francolini, M.; Pietrini, G. LIN7 mediates the recruitment of IRSp53 to tight junctions. Traffic 2009, 10, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Burette, A.C.; Park, H.; Weinberg, R.J. Postsynaptic distribution of IRSp53 in spiny excitatory and inhibitory neurons. J. Comp. Neurol. 2014, 522, 2164–2178. [Google Scholar] [CrossRef]
- Sawallisch, C.; Berhorster, K.; Disanza, A.; Mantoani, S.; Kintscher, M.; Stoenica, L.; Dityatev, A.; Sieber, S.; Kindler, S.; Morellini, F.; et al. The insulin receptor substrate of 53 kDa (IRSp53) limits hippocampal synaptic plasticity. J. Biol. Chem. 2009, 284, 9225–9236. [Google Scholar] [CrossRef]
- Setou, M.; Nakagawa, T.; Seog, D.H.; Hirokawa, N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 2000, 288, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Derin, R.; Li, M.; Bredt, D.S. Characterization of MALS/Velis-1, -2, and -3: A family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J. Neurosci. 1999, 19, 4189–4199. [Google Scholar] [CrossRef]
- Gauthier, K.D.; Rocheleau, C.E. LIN-10 can promote LET-23 EGFR signaling and trafficking independently of LIN-2 and LIN-7. Mol. Biol. Cell 2021, 32, 788–799. [Google Scholar] [CrossRef]
- Andersen, D.S.; Colombani, J.; Palmerini, V.; Chakrabandhu, K.; Boone, E.; Rothlisberger, M.; Toggweiler, J.; Basler, K.; Mapelli, M.; Hueber, A.O.; et al. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature 2015, 522, 482–486. [Google Scholar] [CrossRef]
- Olsen, O.; Wade, J.B.; Morin, N.; Bredt, D.S.; Welling, P.A. Differential localization of mammalian Lin-7 (MALS/Veli) PDZ proteins in the kidney. Am. J. Physiol. Renal Physiol. 2005, 288, F345–F352. [Google Scholar] [CrossRef] [PubMed]
- Misawa, H.; Kawasaki, Y.; Mellor, J.; Sweeney, N.; Jo, K.; Nicoll, R.A.; Bredt, D.S. Contrasting localizations of MALS/LIN-7 PDZ proteins in brain and molecular compensation in knockout mice. J. Biol. Chem. 2001, 276, 9264–9272. [Google Scholar] [CrossRef]
- Matsumoto, A.; Mizuno, M.; Hamada, N.; Nozaki, Y.; Jimbo, E.F.; Momoi, M.Y.; Nagata, K.; Yamagata, T. LIN7A depletion disrupts cerebral cortex development, contributing to intellectual disability in 12q21-deletion syndrome. PLoS ONE 2014, 9, e92695. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Matsumoto, A.; Hamada, N.; Ito, H.; Miyauchi, A.; Jimbo, E.F.; Momoi, M.Y.; Tabata, H.; Yamagata, T.; Nagata, K. Role of an adaptor protein Lin-7B in brain development: Possible involvement in autism spectrum disorders. J. Neurochem. 2015, 132, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Saeki, K.; Jinnouchi, H.; Kazuno, S.; Miura, Y.; Yokomizo, T. The C-terminal region of BLT2 restricts its localization to the lateral membrane in a LIN7C-dependent manner. FASEB J. 2021, 35, e21364. [Google Scholar] [CrossRef]
- Bachmann, A.; Grawe, F.; Johnson, K.; Knust, E. Drosophila Lin-7 is a component of the Crumbs complex in epithelia and photoreceptor cells and prevents light-induced retinal degeneration. Eur. J. Cell Biol. 2008, 87, 123–136. [Google Scholar] [CrossRef]
- Hori, K.; Konno, D.; Maruoka, H.; Sobue, K. MALS is a binding partner of IRSp53 at cell-cell contacts. FEBS Lett. 2003, 554, 30–34. [Google Scholar] [CrossRef]
- Simske, J.S.; Kaech, S.M.; Harp, S.A.; Kim, S.K. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell 1996, 85, 195–204. [Google Scholar] [CrossRef]
- Ide, N.; Hirao, K.; Hata, Y.; Takeuchi, M.; Irie, M.; Yao, I.; Deguchi, M.; Toyoda, A.; Nishioka, H.; Mizoguchi, A.; et al. Molecular cloning and characterization of rat lin-10. Biochem. Biophys. Res. Commun. 1998, 243, 634–638. [Google Scholar] [CrossRef]
- Tseng, T.C.; Marfatia, S.M.; Bryant, P.J.; Pack, S.; Zhuang, Z.; O’Brien, J.E.; Lin, L.; Hanada, T.; Chishti, A.H. VAM-1: A new member of the MAGUK family binds to human Veli-1 through a conserved domain. Biochim. Biophys. Acta 2001, 1518, 249–259. [Google Scholar] [CrossRef]
- Sudo, K.; Ito, H.; Iwamoto, I.; Morishita, R.; Asano, T.; Nagata, K. Identification of a cell polarity-related protein, Lin-7B, as a binding partner for a Rho effector, Rhotekin, and their possible interaction in neurons. Neurosci. Res. 2006, 56, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Shingai, T.; Ikeda, W.; Kakunaga, S.; Morimoto, K.; Takekuni, K.; Itoh, S.; Satoh, K.; Takeuchi, M.; Imai, T.; Monden, M.; et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 2003, 278, 35421–35427. [Google Scholar] [CrossRef] [PubMed]
- Biederer, T.; Sara, Y.; Mozhayeva, M.; Atasoy, D.; Liu, X.; Kavalali, E.T.; Sudhof, T.C. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002, 297, 1525–1531. [Google Scholar] [CrossRef]
- Fukuhara, H.; Masuda, M.; Yageta, M.; Fukami, T.; Kuramochi, M.; Maruyama, T.; Kitamura, T.; Murakami, Y. Association of a lung tumor suppressor TSLC1 with MPP3, a human homologue of Drosophila tumor suppressor Dlg. Oncogene 2003, 22, 6160–6165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rademacher, N.; Schmerl, B.; Lardong, J.A.; Wahl, M.C.; Shoichet, S.A. MPP2 is a postsynaptic MAGUK scaffold protein that links SynCAM1 cell adhesion molecules to core components of the postsynaptic density. Sci. Rep. 2016, 6, 35283. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Tanabe, Y.; Imhof, B.A.; Momoi, M.Y.; Momoi, T. A complex of synaptic adhesion molecule CADM1, a molecule related to autism spectrum disorder, with MUPP1 in the cerebellum. J. Neurochem. 2012, 123, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Hamori, J. Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat. Embryol. 1988, 179, 81–88. [Google Scholar] [CrossRef]
- Fukaya, M.; Watanabe, M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: A study in the adult mouse brain. J. Comp. Neurol. 2000, 426, 572–586. [Google Scholar] [CrossRef]
- Bissen, D.; Foss, F.; Acker-Palmer, A. AMPA receptors and their minions: Auxiliary proteins in AMPA receptor trafficking. Cell Mol. Life Sci. 2019, 76, 2133–2169. [Google Scholar] [CrossRef]
- Won, S.; Levy, J.M.; Nicoll, R.A.; Roche, K.W. MAGUKs: Multifaceted synaptic organizers. Curr. Opin. Neurobiol. 2017, 43, 94–101. [Google Scholar] [CrossRef]
- Yamada, K.; Fukaya, M.; Shimizu, H.; Sakimura, K.; Watanabe, M. NMDA receptor subunits GluRε1, GluRε3 and GluRζ1 are enriched at the mossy fibre-granule cell synapse in the adult mouse cerebellum. Eur. J. Neurosci. 2001, 13, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Wenthold, R.J. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. Eur. J. Neurosci. 2002, 15, 583–587. [Google Scholar] [CrossRef]
- Yamazaki, M.; Fukaya, M.; Hashimoto, K.; Yamasaki, M.; Tsujita, M.; Itakura, M.; Abe, M.; Natsume, R.; Takahashi, M.; Kano, M.; et al. TARPs γ-2 and γ-7 are essential for AMPA receptor expression in the cerebellum. Eur. J. Neurosci. 2010, 31, 2204–2220. [Google Scholar] [CrossRef]
- Kita, K.; Albergaria, C.; Machado, A.S.; Carey, M.R.; Muller, M.; Delvendahl, I. GluA4 facilitates cerebellar expansion coding and enables associative memory formation. Elife 2021, 10, e65152. [Google Scholar] [CrossRef] [PubMed]
- Schmerl, B.; Gimber, N.; Kuropka, B.; Stumpf, A.; Rentsch, J.; Kunde, S.A.; von Sivers, J.; Ewers, H.; Schmitz, D.; Freund, C.; et al. The synaptic scaffold protein MPP2 interacts with GABAA receptors at the periphery of the postsynaptic density of glutamatergic synapses. PLoS Biol. 2022, 20, e3001503. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Chiou, L.C.; Ernst, M.; Fabjan, J.; Savić, M.M.; Lee, M.T. α6-Containing GABAA Receptors: Functional roles and therapeutic potentials. Pharmacol. Rev. 2022, 74, 238–270. [Google Scholar] [CrossRef]
- Tomita, S. Molecular constituents and localization of the ionotropic GABA receptor complex in vivo. Curr. Opin. Neurobiol. 2019, 57, 81–86. [Google Scholar] [CrossRef]
- Shin, S.M.; Skaar, S.; Danielson, E.; Lee, S.H. Aberrant expression of S-SCAM causes the loss of GABAergic synapses in hippocampal neurons. Sci. Rep. 2020, 10, 83. [Google Scholar] [CrossRef]
- Chen, M.; Koopmans, F.; Paliukhovich, I.; van der Spek, S.J.F.; Dong, J.; Smit, A.B.; Li, K.W. Blue native PAGE-antibody shift in conjunction with mass spectrometry to reveal protein subcomplexes: Detection of a cerebellar α1/α6-Subunits containing γ-aminobutyric acid type A receptor subtype. Int. J. Mol. Sci. 2023, 24, 7632. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, N.; Chandra, D.; Homanics, G.E.; Olsen, R.W.; Houser, C.R. Altered localization of the δ subunit of the GABAA receptor in the thalamus of α4 subunit knockout mice. Neurochem. Res. 2014, 39, 1104–1117. [Google Scholar] [CrossRef]
- Kralic, J.E.; Sidler, C.; Parpan, F.; Homanics, G.E.; Morrow, A.L.; Fritschy, J.M. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J. Comp. Neurol. 2006, 495, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.C.; Jenkins, P.M.; Tanaka, M.; Mooney, R.; Bennett, V. Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proc. Natl. Acad. Sci. USA 2015, 112, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Loebrich, S.; Bahring, R.; Katsuno, T.; Tsukita, S.; Kneussel, M. Activated radixin is essential for GABAA receptor α5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006, 25, 987–999. [Google Scholar] [CrossRef]
- Zhou, X.; Gueydan, M.; Jospin, M.; Ji, T.; Valfort, A.; Pinan-Lucarre, B.; Bessereau, J.L. The netrin receptor UNC-40/DCC assembles a postsynaptic scaffold and sets the synaptic content of GABAA receptors. Nat. Commun. 2020, 11, 2674. [Google Scholar] [CrossRef]
- Lin, E.I.; Jeyifous, O.; Green, W.N. CASK regulates SAP97 conformation and its interactions with AMPA and NMDA receptors. J. Neurosci. 2013, 33, 12067–12076. [Google Scholar] [CrossRef]
- Ye, F.; Zeng, M.; Zhang, M. Mechanisms of MAGUK-mediated cellular junctional complex organization. Curr. Opin. Struct. Biol. 2018, 48, 6–15. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.A.; Grant, S.G. Supramolecular organization of NMDA receptors and the postsynaptic density. Curr. Opin. Neurobiol. 2017, 45, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Jimbo, E.; Tanabe, Y.; Yu, Z.; Kojima, K.; Mori, M.; Li, H.; Iwamoto, S.; Yamagata, T.; Momoi, M.Y.; Momoi, T. The association of GPR85 with PSD-95-neuroligin complex and autism spectrum disorder: A molecular analysis. Mol. Autism 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Ganapathiraju, M.K.; Thahir, M.; Handen, A.; Sarkar, S.N.; Sweet, R.A.; Nimgaonkar, V.L.; Loscher, C.E.; Bauer, E.M.; Chaparala, S. Schizophrenia interactome with 504 novel protein-protein interactions. NPJ Schizophr. 2016, 2, 16012. [Google Scholar] [CrossRef]
- Mullins, N.; Forstner, A.J.; O’Connell, K.S.; Coombes, B.; Coleman, J.R.I.; Qiao, Z.; Als, T.D.; Bigdeli, T.B.; Borte, S.; Bryois, J.; et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021, 53, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 2019, 179, 1469–1482.e11. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Tobler, R.; Souilmi, Y.; Huber, C.D.; Bean, N.; Turney, C.S.M.; Grey, S.T.; Cooper, A. The role of genetic selection and climatic factors in the dispersal of anatomically modern humans out of Africa. Proc. Natl. Acad. Sci. USA 2023, 120, e2213061120. [Google Scholar] [CrossRef]
- Khoury, S.; Wang, Q.P.; Parisien, M.; Gris, P.; Bortsov, A.V.; Linnstaedt, S.D.; McLean, S.A.; Tungate, A.S.; Sofer, T.; Lee, J.; et al. Multi-ethnic GWAS and meta-analysis of sleep quality identify MPP6 as a novel gene that functions in sleep center neurons. Sleep 2021, 44, zsaa211. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G. The molecular evolution of the vertebrate behavioural repertoire. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, N.; Saitoh, Y.; Saito, M.; Yamada, T.; Kamijo, A.; Yoshizawa, T.; Sakamoto, T. Recent Progress on Genetically Modified Animal Models for Membrane Skeletal Proteins: The 4.1 and MPP Families. Genes 2023, 14, 1942. https://doi.org/10.3390/genes14101942
Terada N, Saitoh Y, Saito M, Yamada T, Kamijo A, Yoshizawa T, Sakamoto T. Recent Progress on Genetically Modified Animal Models for Membrane Skeletal Proteins: The 4.1 and MPP Families. Genes. 2023; 14(10):1942. https://doi.org/10.3390/genes14101942
Chicago/Turabian StyleTerada, Nobuo, Yurika Saitoh, Masaki Saito, Tomoki Yamada, Akio Kamijo, Takahiro Yoshizawa, and Takeharu Sakamoto. 2023. "Recent Progress on Genetically Modified Animal Models for Membrane Skeletal Proteins: The 4.1 and MPP Families" Genes 14, no. 10: 1942. https://doi.org/10.3390/genes14101942
APA StyleTerada, N., Saitoh, Y., Saito, M., Yamada, T., Kamijo, A., Yoshizawa, T., & Sakamoto, T. (2023). Recent Progress on Genetically Modified Animal Models for Membrane Skeletal Proteins: The 4.1 and MPP Families. Genes, 14(10), 1942. https://doi.org/10.3390/genes14101942






